Abstract
Waterlogging events affect soil properties, which alter plant nutrient availability and result in an increased solubility of micronutrients. Until now, it has not been conclusively determined whether plants take up increased concentrations of plant-available Mn, Fe, Cu, or Zn during a period of waterlogging. The aim of this study was to analyze (1) if the micronutrient concentrations increase in plant tissues after waterlogging or (2) rather lead to micronutrient deficiencies, and (3) if this process depends on the developmental stage in which the plant was flooded. Winter wheat and rapeseed were cultivated in large containers and water-logged at two developmental stages: DC 31 (first node visible) and DC 51 (beginning of ear emergence/floral bud appearance). Early waterlogging did not result in microelement toxicities neither in winter wheat nor in rapeseed, although the Mn concentration in rapeseed shoots was significantly increased. On the contrary, in rapeseed, early waterlogging resulted in significantly decreased Cu and Zn concentrations. After late waterlogging, plants accumulated high amounts of Mn and Fe (wheat) or Mn, Cu, and Zn (rapeseed), leading to toxic levels. We conclude that the occurrence of micronutrient deficiencies or toxicities depends on the developmental stage in which the plant was flooded.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
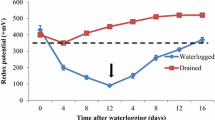
Mineral nutrients are essential for plant growth and development. In plant production, a sufficient supply of mineral nutrients to crop plants is crucial for yield formation. The availability of nutrients depends on the physical, chemical and biological properties of soil. Among these properties, gas diffusion rate, oxidation–reduction status (Eh) and occurrence of soil bacteria affect the solubility of nutrients (Ponnamperuma 1972; Armstrong 1979). In water-logged soils, the gas diffusion rate is significantly reduced resulting in O2 depletion and accumulation of CO2 (Ponnamperuma 1972). As a consequence of O2 shortage, reducing soil compounds increase and Eh declines (Sajwan and Lindsay 1986). The sequential reduction of electron acceptors occurs as follows: oxygen reduction is initiated at < 550 mV, subsequently followed by microbial denitrification and reduction of Mn4+ (< 500 mV). Below 330 mV, the soil is characterized as completely anaerobic. Any further decline of the Eh below < 150 mV results in reduction of Fe3+ to Fe2+. At Eh of < − 100 mV sulfate is reduced to sulfides (Husson 2013).
Wetland plants, such as rice, are tolerant to waterlogging because they developed different morphological and anatomical adaptations, for example, adventitious roots and aerenchyma, to tolerate an O2-deficient root environment (Bailey-Serres and Voesenek 2008). If plants are not adapted to water-logged conditions, for example, wheat and rapeseed among others, growth and metabolism will quickly be inhibited (Huang et al. 1995; Bailey-Serres and Voesenek 2008; Zhang et al. 2016, 2017). Plant roots switch from respiration to anaerobic fermentation causing restrictions in ATP synthesis (Colmer and Voesenek 2009). The affected energy status of the plants limits the capacity to absorb water and nutrients (Colmer and Voesenek 2009) and results in reduced root and shoot dry mass (Huang et al. 1995; Malik et al. 2002). Additionally, plants may suffer from ion toxicities due to the declined soil redox potential, leading to higher concentrations of Mn2+ and Fe3+ in the soil solution. Several studies reported increased concentrations of Mn2+ and Fe3+ in wheat, rapeseed and maize during waterlogging (Stieger and Feller 1994; Setter et al. 2009; Ashraf and Mehmood 1990; Ashraf and Rehman 1999). Khabaz-Saberi et al. (2006) showed that Australian wheat varieties had two- to ten-fold higher Mn and Fe concentrations in the shoot, when suffering from waterlogging. In rapeseed shoots and roots, iron concentration significantly increased during waterlogging, whereas Mn concentration was increased in the roots, but not in the shoots (Ashraf and Mehmood 1990). In leaves of maize water-logged for 34 days, Mn and Fe concentrations increased 20- to 80-fold (Ashraf and Rehman 1999). In contrast to these studies, Steffens et al. (2005) reported that waterlogging caused a sub-optimal nutrient supply for N, P, K, Mn, Cu, and Zn of wheat and barley. They also showed that waterlogging did not induce Mn- or Fe-toxicity, although a higher plant availability and uptake was expected due to reduction processes in soil (Steffens et al. 2005). Studies dealing with responses of other micronutrients than Mn or Fe to waterlogging are scarce and inconsistent (Stieger and Feller 1994; Steffens et al. 2005; Setter et al. 2009). Additionally, in some of the studies, the waterlogging treatment took relatively long [4 weeks (Ashraf and Mehmood 1990); 34 days (Ashraf and Rehman 1999); 38 days (Stieger and Feller 1994)], whereas in other studies very young plants were water-logged [13 days old (Steffens et al. 2005); 22 days old (Setter et al. 2009)], making the studies difficult to compare. Furthermore, the studies do not include a recovery phase and plants were harvested directly at the end of the waterlogging treatment (Stieger and Feller 1994; Steffens et al. 2005). Including recovery phases after waterlogging periods could answer the question of whether plants regain sufficient amounts of micronutrients if they suffer deficiency during waterlogging, or if micronutrients further accumulate in the plant after waterlogging.
For this reason, the objective of this study was to analyze the micronutrient concentrations of wheat and rapeseed plants after a 2-week waterlogging period occurring at the stem elongation stage, at the beginning of ear emergence (wheat), or floral bud appearance (rapeseed), and to answer whether this results in microelement toxicities or deficiencies in winter wheat and winter rapeseed at harvest.
Materials and Methods
Plant Material
All experiments were conducted with the winter wheat (Triticum aestivum L.) cultivars JB Asano and Kredo and the rapeseed (Brassica napus L.) cultivar Avatar.
Experimental Setup
The experiment was carried out in the growing season 2015–2016 in an unheated greenhouse at the experimental station of the Institute of Plant Nutrition and Soil Science, Kiel University, Kiel (54°20′50″, 10°6′55″E). Winter wheat and rapeseed plants were grown to maturity over their entire lifecycle in large containers (height: 0.9 m, area: 0.16 m2, volume: 120 L, according to Hohmann et al. 2016). The containers were filled with soil collected at the experimental farm “Hohenschulen” of Kiel University. Fertilizer application followed standard applications for the field. Plant protection was applied as required. For detailed information see Wollmer et al. (2018).
Treatments
Waterlogging treatments were carried out at different periods during the plants’ life cycle. The first treatment started when the first node was visible (BBCH 31, early waterlogging, W1), whereas the second treatment started at the beginning of ear emergence (winter wheat) or at floral bud appearance (rapeseed) (BBCH 51, late waterlogging, W2). Waterlogging treatments were imposed for 2 weeks by closing the faucets deployed at the bottom of the containers and subsequent watering with water supernatant approx. 2 cm above the soil surface. Re-irrigation was performed daily to keep the containers flooded. The corresponding control (W0) was set at 60% water holding capacity (WHC) throughout the entire crop life cycle. At the end of the waterlogging treatments, faucets were opened to allow drainage of the soil to the respective target WHC of 60%. After drainage, all containers were maintained at 60% WHC until seed maturity. Water supply was monitored by weighing the containers every second day. If necessary, water was added to retain the respective target soil water capacity. Containers were randomized twice a week.
Plant Sampling and Analysis
At the end of the waterlogging treatments, plant samples were harvested and fresh and dry weights were determined. After W1, whole wheat plants were used for analysis, whereas rapeseed plants were separated into shoots and flowers. After W2, wheat plants were separated into leaves plus stem and spikes, rapeseed plants were again separated into shoots and flowers. To analyze if microelements accumulate in the recovery phase after subsequent waterlogging, samples of winter wheat from W1 were taken 4 weeks after the W1 treatment (recovery phase, second sampling date). Finally, all plants grown in one container were harvested at seed maturity. Each plant was separated in to straw, chaff with rachis or pod-walls, and grains. Biomasses of the different fractions were quantified.
Dry weight was determined by oven-drying at 65 °C to constant weight. Samples were milled to a fine powder for further analysis.
For mineral analysis, 200 mg of finely ground plant material was digested with 10 mL 69% HNO3 at 190 °C for 45 min in a microwave oven (1800 W, MARS 6, Xpress, CEM, Matthews, MC, USA) and concentrations of micronutrients were quantified using inductively coupled plasma–mass spectrometry (ICP–MS; Agilent Technologies 7700 Series, Böblingen, Germany) according to the method described by Jezek et al. (2015).
Statistics
Statistical analysis was based on four to five (winter wheat) or three biological replicates (rapeseed). Data were statistically analyzed using R software (2018). Effects of treatments were tested using a statistical mixed model. The data were assumed to be normally distributed and to be heteroscedastic after performance of a graphical residual analysis. Based on this model, an analysis of variances (ANOVA) was performed, followed by multiple contrast tests to compare the several levels of influence factors, respectively.
Results
Micronutrient Concentrations of Winter Wheat and Winter Rapeseed After Early Waterlogging (W1)
When winter wheat plants were water-logged for 2 weeks at BBCH 31, no significant differences in Mn, Fe, or Cu concentrations could be measured in both cultivars (Fig. 1a–c). Zinc concentration was significantly decreased after W1 in Kredo compared to the corresponding control, but not in JB Asano (Fig. 1d).
a Manganese, b Fe, c Cu and d Zn concentrations of the whole wheat plant after early waterlogging. W0 = control, W1 = water-logged at DC 31. Bars representing means + standard errors (n = 4, except JB Asano in W1 where n = 5). Asterisks (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001) indicate significant differences between drained and water-logged plants
Under W1, the Mn concentration of rapeseed shoots significantly increased by 40% compared to the non-waterlogged control (Fig. 2a). In rapeseed flower, a decrease in Mn concentration was visible, but not significant (Fig. 2a). The Fe concentration was not significantly reduced by waterlogging in rapeseed shoots and flowers (Fig. 2b). In rapeseed shoots, waterlogging significantly decreased Cu and Zn concentrations by 43% and 55%, respectively (Fig. 2c, d). Reductions of Cu and Zn concentrations in flower were non-significant (Fig. 2c, d).
a Manganese, b Fe, c Cu and d Zn concentrations of the rapeseed plant separated into vegetative shoot and flower after early waterlogging. W0 = control, W1 = water-logged at DC 31. Bars representing means + standard errors (n = 3). Asterisks (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001) indicate significant differences between drained and water-logged plants
Micronutrient Concentrations of W1 Winter Wheat Plants in the Recovery Phase
At the second sampling date, 4 weeks after the first waterlogging treatment, concentrations of all measured micronutrients in shoots and spikes of JB Asano W1 plants did not significantly differ from their corresponding controls (Fig. 3a–d). In the Kredo, a significant increase of 48% in the Mn concentration in shoots of W1 plants could be measured compared to the non-waterlogged control (Fig. 3a). In contrast, Fe concentrations of Kredo W1 shoots significantly decreased during the recovery period (Fig. 3b). Non-significant differences were observed in the Cu and Zn concentrations in Kredo shoots of W1 plants compared to the corresponding control (Fig. 3c, d). Micronutrient concentrations of Kredo spikes were unaffected by early waterlogging and were comparable to the corresponding controls (Fig. 3a–d).
a Manganese, b Fe, c Cu and d Zn concentrations of the whole wheat plant separated into vegetative shoot and spike after late waterlogging. W0 = control, W1 = water-logged at DC 31, W2 = water-logged at DC 51. Bars representing means + standard errors (n = 4, except JB Asano in W1 and W2 where n = 5). Asterisks (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001) indicate significant differences between drained and water-logged plants
To avoid a too small sample size, no samples of rapeseed plants were taken in the recovery period due to space limitations and a resulting maximum of 12 cultivated plants per container.
Micronutrient Concentrations in Winter Wheat and Winter Rapeseed after Late Waterlogging (W2)
When winter wheat plants were water-logged at ear emergence (BBCH 51), the Mn concentration in shoots of JB Asano and Kredo significantly increased by 46% and 63%, respectively, compared to the corresponding control (Fig. 3a). Spike Mn concentrations were significantly increased by 43%, in JB Asano whereas Mn concentrations of Kredo spikes showed no significant differences between W0 and W2 plants (Fig. 3a). Analyzing the Fe concentration of the shoots revealed no significant differences between waterlogging treatments in both cultivars (Fig. 3b). In JB Asano spikes, the Fe concentration was significantly increased after W2 compared to W0 (Fig. 3b), whereas Kredo spike Fe concentrations showed no significant differences (Fig. 3b). Concentrations of Cu and Zn were unaffected by late waterlogging treatment in shoots and spikes of both cultivars compared to the non-waterlogged controls (Fig. 3c, d).
Waterlogging of rapeseed at the floral bud appearance stage (BBCH 51) did not result in any significant micronutrient changes compared to the controls (Fig. 4a–d).
a Manganese, b Fe, c Cu and d Zn concentrations of the rapeseed plant separated into vegetative shoot and flower after late waterlogging. W0 = control, W2 = water-logged at DC 51. Bars representing means + standard errors (n = 3). Asterisks (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001) indicate significant differences between drained and water-logged plants
Micronutrient Concentrations in Winter Wheat and Winter Rapeseed After Harvest at Maturity
At seed maturity, wheat plants were harvested and separated into straw, chaff, and grains. In JB Asano, the Mn concentrations in straw, chaff, and grains were not significantly different between W0 and W1 plants (Fig. 5a). After W2, the highest Mn concentration could be measured in straw of JB Asano, which was 4.5 times higher than the corresponding control (Fig. 5a). In JB Asano chaff, the Mn concentration increased by 40% in W2 plants compared to the corresponding controls, whereas Mn concentration in the grains was not significantly affected by late waterlogging (Fig. 5a). In Kredo straw, the Mn concentration significantly increased in W1 plants by 60%, but was highest in W2 plants, which was five times higher than in the control and two times higher than in W1 plants (Fig. 5a). The Mn concentration in Kredo chaff was not affected in plants water-logged at W1 nor at W2 compared to W0 (Fig. 5a). Grain Mn concentration of Kredo was not significantly different between W0 and W1 plants, but was significantly increased after W2 (Fig. 5a).
a Manganese, b Fe, c Cu and d Zn concentrations of the whole wheat plant separated in straw, chaff and grains at harvest after maturity. W0 = control, W1 = water-logged at DC 31, W2 = water-logged at DC 51. Bars representing means + standard errors (n = 4, except JB Asano in W1 and W2 where n = 5). Asterisks (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001) indicate significant differences between drained and water-logged plants
Analysis of Fe concentrations revealed no significant differences in JB Asano straw of W0 and W1 plants, but a significant increase of 58% after W2 was measured compared to the non-waterlogged control (Fig. 5b). Iron concentrations of JB Asano chaff and grains were not significantly different in W1 and W2 plants compared to the corresponding controls (Fig. 5b). Analyzing Fe concentrations in Kredo straw revealed no significant differences between the control and the waterlogging treatments, although W2 plants had Fe concentrations that were 2.5 times higher than the control plants (Fig. 5b). The reason for that was a relatively high variation in the obtained single data. In Kredo chaff of W2 plants, a significant decrease of Fe concentration was measured compared to W0 plants, whereas chaff of W1 plants had Fe concentrations comparable to the control (Fig. 5b). In the grains of Kredo, Fe concentration was not significantly increased due the waterlogging treatments (Fig. 5b).
In JB Asano straw, the Cu concentration significantly increased after late waterlogging treatment, but not after early waterlogging, whereas Cu concentrations of chaff and grain were not affected by any waterlogging treatment (Fig. 5c). In contrast, Kredo plants had significantly decreased Cu concentrations in chaff after late waterlogging, but straw and grain Cu concentrations were not affected by W1 nor by W2 (Fig. 5c).
Zinc concentrations in JB Asano and Kredo straw, chaff, and grains showed no significant differences between the waterlogging treatments and the corresponding controls (Fig. 5d).
In rapeseed plants, the Mn concentration of the straw was significantly increased by approx. 80% after the late waterlogging treatment compared to the control, but not after W1 (Fig. 6a). In chaff, the highest Mn concentration was measured in W2 plants, whereas the Mn concentration in chaff of W1 plants was comparable to the control (Fig. 6a). In rapeseed grains, analysis of Mn concentrations showed non-significant differences between the waterlogging treatments and the control (Fig. 6a). In rapeseed straw, chaff, and grains, no significant differences in Fe concentrations could be measured between the different waterlogging treatments (Fig. 6b). After W2, Cu and Zn concentrations increased significantly in straw of rapeseed plants compared to the control and the early waterlogging treatment, whereas chaff and grains showed comparable Cu and Zn concentrations independent of the waterlogging treatment (Fig. 6c, d).
a Manganese, b Fe, c Cu and d Zn concentrations of the whole rapeseed plant separated in straw, chaff, and grains at harvest after maturity. W0 = control, W1 = water-logged at DC 31, W2 = water-logged at DC 51. Bars representing means + standard errors (n = 3). Asterisks (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001) indicate significant differences between drained and water-logged plants
Discussion
Waterlogging events can result in altered soil nutrient availability (Ponnamperuma 1972). The solubility of micronutrients, such as Mn- and Fe-oxides, increases and their concentrations can reach toxic levels for plants (Ponnamperuma 1972; Khabaz-Saberi et al. 2006). It has not yet been conclusively determined if plants take up increased concentrations of plant-available Mn, Fe, Cu, or Zn during a period of waterlogging and if this results in toxicities in the plant tissue. To date, studies dealing with waterlogging and nutrient status of plants are scarce. For this reason, Mn, Fe, Cu, and Zn concentrations were determined in wheat and rapeseed plants after waterlogging at two developmental stages for 2 weeks and at maturity.
In this study it could be shown that the micronutrient concentrations (Mn, Fe, Cu, Zn) of early water-logged plants of both wheat varieties were not significantly different from the levels of control plants with the exception of Zn concentration of Kredo, which was significantly reduced after waterlogging (Fig. 1a, b). These results contrast with a study by Khabaz-Saberi et al. (2006), who showed that Australian wheat varieties had two to ten times increased Mn and Fe concentrations in the shoot when suffering from waterlogging, resulting in growth reduction. In other studies, increased Mn and Fe concentrations were also found after waterlogging events (Stieger and Feller 1994; Setter et al. 2009; Khabaz-Saberi et al. 2012). However, Steffens et al. (2005) were unable to detect increased micronutrient concentrations after a 15-day waterlogging phase, both in the summer wheat variety Thassos and in the spring barley variety Ingrid. Comparable to the results obtained in this study, the concentrations of Cu and Zn were significantly reduced after waterlogging (Steffens et al. 2005). The reason for the decrease in nutrient concentration is described by Steffens et al. (2005): the reduced activity of the plasma membrane H+-ATPase is triggered by low ATP concentrations. Furthermore, they indicate that due to partial depolarization of the membrane potential (triggered by the low H+-ATPase activity), the uptake of inorganic cations is more impaired by waterlogging than the uptake of inorganic anions (Steffens et al. 2005). This is not only true for crops such as wheat and barley, but also for the oilseed crop rapeseed. In this experiment, Cu und Zn concentrations of rapeseed shoots significantly decreased after waterlogging (Fig. 2c, d). However, in contrast to wheat, the Mn concentration of rapeseed shoots significantly increased after the 2-week waterlogging period (Fig. 2a).
The assumption that the micronutrient levels in the plants would rise sharply after the end of the waterlogging period was not fully confirmed. After the regeneration phase, the Mn concentration in the shoots of JB Asano did not differ from that of the controls, but in Kredo shoots Mn concentrations significantly increased (Fig. 3a). However, the Cu and Zn concentrations in the shoots of early water-logged wheat plants of both cultivars were not significantly different from the control (Fig. 3c, d). This was also consistent with the results of the soil analysis (data not shown). Although the concentration of plant-available Mn in the soil increased extremely after waterlogging, the concentrations of Cu and Zn decreased. For Cu, this coincides with a study by Zhang et al. (2017) who showed that copper ion binding was also reduced due to waterlogging on the molecular level. The soil Fe concentration was also decreased after the waterlogging period, which resulted in a significant reduction of Fe concentrations in the shoots of Kredo, but not in the shoots of JB Asano. Apart from the decreased soil concentrations of Fe, Cu and Zn, it may also be possible that the nutrient uptake by the roots was slowed down due to a lack of ATP supply to the roots. Due to the conversion of metabolism from respiration to fermentation, the roots are no longer able to provide sufficient ATP as a result of reduced expression of various ATP synthase subunits (Zhang et al. 2017), which in consequence severely limits the ion transport across the plasma membrane by H+-ATPases (Elzenga and van Veen 2010). This theory is also consistent with Steffens et al. (2005) and, therefore, it can also be assumed that in this study, reduced H+-ATPase activity is the reason. However, measurements of the ATP concentration and enzyme activity were not part of this work, because these measurements were conducted by Steffens et al. (2005) already.
For transport into the shoot, the absorbed nutrients are translocated into the xylem by being transported from the xylem parenchyma cells into the xylem vessels. Nutrient transport is also driven by H+-ATPases, which in turn require ATP, whose provision is inhibited under waterlogging conditions (Colmer and Greenway 2011). In addition, the hydraulic conductivity of the roots is reduced by waterlogging, which inhibits water absorption (Holbrook and Zwieniecki 2003). To prevent loss of water in the plant, the stomata are closed (Bradford and Hsiao 1982; Mielke et al. 2003). This is a common mechanism during waterlogging periods and has been found in tomato plants (Bradford and Hsiao 1982), the tropical tree Genipa americana (Mielke et al. 2003), and cotton (Zhang et al. 2015), as well as in wheat (Shao et al. 2013) and barley (Yordanova et al. 2005). However, stomatal closure also reduces transpiration (Mielke et al. 2003; Yordanova et al. 2005; Shao et al. 2013), which in turn reduces the transport of water and nutrients from the xylem into the upper leaf organs.
After late waterlogging at BBCH 51, significantly increased Mn concentrations in shoots of late water-logged wheat plants could be measured in both varieties (Fig. 3a). Manganese concentrations of the rapeseed shoots were also increased, but significance could not be measured, most probably because of the widely varying single values, although the tendency towards an increasing Mn concentration was obvious. One reason for the increased Mn concentration in both wheat cultivars and in rapeseed could have been increased soil temperatures during and after late flooding. In the case of waterlogging, higher soil temperatures lead to a faster decrease in the oxygen content of the soil and, thus, also of the redox potential of the soil (Ponnamperuma 1984). This results in a faster release of soil-bound nutrients, for example, Mn2+ or Fe3+ (Ponnamperuma 1984), which increases the nutrient availability for the plant. In this experiment, the increased soil temperatures resulted from the fact that the experiments were carried out in a cold greenhouse with non-constant climatic conditions, in which altogether slightly higher temperatures were present than under field conditions. The dark green color of the containers could have further contributed to a faster warming of the soil. This effect was also obvious at maturity, when plants were harvested. Manganese and Fe concentrations were significantly increased in the late water-logged wheat varieties, at least in JB Asano and in rapeseed (Figs. 5a, b, 6a). Interestingly, significantly increased concentrations of Cu and Zn were measured in the rapeseed straw (Fig. 6c,d).
Analyzing the results of both plant species, it should be noted that the highest Mn and Fe concentrations were, in most cases, measured in the shoots of the plants and not in other organs such as flower, chaff, or grains. This supports the hypothesis that the nutrient transport in the xylem was reduced and nutrients thus accumulated in the vegetative organs.
When translating results onto the field, it is crucial to consider not only the time at which the waterlogging occurs, but also the temperature. If waterlogging events occur during an extremely warm spring or summer, it is likely that micronutrients will increase in the plant. If there are waterlogging events during cooler temperature periods, lower micronutrient contents are to be expected in the plant.
References
Armstrong W (1979) Aeration in higher plants. Adv Bot Res 7:225–332
Ashraf M, Mehmood S (1990) Effects of waterlogging on growth and some physiological parameters of four Brassica species. Plant Soil 121:203–209
Ashraf M, Rehman H (1999) Mineral nutrient status of corn in relation to nitrate and long-term waterlogging. J Plant Nutr 22:1253–1268
Bailey-Serres J, Voesenek LACJ (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59:313–339
Bradford KJ, Hsiao TC (1982) Stomatal behavior and water relations of waterlogged tomato plants. Plant Physiol 70:1508–1513
Colmer TD, Greenway H (2011) Ion transport in seminal and adventitious roots of cereals during O2 deficiency. J Exp Bot 62:39–57
Colmer TD, Voesenek LACJ (2009) Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 36:665–681
Elzenga JTM, van Veen H (2010) Waterlogging and plant nutrient uptake. In: Mancuso S, Shabala S (eds) Waterlogging signalling and tolerance in plants. Springer, Berlin, pp 23–35
Hohmann M, Stahl A, Rudloff J, Wittkop B, Snowdon RJ (2016) Not a load of rubbish: simulated field trials in large-scale containers. Plant Cell Environ 39(9):2064–2073
Holbrook NM, Zwieniecki MA (2003) Plant biology—water gate. Nature 425:361
Huang B, Johnson JW, Nesmith S, Bridges DC (1995) Growth, physiological and anatomical responses of two wheat genotypes to waterlogging and nutrient supply. J Exp Bot 45:193–202
Husson O (2013) Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: a transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 362:389–417
Jezek M, Geilfus CM, Bayer A, Mühling KH (2015) Photosynthetic capacity, nutrient status, and growth of maize (Zea mays L.) upon MgSO4 leaf-application. Front Plant Sci 5:781. https://doi.org/10.3389/fpls.2014.00781
Khabaz-Saberi H, Setter TL, Waters I (2006) Waterlogging induces high to toxic concentrations of iron, aluminum, and manganese in wheat varieties on acidic soil. J Plant Nutr 29:899–911
Khabaz-Saberi H, Barker SJ, Rengel Z (2012) Tolerance to ion toxicities enhances wheat (Triticum aestivum L.) grain yield in waterlogged acidic soils. Plant Soil 354:371–381
Malik AI, Colmer TD, Lambers H, Setter TL, Schortemeyer M (2002) Short-term waterlogging has long-term effects on the growth and physiology of wheat. New Phytol 153:225–236
Mielke MS, de Alemeida AAF, Gomes FP, Aguilar MAG, Mangabeira PAO (2003) Leaf gas exchange, chlorophyll fluorescence and growth responses of Genipa americana seedlings to soil flooding. Environ Exp Bot 50:221–231
Ponnamperuma FN (1972) The chemistry of submerged soil. Adv Agron 24:29–96
Ponnamperuma FN (1984) Effects of flooding on soils. In: Flooding and plant growth, pp 9–45
Sajwan KS, Lindsay WL (1986) Effects of redox on zinc deficiency in paddy rice. Soil Sci Soc Am J 50:1264–1269
Setter TL, Waters I, Sharma KS, Singh KN, Kulshreshtha N, Yaduvanshi NPS, Ram PC, Singh BN, Rane J, McDonald G, Khabaz-Saberi H, Biddulph TB, Wilson R, Barclay I, McLean R, Cakir M (2009) Review of wheat improvement for waterlogging tolerance in Australia and India: the importance of anaerobiosis and element toxicities associated with different soils. Ann Bot 103:221–235
Shao GC, Lan JJ, Yu SE, Liu N, Guo RQ, She DL (2013) Photosynthesis and growth of winter wheat in response to waterlogging at different growth stages. Photosynthetica 51:429–437
Steffens D, Hütsch BW, Eschholz T, Lošák T, Schubert S (2005) Waterlogging may inhibit plant growth primarily by nutrient deficiency rather than nutrient toxicity. Plant Soil Environ 51:545–552
Stieger PA, Feller U (1994) Nutrient accumulation and translocation in maturing wheat plants grown on waterlogged soil. Plant Soil 160:87–95
Wollmer AC, Pitann B, Mühling KH (2018) Waterlogging events during stem elongation or flowering affect yield of oilseed rape (Brassica napus L.) but not seed quality. J Agro Crop Sci 204:165–174
Yordanova RY, Uzunova AN, Popova LP (2005) Effects of short-term soil flooding on stomata behavior and leaf gas exchange in barley plants. Biol Plant 49:317–319
Zhang Y, Song X, Yang G, Li Z, Lu H, Kong X, Eneji AE, Dong H (2015) Physiological and molecular adjustment of cotton to waterlogging at peak-flowering in relation to growth and yield. Field Crop Res 179:164–172
Zhang Y, Chen Y, Lu H, Kong X, Dai J, Li Z, Dong H (2016) Growth, lint yield and changes in physiological attributes of cottonunder temporal waterlogging. Field Crop Res 194:83–93
Zhang Y, Kong X, Dai J, Luo Z, Li Z, Lu H, Xu S, Tang W, Li W, Xin C, Dong H (2017) Global gene expression in cotton (Gossypium hirsutum L.) leaves to waterlogging stress. PLoS ONE 12:e0185075
Acknowledgements
Anna-Catharina Wollmer gratefully acknowledges the financial support provided by the Vereinigte Hagelversicherung VVaG, Gießen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wollmer, AC., Pitann, B. & Mühling, KH. Timing of Waterlogging Is Crucial for the Development of Micronutrient Deficiencies or Toxicities in Winter Wheat and Rapeseed. J Plant Growth Regul 38, 824–830 (2019). https://doi.org/10.1007/s00344-018-9893-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-018-9893-9