Abstract
Potassium (K+) is a major limiting element of plant growth, and crops often suffer from low-K+ (LK) stress. Although nitric oxide (NO) is a signaling molecule involved in plant root adaptation to the environment, it remains unclear whether it participates in root growth regulated by LK conditions. Two tobacco cultivars (Nicotiana tabacum L.) exhibiting variant growth features under LK were used in this study. We investigate the effects of LK on root growth, NO accumulation, nitrate reductase (NR) activity and effects of a NO Donor (SNP and NONOate), NO scavenger (cPTIO), NR inhibitor (tungstate), and NO synthase inhibitor (L-NAME) on elongation of first-order lateral roots (LR). Compared with control treatment, the LK-tolerant cultivar NC89 maintained plant growth under LK at 14 days, whereas the dry weight was reduced significantly in the LK-susceptible cultivar Yunyan1. Low-K+-inhibited root growth, mostly by impairing first-order LR formation and elongation was only recorded in cv. Yunyan1. NO accumulation increased in root tips even when cv. Yunyan1 was subjected to LK at day 1. LK-induced NO was generated by the NR pathway during early LK. Application of SNP and NONOate to control-treated plants decreased first-order LR elongation to levels similar to LK treatment in cv. Yunyan1, whereas cPTIO, L-NAME, and tungstate application had the opposite effect. Further results suggested that NO might be involved in auxin-mediated LR elongating as plants respond to LK. In conclusion, NO generated by the NR pathway may be involved in the inhibition by LK stress of first-order LR elongation in tobacco plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potassium (K+) is a major nutrient essential for plant growth and development. It accounts for up to 10% of plant dry weight and is (quantitatively) the most important cation in plant cells (Leigh and Wyn 1984). However, the K+ concentration in soil solutions is generally within the range of 0.025–5.0 mM (Barber 1995), but in the rhizosphere, the concentration is usually less than 0.3 mM (Schroeder and others 1994). Even in fertilized fields, K+ availability varies with environmental conditions, such as drought and soil density (Kuchenbuch and others 1986; Liebersbach and others 2004). Therefore, plants may frequently experience K+ deficiency (LK).
The ability of plants to respond to nutrient deficiencies through changes in root architecture is a basic element of adaptation to the environment. Physiological, metabolic, and morphological root adaptations to LK have been recorded in many plant species [for example, Arabidopsis (Armengaud and others 2004; Schachtman and Shin 2007; Jung and others 2009), cotton (Zhang and others 2009), barley (Drew 1975), and rice (Ma and others 2012)]. In a classic study of Drew (1975), increased lateral root (LR) growth in barley seedlings was investigated when K+ was supplied only to selected portions of the root system. In contrast, seedlings of the model plant Arabidopsis typically respond to LK by dramatically reducing LR initiation and elongation (Armengaud and others 2004; Schachtman and Shin 2007; Gruber and others 2013). Interestingly, Kellermeier and others (2013) reported that in response to low K+, there seems to be an antagonism between lateral and primary root growth in Arabidopsis, with the resulting root architecture dependent on accession. Although plant root responses to K+ deficiency are well documented at the physiological level, the regulatory mechanisms underlying these changes are still obscure.
Root growth is regulated by extrinsic environmental signals and intrinsic developmental programs. More and more data have revealed that root morphology under K+-deficient conditions is regulated by phytohormones such as ethylene and auxin (Muday and others 2012; Wang and Wu 2013; Song and others 2015). Ethylene is induced by K+-deficiency stress (Shin and Schachtman 2004) and enhances auxin transport toward roots by increasing PIN3 and PIN7 transcription in the central cylinder, resulting in auxin accumulation in the meristematic zone (Muday and others 2012). The signaling molecule nitric oxide (NO) also plays a pivotal role in root growth modulation (adventitious root formation, Pagnussat and others 2003; LR development, Correa-Aragunde and others 2004; Sun and others 2015; root hair formation, Lombardo and others 2006; primary root elongation, Zhao and others 2007; Fernández-Marcos and others 2011; Bai and others 2014; Manoli and others 2014; Sun and others 2016). Fernández-Marcos and others (2011) suggested that higher levels of NO reduced root meristem activity. Conversely, Sanz and others (2014) reported that depletion of NO reduced primary root elongation and NO-deficient mutant roots had small root meristems, suggesting an important role for NO in the regulation of stem cell decisions. Lira-Ruan and others (2013) reported that NO has a dual action on Arabidopsis LR branching, slightly promoting it in the root portion formed before the treatment and strongly inhibiting it in the root portion formed during the treatment. Studies have shown the involvement of NO in root development, modulated by nutrient deficiency (Zhao and others 2007; Chen and others 2010; Wang and others 2010; Meng and others 2012; Trevisan and others 2014). Interestingly, NO is important in the shared signaling pathway of the P- and Fe-deficiency-induced formation of cluster roots in white lupin and the N- and P-deficiency-induced elongation of seminal roots in rice (Meng and others 2012; Sun and others 2016). Therefore, it could be hypothesized that NO is also involved in a signaling pathway in K+-deficiency-regulated root growth. However, the role of NO in the LK-induced signaling pathway requires further investigation.
Tobacco (Nicotiana tabacum L.) is an economically important crop worldwide. K+ is deficient in most tobacco-producing areas in China, thus supplemental K+ fertilization is often required to achieve or maintain yields. From the sustainable and eco-friendly viewpoint of modern agriculture, the use of K+-efficient genotypes tolerant to K+ deficiency may be used to exploit the biological potential of plants. In this study, two tobacco cultivars exhibiting variant growth features under LK stress were examined to elucidate the underlying tolerance mechanism of tobacco to K+ deficiency. We found that an LK-tolerant tobacco cultivar (NC89) could maintain higher K+ concentrations than an LK-susceptible tobacco cultivar (Yunyan1) during the experimental period. Furthermore, cv. NC89 could maintain plant growth, including root morphology, whereas LK-induced inhibition was observed in cv. Yunyan1 under LK treatment. Our results suggest that NO plays an important role in modulating the elongation of first-order LRs in tobacco plants as an adaptation to K+-deficiency.
Materials and Methods
Plant Growth
Two tobacco genotypes (Nicotiana tabacum L.), low-K+-tolerant “NC89” and low-K+-susceptible “Yunyan1” seeds, kindly provided by the National Infrastructure for Crop Germplasm Resources (Tobacco, Qingdao), were used as experimental subjects in this study. Two tobacco genotypes were selected from differential responses of 35 tobacco genotypes to K+ deficiency in preliminary experiments with three independent biological replicates (unpublished data).
Seeds were germinated in trays filled with a mixture of peat and vermiculite (V:V, 1:1) held in a greenhouse under natural illumination and day/night temperatures of 28/22 °C. Twenty-five-day-old seedlings of uniform size and vigor were transplanted into holes in lids of 7 L pots (ten holes per lid and one seedling per hole). One-quarter strength Hoagland’s nutrient solution (Hoagland and Arnon 1950) was supplied for 14 and 21 days. Seedlings were then subjected to two treatments: K+ deficiency (LK, 0.01 mM) or normal nutrition (control, 2 mM). Potassium was supplied in the nutrient medium as K2SO4. The nutrient solution (pH 6) comprised 1.25 mM Ca(NO3)2, 0.25 mM NaH2PO4, 0.5 mM MgSO4·7H2O, 20.0 µM Fe-EDTA, 9.1 µM MnCl2, 0.5 µM (NH4)6Mo7O24, 46 µM H3BO3, 0.8 µM ZnSO4, and 0.3 µM CuSO4. The nutrient solution was replaced daily and aerated for 30 min to maintain optimal oxygen content. Each treatment was replicated fourfold and arranged in a completely randomized design to avoid edge effects. In addition, all experiments included three independent biological replicates. The plants were harvested after 14 or 21 days of treatment. Root samples were snap-frozen in liquid N2 and stored at −70 °C until further measurement.
Preliminary experiments were conducted to determine the appropriate application levels for cvs NC89 and Yunyan1. Pharmacological responsiveness to sodium nitroprusside (SNP) differed between the two tobacco cultivars (Supplementary Figs. 1, 2). For example, application of 1 or 2.5 µM SNP in addition to control nutrition inhibited the first-order LR length of cv. Yunyan1 to a level similar to that of LK treatment, whereas application of SNP (≥7.5 µM) inhibited first-order LR length in cv. NC89 compared with control nutrition. Thus, two concentrations of SNP (7.5 µM for cv. NC89 and 2.5 µM for cv. Yunyan1) were used in subsequent experiments. Similarly, two concentrations of another NO donor, diethylamine NONOate (NONOate, 100 µM for cv. NC89 and 50 µM for cv. Yunyan1) were used in subsequent experiments. Also, 80 µM 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), 50 µM sodium tungstate dihydrate (Tu), 100 µM NG-nitro-l-arginine methyl ester (L-NAME), 20 nM α-Naphthylacetic acid (NAA, exogenous auxin), and 60 nM N-1-Naphthylphthalamic acid (NPA, auxin transport inhibitor) were applied every day with the change of solution. Except for NAA (dissolved in 1 M NaOH) and NPA (dissolved in dimethyl sulphoxide), other chemicals were applied to the plant-growth media after being dissolved in distilled water. These chemicals were from Sigma-Aldrich, Inc. except L-NAME from J&K Scientific Ltd.
Measurement of Root System Architecture
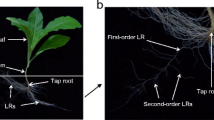
Although tobacco is a dicotyledon, its taproot is not as well differentiated as those of typical dicotyledonous species (Song and others 2015). During the course of treatments in hydroponic culture, first- and second-order LR development differed significantly, but no changes were observed in taproots. Accordingly, we investigated the effect of K+ deficiency on LRs and did not evaluate the taproots.
We measured total root length and volume using a WinRHIZO scanner-based image analysis system (Regent Instruments, Montreal, QC, Canada). The lengths of first-order LRs were measured using a ruler. Average lengths of second-order LRs were determined using a root analysis apparatus. The number of first-order LRs was counted by eye. We calculated second-order LR density by dividing the counts of second-order LRs by the lengths of first-order LRs.
Measurement of K+ Concentration
K+ concentration was determined in the leaves and roots of tobacco seedlings following the procedures described by Song and others (2015). Desiccated samples were ground into powder. About 50 mg of the powder was digested in H2SO4 and H2O2 at 270 °C. After cooling, the sample was diluted to 100 mL with distilled water. K+ concentrations were determined by inductively coupled plasma emission spectrometry (Optima 2100DV, Perkin Elmer, Waltham, MA, USA).
Measurement of NO by Using Two Methods
On the one hand, NO in the root tips was imaged using diaminofluorescein-FM diacetate (DAF-FM DA) and an epifluorescence microscopy. The root tips were loaded with 10 µM DAF-FM DA in 20 mM HEPES-NaOH buffer (pH 7.5). After incubating in darkness for 30 min, the root tips were washed three times in fresh buffer and immediately visualized using a stereomicroscope with a color CCD camera, excitation at 488 nm, and emission at 495–575 nm (Olympus MVX10). Signal intensities of green fluorescence in the images were quantified according to Xie and others (2013) using Photoshop software (Adobe Systems, San Jose, CA, USA). Data are presented as relative units of pixel intensities via region of interest analysis, provided by the Photoshop software. The background of green fluorescence in the root tip was load without treatment of 10 µM DAF-FM DA; the final NO fluorescence data were calculated as green fluorescence with treatment of 10 µM DAF-FM DA minus the background data.
On the other hand, NO production was determined by using Griess reagent. NO production was determined using the method described by Zhou and others (2005). Absorbance was assayed at 540 nm; NO content was calculated by comparison to a standard curve of NaNO2.
Determination of Nitrate Reductase (NR) Activity
Nitrate reductase activity in tobacco roots was measured using the method described by Ogawa and others (1999) with some modification. The assay mixture for NR activity contained 25 mM potassium phosphate buffer (pH 7.5), 10 mM KNO3, 0.2 mM NADH, 5 mM NaHCO3, and 5 µL of extract in a final volume of 0.5 mL. The assays were conducted at 30 °C for 15 min. The reaction was terminated by the addition of 50 µL of 0.5 M Zn(CH3COO)2 and the excess NADH was oxidized by the addition of 50 µL of 0.15 mM phenazine methosulfate. The mixture was centrifuged at 10,000 g for 5 min. The amount of NO2 − produced was measured by combining 500 µL of the supernatant with 250 µL of 1% sulfanilamide prepared in 1.5 N HCl and 250 µL of 0.02% N-(1-Naphthyl)ethylene-diamine dihydrochloride and reading at 540 nm in a spectrophotometer.
qRT-PCR Analysis
Transcript levels of the tobacco NR genes (NtNIA1, LOC104097998; NtNIA2, LOC104248549), K+ transporter and channel genes (NtHAK1, DQ841950.1; NtHKT1, LOC104095513; NtNKT1, AB196790.1), and marker gene of the cell cycle activity within the root meristem (NtCYCB1;1, Z37978.2) were compared to that of NtL25 (L18908.1), which is a stable reference gene (Schmidt and Delaney 2010). RNA extraction, reverse transcription, and quantitative real-time PCR (qRT-PCR) were performed following procedures described by Livak and Schmittgen (2001) and Sun and others (2016). Primer sets are listed in Supplementary Table 1.
Data Analysis
Experimental data were pooled for calculations of means and standard error (SE) and subjected to student’s t-test or one-way analysis of variance (ANOVA) followed by multiple comparison tests (LSD). All statistical procedures were performed using the SPSS ver. 11.0 software (SPSS Inc., Chicago, IL, USA). In all analyses, significant differences were determined at p < 0.05.
Results
Responses to K+ Deficiency Differ Between the Two Tobacco Cultivars
In comparison with normal treatment (CK), shoot and root growth were significantly reduced by the K+ deficiency treatment (LK) at 14 days only in cv. Yunyan1 (Fig. 1a, b). K+ deficiency markedly reduced the shoot and root dry weight of cv. Yunyan 1 by 46 and 42%, respectively. Interestingly, we detected no significant differences in plant growth of cv. NC89 between the two treatments. To further analyze the effect of K+ deficiency on growth of tobacco plants, we prolonged the growth duration. At 21 days, LK decreased shoot and root dry weight in cv. NC89 by 21 and 23%, respectively, whereas cv. Yunyan1 growth was inhibited by about 50% compared with the control treatment (Fig. 1c). Accordingly, cv. NC89 was recognized as a LK-tolerant cultivar and cv. Yunyan1 as an LK-susceptible cultivar.
Phenotype of two tobacco cultivars responding to K+ deficiency. Seedlings were subjected to K+ deficiency (LK, 0.01 mM) or provided with normal nutrition (control, 2 mM) for 14 days (a, b) or 21 days (c) in hydroponic culture. Bar = 5 cm. Values are means of four replications ±SE and bars with different letters indicate significant differences at p < 0.05, as determined by ANOVA followed by the LSD test
K+ deficiency significantly decreased K+ concentration in the roots and shoots of tobacco at 14 days (Fig. 2a). The decrease in K+ concentration was higher in cv. Yunyan1 than in cv. NC89 under K+ deficiency. Furthermore, a similar tendency was observed in relative expression of two high-affinity K+ transporters (HAK1 and HKT1) rather than that of a putative inwardly directed K+ channel (NKT1) (Fig. 2b), which might explain the greater resistance to K+ deficiency of cv. NC89 than cv. Yunyan1.
K+ concentration and expression of K+ transporters and channel in two tobacco cultivars in response to K+ deficiency. Seedlings were subjected to K+ deficiency (LK, 0.01 mM) or provided with normal nutrition (Control, 2 mM) for 14 days in hydroponic culture. a K+ concentration in shoots and roots, b relative gene expression of two high-affinity K+ transporters (HAK1 and HKT1) and one inward channel (NKT1). Values are means of four replications ±SE and bars with different letters indicate significant differences at p < 0.05, as determined by ANOVA followed by the LSD test (a) and by Student’s t-test (b)
K+ Deficiency Inhibited the Formation and Elongation of First-Order LRs Only in cv. Yunyan1
K+ deficiency significantly decreased root growth in cv. Yunyan1 relative to the control treatment at 14 days, whereas no difference was recorded in the root growth of cv. NC89 (Fig. 3). K+ deficiency markedly reduced total root volume and length in cv. Yunyan1 relative to the control by 44 and 37%, respectively. Further analysis showed that K+ deficiency decreased the number and average length of first-order LRs by 36 and 37%, respectively, compared with the control. However, we detected no significant differences in the density and average length of second-order LRs between the two treatments, similar to our previous report (Song and others 2015).
Root morphology of two tobacco cultivars in response to K+ deficiency. Seedlings were subjected to K+ deficiency (LK, 0.01 mM) or provided with normal nutrition (Control, 2 mM) for 14 days in hydroponic culture. LR lateral root. Values are means of four replications ±SE and bars with different letters indicate significant differences at p < 0.05, as determined by ANOVA followed by the LSD test
K+ Deficiency Increases the NO Level in cv. Yunyan1, but not in cv. NC89
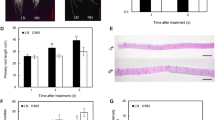
The signaling molecule NO plays a pivotal role in root growth modulation (Pagnussat and others 2003; Correa-Aragunde and others 2004; Zhao and others 2007; Fernández-Marcos and others 2011; Bai and others 2014; Manoli and others 2014; Sun and others 2015). To determine whether NO participates in the elongation of first-order LRs in the presence of K+ deficiency, we measured NO-associated green fluorescence in the root tips of first-order LRs of two tobacco cultivars at 1 and 14 days (Fig. 4). The NO-associated green fluorescence in the root tip and relative NO content was visibly increased by the K+-deficient condition only in cv. Yunyan1 even at day 1, compared with normal nutrition (Fig. 4a, c, d). Fluorescence signal intensity showed LK-induced accumulation of NO in the root tips by 71% at day 1 and by 114% at day 14 in cv. Yunyan1. No difference was recorded in cv. NC89 under the two treatments (Fig. 4a–c).
Accumulation of nitric oxide (NO) in the root tip of first-order lateral roots in two tobacco cultivars in response to K+ deficiency. Seedlings were subjected to K+ deficiency (LK, 0.01 mM) or provided with normal nutrition (control, 2 mM) for 14 days in hydroponic culture. a NO production shown as green fluorescence in the root tips at days 1 and 14, Bar = 1 mm, b, c NO production in cv. NC89 (b) and cv. Yunyan1 (c) expressed as relative fluorescence intensity, d NO content detected by using Griess reagent in cvs NC89 and Yunyan1 (d) at day 1, which was expressed as relative NO content. Values are means of four replications ±SE and bars with different letters indicate significant differences compared to control at the same time point at p < 0.05, as determined by Student’s t-test
The LK-Induced Increase in NO Level was due to the Nitrate Reductase Pathway
Nitrate reductase has been identified as an important enzymatic pathway for NO production in plants (Wilson and others 2008). Relative NR activity was assessed in the roots of tobacco plants at days 1, 2, 7, and 14 (Fig. 5a, b). The time-course of NR activity differed between the two tobacco cultivars. For example, in cv. Yunyan1, relative NR activity was induced by 83 and 46% by LK treatment at days 1 and 2, and decreased thereafter. qRT-PCR analysis showed that NIA1 and NIA2 RNA levels were increased considerably in the roots of cv. Yunyan1 (Fig. 5c). However, no difference was observed in relative NR activity in cv. NC89 between the two treatments. This suggests that LK-induced NO in an LK-susceptible tobacco cultivar was generated via the NR pathway at the start of treatment.
Nitrate reductase (NR) activity and qRT-PCR analysis of NR gene expression levels in the roots. Seedlings were subjected to K+ deficiency (LK, 0.01 mM) or provided with normal nutrition (Control, 2 mM) for 14 days in hydroponic culture. a cv. NC89, b cv. Yunyan1, c relative expression levels of NR genes (NIA1 and NIA2) in cv. Yunyan1. Values are means of four replications ±SE and bars with different letters indicate significant differences compared to control at the same time point or for the same gene at p < 0.05, as determined by Student’s t-test
Exogenous Application of an NO Donor and Scavenger Affect First-Order LR Elongation
We examined the responses of first-order LR elongation to the application of the (i) NO donor SNP and NONOate, (ii) NO scavenger cPTIO, (iii) NOS inhibitor L-NAME, and (iv) NR inhibitor tungstate to determine whether the increased NO level (resulting from K+ deficiency) was responsible for belowground changes in tobacco root morphology (Figs. 6, 7). In cv. NC89, the first-order LR length decreased significantly at SNP concentrations of at least 7.5 µM (Fig. S1). In cv. Yunyan1, the first-order LR length decreased upon application of 1.25–10 µM SNP and upon 2.5 µM SNP application decreased to a similar level to that under LK treatment (Fig. S2). Interestingly, a different response to another NO donor (NONOate) was recorded in the two tobacco cultivars. Thus, two SNP concentrations (7.5 µM, cv. NC89; 2.5 µM, cv. Yunyan1) and two NONOate concentrations (100 µM, cv. NC89; 50 µM, cv. Yunyan1) were used in subsequent analyses. Application of SNP and NONOate markedly induced NO accumulation and decreased first-order LR length in both tobacco cultivars. Furthermore, in cv. Yunyan1, application of SNP and NONOate affected NO accumulation and average length of first-order LR to a similar level as that under LK stress. In two rice cultivars, the application of cPTIO, L-NAME, or tungstate markedly decreased NO accumulation and increased first-order LR length, in contrast to the application of SNP and NONOate. Thus, increased NO levels were related to the shifts in first-order LR elongation modulated by K+ deficiency.
Accumulation of nitric oxide (NO) and average length of first-order lateral roots in the NC89 tobacco cultivar. Seedlings were grown in hydroponic medium containing normal nutrition (Control, 2 mM) or K+ deficiency (LK, 0.01 mM) in addition to sodium nitroprusside (SNP, 7.5 µM), diethylamine NONOate (NONOate, 100 µM), 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO, 80 µM), NG-Nitro-L-arginine methyl ester (L-NAME, 100 µM), and tungstate (Tu, 50 µM) for 14 days. a, b Photographs of NO production shown as green fluorescence in the root tips (a), and NO production expressed as relative fluorescence intensity compared to control (b), c average length of the first-order lateral roots (LRs). Values are means of four replications ±SE and bars with different letters indicate significant differences at p < 0.05, as determined by ANOVA followed by the LSD test
Accumulation of nitric oxide (NO) and average length of first-order lateral roots in Yunyan1 tobacco cultivar. Seedlings were grown in hydroponic medium containing normal nutrition (Control, 2 mM) or K+ deficiency (LK, 0.01 mM) in addition to sodium nitroprusside (SNP, 2.5 µM), diethylamine NONOate (NONOate, 50 µM), 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO, 80 µM), NG-nitro-l-arginine methyl ester (L-NAME, 100 µM), and tungstate (Tu, 50 µM) for 14 days. a, b Photographs of NO production shown as green fluorescence in the root tips (a), and NO production expressed as relative fluorescence intensity compared to control (b), c average length of first-order lateral roots (LRs). Values are means of four replications ±SE and bars with different letters indicate significant differences at p < 0.05, as determined by ANOVA followed by the LSD test
Involvement of NO in Auxin-Mediated K+-Deficiency-Inhibited First-Order Elongating
It has been reported that auxin was also involved in the regulation of K+-deficiency-induced root elongating (Song and others 2015), the relationship between NO and auxin was therefore investigated. Application of NAA (exogenous auxin) and NPA (auxin transport inhibitor) increased and decreased, respectively, first-order LR elongation under both K+ treatments (Song and others 2015). As shown in Fig. 8, application of NAA decreased NO accumulation and increased first-order LR length under two K+ treatments, as compared with control or LK-treated plants; furthermore, SNP co-incubation maintained higher NO accumulation and less first-order LR length in NAA-treated plants under both K+ treatments. Conversely, NPA co-incubation did not restore the NO generation and first-order LR length in cPTIO-treated LK plants. The above results suggested that NO might be involved in IAA-mediated K+-deficiency-induced root elongating.
Crosstalk between auxin and NO in regulating K+-deficiency-induced first-order lateral root (LR) elongation. Seedlings of cv. Yunyan1 were grown in hydroponic medium containing normal nutrition (Control, 2 mM) or K+ deficiency (LK, 0.01 mM) in addition to sodium nitroprusside (SNP, 2.5 µM), 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO, 80 µM), α-Naphthylacetic acid (NAA, 20 nM), and N-1-Naphthylphthalamic acid (NPA, 60 nM) for 14 days. a, b Photographs of NO production shown as green fluorescence in the root tips (a), and NO production expressed as relative fluorescence intensity compared to control (b), c average length of first-order LRs. Values are means of four replications ±SE and bars with different letters indicate significant differences at p < 0.05, as determined by ANOVA followed by the LSD test
Discussion
Potassium is a major limiting element of plant growth, and crops often suffer from K+-deficiency stress. The identification of crop cultivars with LK-tolerance and K+-efficient utilization in low-input farming systems continues to be an important goal for plant scientists (Fan and others 2014; Wang and Wu 2013, 2015). In this study, two tobacco genotypes exhibited variant growth features under K+-deficiency treatment. In comparison to normal nutrition, an LK-tolerant cultivar (NC89) maintained shoot and root growth under LK-stress treatment at 14 days, whereas biomass accumulation was inhibited by about 44% in an LK-susceptible cultivar (Yunyan1). As expected, plant growth was decreased by LK treatment in the two tobacco cultivars with prolonged growth duration, but cv. NC89 still maintained a higher dry weight than cv. Yunyan1 at 21 days (Fig. 1c). Furthermore, cv. NC89 maintained higher shoot and root K+ concentrations under K+ deficiency than cv. Yunyan1. This was consistent with the results from two watermelon cultivars (Fan and others 2014). Several KUP/HAK/KT transporters and one inward K+ channel (AKT1) from diverse plant species have been reported as high-affinity K+ transporters and K+ channels involved in K+ uptake under LK conditions (Spalding and others 1999; Pyo and others 2010; Ma and others 2012; Wang and others 2012; Wang and Wu 2013). Although it has been reported that the transcripts of group I of the KUP/HAK/KT family increase under K+ restriction in roots of many plant species (Santa-María and others 1997; Gierth and others 2005; Guo and Crawford 2005; Wang and Wu 2013), Yang and others (2014) found that OsHAK5 expression increased substantially within 24 h after K+ removal whereas it were not significantly affected or decreased with prolonged K+ starvation in rice roots which was consistent with the result of Okada and others (2008). We also found that relative expression of two high-affinity K+ transporters (HAK1 and HKT1) and a putative inwardly directed K+ channel (NKT1) decreased significantly by 14-days K+ starvation in two tobacco roots. Interestingly, compared with control tobacco plants, a higher expression level of two high-affinity K+ transporters was observed in cv. NC89 than in cv. Yunyan1 (Fig. 2b). A similar result has been reported by Ruan and others (2015) that a greater number of high-affinity K+ transporter genes were upregulated in an LK-tolerant compared to an LK-susceptible wheat cultivar. This likely explains the higher K+ concentration in the LK-tolerant compared to the LK-susceptible cultivar.
The root is the first plant organ that detects nutrient deficiency in the external environment; thus the ability of plant roots to respond to nutrient deficiency is a basic element of adaptation to the environment. Because K+ is the main osmotic cation in plant root cells, it is reasonable to surmise that K+ deficiency would arrest root growth (Armengaud and others 2004; Schachtman and Shin 2007; Jung and others 2009; Zhang and others 2009; Ma and others 2012; Gruber and others 2013; Kellermeier and others 2013). However, the mechanism by which K+ deficiency inhibits root growth needs more investigation. In the model plant Arabidopsis, Kellermeier and others (2013) suggested two extreme strategies of root morphological adaptation to low K+; in strategy I, primary root growth is maintained but LR elongation is compromised, whereas in strategy II, arrested primary root elongation favors LR branching. Tobacco, an economically important crop worldwide, has a root architecture different from those of most dicotyledonous plants (Hochholdinger and others 2004). LRs make up the bulk of the root system in tobacco seedlings, due to the lack of a taproot. In the present study, inhibition of root growth in the LK-susceptible cultivar was due mainly to decreased formation and elongation of first-order LRs rather than second-order LRs, consistent with the findings in another tobacco cultivar (Song and others 2015). These findings suggest that the strategy of tobacco root morphological adaptation to low K+ was to arrest first-order LR growth but maintain second-order LR.
Increasing data have revealed that root growth in response to K+ deficiency is regulated by ethylene and auxin in Arabidopsis (Muday and others 2012; Wang and Wu 2013). And our previous results showed that K+ deficiency inhibited first-order LR elongation in tobacco plants by shifting auxin distribution (Song and others 2015). It has been reported that NO functions as a signaling molecule in the regulation of root development when plants are subjected to nutrient deficiency (Zhao and others 2007; Meng and others 2012; Sun and others 2016). Studies conducted during the induction of diverse plant responses have demonstrated an interaction between NO and plant hormones such as auxin and ethylene (Kolberrt and others 2008; Terrile and others 2012; Freschi 2013). However, whether NO participates in LR growth regulated by K+-deficiency remains unclear. In this study, first-order LR elongation was decreased by 7.5 μM SNP (or 100 μM NONOate) in NC89 and 2.5 μM SNP (or 50 μM NONOate) in cv. Yunyan1, confirming that the appropriate amount of NO could reduce the elongation of first-order LRs in tobacco plants. Interestingly, the accumulation of NO and the length of first-order LRs were modulated by the application of 2.5 μM SNP (or 100 μM NONOate) in cv. Yunyan1, to levels similar to those under LK, and were considerably inhibited by the application of cPTIO under LK. Furthermore, the application of SNP to control plants could decrease the expression level of CYCB1;1, a marker gene of cell cycle activity within the root meristem (Fig. S3), suggesting an important role for NO in the regulation of stem cell decisions. This was consistent with the result of Fernández-Marcos and others (2011). As for the crosstalk between NO and auxin, although Terrile and others (2012) reported the importance of NO in auxin signaling pathways, in most of the reports NO was identified to function downstream of auxins, apparently through linear signaling pathways (Kolbert and others 2008; Freschi 2013). In this study, we also found that NO might be involved in auxin-regulated first-order LR elongation in response to K+-deficient condition. This suggests that NO induced by K+-deficiency was negatively correlated with elongation of first-order LRs.
Interestingly, because the previous study has shown that NO negatively regulates AKT1-mediated K+ uptake in Arabidopsis (Xia and others 2014), it is easy to speculate that LK-induced NO accumulation would have a feedback modulation of K+ uptake through downregulating several K+ transporters. This suggested the possible roles of NO on K+ uptake in response to LK condition rather than its roles on root growth.
Nitrate reductase and a putative NOS enzyme represent potential enzymatic sources of NO production in plants (Wilson and others 2008). Plant NOS has not yet been identified (Moreau and others 2008; Gas and others 2009; Ree and others 2011), although experiments using inhibitors of the animal NOS enzyme have provided some evidence of the role of the l-arginine pathway in NO production (Zhao and others 2007). It has been reported that NR is involved in NO production in response to biotic and abiotic stresses (Srivastava and others 2009; Zhao and others 2009; Chen and others 2010; Kolbert and others 2010). Results from Bright and others (2006) and Zhao and others (2009) supported that NIA1 is a key component in NR-mediated NO production. In this study, NO was shown to be initially generated by a NIA1- and NIA2-dependent NR pathway under LK conditions in an LK-susceptible tobacco cultivar. Sun and others (2016) reported that root NR activity was also induced by N deficiency during the early phase of treatment relative to normal nutrition. Actually, K+-coupled transport of nitrate in plants was proposed more than 40 years ago (Ben-zioni and others 1970, 1971). Xia and others (2015) also suggested a close relationship between nitrate and K+ transport in plants. Compared with the LK-tolerant tobacco cultivar (NC89), a larger decrement of K+ concentration in the LK-susceptible tobacco cultivar (Yunyan1) might lead to more nitrate accumulating in roots initially under LK condition. And consequently, a higher increment of LK-induced NR activity in the roots was observed in cv. Yunyan1 than in cv. NC89, which decreased thereafter relative to the control (Fig. 5b). qRT-PCR analysis showed that NIA1 and NIA2 expression was induced by three and four times in the roots of cv. Yunyan1 at day 1. Moreover, NO accumulation was reduced and the length of first-order LRs was increased after the application of tungstate in both tobacco cultivars. These results suggest that NIA1- and NiA2-dependent NR was involved in initially LK-induced NO generation in LK-susceptible tobacco plants. Although the NOS protein has not yet been identified in plants (Moreau and others 2008; Gas and others 2009; Ree and others 2011), an inhibitor of the animal NOS enzyme (L-NAME) inhibited NO accumulation and induced root elongation in cv. Yunyan1 under LK conditions. This suggested that the NOS pathway might be involved in LK-induced NO production.
In conclusion, two tobacco cultivars exhibited variant growth features under LK stress. The LK-tolerant tobacco cultivar (NC89) maintained a higher K+ concentration in the shoots and roots than the LK-susceptible tobacco cultivar (Yunyan1). Furthermore, our findings suggest that NO plays an important role in inhibiting first-order LR elongation in LK-susceptible cultivars when plants are responding to K+ deficiency.
References
Armengaud P, Breitling R, Amtmann A (2004) The potassium-dependent transcriptome of Arabidopsis reveals a prominent role of jasmonic acid in nutrient signaling. Plant Physiol 136:2556–2576. doi:10.1104/pp.104.046482
Bai S, Yao T, Li M et al (2014) PIF3 is involved in the primary root growth inhibition of Arabidopsis induced by nitric oxide in the light. Mol Plant 4:616–625. doi:10.1093/mp/sst142
Barber SA (1995) Potassium. In: Soil nutrient bioavailability: a mechanistic approach. Wiley, New York, pp 231–232
Ben-zioni A, Vaadia Y, Herman Lips S (1970) Correlations between nitrate reduction, protein synthesis and malate accumulation. Physiol Plant 23:1039–1047. doi:10.1111/j.1399-3054.1970.tb08878.x
Ben-zioni A, Vaadia Y, Herman Lips S (1971) Nitrate uptake by roots as regulated by nitrate reduction products of the shoot. Physiol Plant 24:288–290. doi:10.1111/j.1399-3054.1971.tb03493.x
Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45:113–122. doi:10.1111/j.1365-313X.2005.02615.x
Chen WW, Yang JL, Qin C et al (2010) Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol 154:810–819. doi:10.1104/pp.110.161109
Correa-Aragunde N, Graziano M, Lamattina L (2004) Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218:900–905. doi:10.1007/s00425-003-1172-7
Drew MC (1975) Comparison of the effects of a localized supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot in barley. New Phytol 75:490–749. doi:10.1111/j.1469-8137.1975.tb01409.x
Fan M, Huang Y, Zhong Y et al (2014) Comparative transcriptome profiling of potassium starvation responsiveness in two contrasting watermelon genotypes. Planta 239:397–410. doi:10.1007/s00425-013-1976-z
Fernández-Marcos M, Sanz L, Lewis DR, Muday GK, Lorenzo O (2011) Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)-dependent acropetal auxin transport. Proc Natl Acad Sci USA 108:18506–18511. doi:10.1073/pnas.1108644108
Freschi L (2013) Nitric oxide and phytohormone interactions: current status and perspectives. Front Plant Sci 4:398. doi:10.3389/fpls.2013.00398
Gas E, Flores-Pérez Ú, Sauret-Güeto S et al (2009) Hunting for plant nitric oxide synthase provides new evidence of a central role for plastids in nitric oxide metabolism. Plant Cell 21:18–23. doi:10.1105/tpc.108.065243
Gierth M, Mäser P, Schroeder JI (2005) The Potassium Transporter AtHAK5 Functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiol 137:1105–1114. doi:10.1104/pp.104.057216
Gruber BD, Giehl RFH, Friedel S, Wirén N (2013) Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol 163:161–179. doi:10.1104/pp.113.218453
Guo FQ, Crawford NM (2005) Arabidopsis nitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. Plant Cell 17:3436–3450. doi:10.1105/tpc.105.037770
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. In: Circular California Agricultural Experiment Station 2nd edit. pp 32
Hochholdinger F, Park WJ, Sauer M, Woll K (2004) From weeds to crops: genetic analysis of root development in cereals. Trends Plant Sci 9:42–48. doi:10.1016/j.tplants.2003.11.003
Jung JY, Shin R, Schachtman DP (2009) Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis. Plant Cell 21:607–621. doi:10.1105/tpc.108.063099
Kellermeier F, Chardon F, Amtmann A (2013) Natural variation of Arabidopsis root architecture reveals complementing adaptive strategies to potassium starvation. Plant Physiol 161:1421–1432. doi:10.1104/pp.112.211144
Kolbert Z, Bartha B, Erdei L (2008) Exogenous auxin-induced NO synthesis is nitrate reductase- associated in Arabidopsis thaliana root primordial. J Plant Physiol 165:967–975. doi:10.1016/j.jplph.2007.07.019
Kolbert Z, Ortega L, Erdei L (2010) Involvement of nitrate reductase (NR) in osmotic stress-induced NO generation of Arabidopsis thaliana L. roots. J Plant Physiol 167:77–80. doi:10.1016/j.jplph.2009.08.013
Kuchenbuch R, Claassen N, Jungk A (1986) Potassium availability in relation to soil-moisture. Plant Soil 95:221–231. doi:10.1007/BF02375075
Leigh RA, Wyn JRG (1984) A hypothesis relating critical potassium concentrations for growth to the distribution and function of this ion in the plant cell. New Phytol 97:1–13. doi:10.1111/j.1469-8137.1984.tb04103.x
Liebersbach H, Steingrobe B, Claassen N (2004) Roots regulate ion transport in the rhizosphere to counteract reduced mobility in dry soil. Plant Soil 260:79–88. doi:10.1023/B:PLSO.0000030191.92338.6a
Lira-Ruan V, Mendivil SN, Dubrovsky JG (2013) Heuristic aspect of the lateral root initiation index: a case study of the role of nitric oxide in root branching. Appl Plant Sci 1(10):1300029. doi:10.3732/apps.1300029
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–△△C T method. Methods 25:402–408. doi:10.1006/meth.2001.1262
Lombardo MC, Graziano M, Polacco JC, Lamattina L (2006) Nitric oxide functions as a positive regulator of root hair development. Plant Signal Behav 1:28–33. doi:10.4161/psb.1.1.2398
Ma L, Wu WH, Wang Y (2012) Transcriptome analysis of rice root responses to potassium deficiency. BMC Plant Biol 12:161. doi:10.1186/1471-2229-12-161
Manoli A, Begheldo M, Genre A, Lanfranco L, Trevisan S, Quaggiotti S (2014) NO homeostasis is a key regulator of early nitrate perception and root elongation in maize. J Exp Bot 65:185–200. doi:10.1093/jxb/ert358
Meng ZB, Chen LQ, Suo D, Li GX, Tang CX, Zheng SJ (2012) Nitric oxide is the shared signalling molecule in phosphorus-and iron-deficiency-induced formation of cluster roots in white lupin Lupinus albus. Ann Bot 109:1055–1064. doi:10.1093/aob/mcs024
Moreau M, Lee GI, Wang Y, Crane BR, Klessig DF (2008) AtNOS/A1 is a functional Arabidopsis thaliana cGTPase and not a nitric oxide synthase. J Biol Chem 283:32957–32967. doi:10.1074/jbc.M804838200
Muday GK, Rahman A, Binder BM (2012) Auxin and ethylene: collaborators or competitors? Trends Plant Sci 17:181–195. doi:10.1016/j.tplants.2012.02.001
Ogawa T, Fukuoka H, Yano H, Ohkawa Y (1999) Relationships between nitrite reductase activity and genotype-dependent callus growth in rice cell cultures. Plant Cell Rep 18:576–581. doi:10.1007/s002990050625
Okada T, Nakayama H, Shinmyo A, Yoshida K (2008) Expression of OsHAK genes encoding potassium ion transporters in rice. Plant Biotechnol 25:241–245. doi:10.5511/plantbiotechnology.25.241
Pagnussat GC, Lanteri ML, Lamattina L (2003) Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol 132:1241–1248. doi:10.1104/pp.103.022228
Pyo YJ, Gierth M, Schroeder JI, Cho MH (2010) High-affinity K+ transport in Arabidopsis: AtHAK5 and AKT1 are vital for seedling establishment and postgermination growth under low-potassium conditions. Plant Physiol 153:863–875. doi:10.1104/pp.110.154369
Ree KV, Gehl B, Chehab EW et al (2011) Nitric oxide accumulation in Arabidopsis is independent of NOA1 in the presence of sucrose. Plant J 68:225–233. doi:10.1111/j.1365-313X.2011.04680.x
Ruan L, Zhang J, Xin X et al (2015) Comparative analysis of potassium deficiency-responsive transcriptomes in low potassium susceptible and tolerant wheat (Triticum aestivum L). Sci Rep 5:10090. doi:10.1038/srep10090
Santa-María GE, Rubio F, Dubcovsky J et al (1997) The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell 9:2281–2289. doi:10.1105/tpc.9.12.2281
Sanz L, Fernández-Marcos M, Modrego A et al (2014) Nitric oxide plays a role in stem cell niche homeostasis through its interaction with auxin. Plant Physiol 166:1972–1984. doi:10.1104/pp.114.247445
Schachtman DP, Shin R (2007) Nutrient sensing and signaling: NPKS. Annu Rev Plant Biol 58:47–69. doi:10.1146/annurev.arplant.58.032806.103750
Schmidt GW, Delaney SK (2010) Stable internal reference genes for normalization of real-time RT-PCR in tobacco (Nicotiana tabacum) during development and abiotic stress. Mol Genet Genom 283:233–241. doi:10.1007/s00438-010-0511-1
Schroeder JI, Ward JM, Gassmann W (1994) Perspectives on the physiology and structure of inward-rectifying K+ channels in higher plants: biophysical implications for K+ uptake. Annu Rev Biophys Biomol Struct 23:441–471. doi:10.1146/annurev.bb.23.060194.002301
Shin R, Schachtman DP (2004) Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc Natl Acad Sci USA 101:8827–8832. doi:10.1073/pnas.0401707101
Song W, Liu S, Meng L et al (2015) Potassium deficiency inhibits lateral root development in tobacco seedlings by changing auxin distribution. Plant Soil 396:163–173. doi:10.1007/s11104-015-2579-1
Spalding EP, Hirsch RE, Lewis DR, Qi Z, Sussman MR, Lewis BD (1999) Potassium uptake supporting plant growth in the absence of AKT1 channel activity. J Gen Physiol 113:909–918. doi:10.1085/jgp.113.6.909
Srivastava N, Gonugunta VK, Puli MR, Raghavendra AS (2009) Nitric oxide production occurs downstream of reactive oxygen species in guard cells during stomatal closure induced by chitosan in abaxial epidermis of Pisum sativum. Planta 229:757–765. doi:10.1007/s00425-008-0855-5
Sun H, Li J, Song W et al (2015) Nitric oxide generated by nitrate reductase increases nitrogen uptake capacity by modulating lateral root formation and inorganic nitrogen uptake rate in rice. J Exp Bot 66:2449–2459. doi:10.1093/jxb/erv030
Sun H, Bi Y, Tao J et al (2016) Strigolactones are required for nitric oxide to induce root elongation in response to nitrogen and phosphate-deficiency in rice. Plant Cell Environ 39:1473–1484. doi:10.1111/pce.12709
Terrile MC, París R, Calderón-Villalobos LIA et al (2012) Nitric oxide influences auxin signaling through S-nitrosylation of the Arabidopsis TRANSPORT INHIBITOR RESPONSE 1 auxin receptor. Plant J 70:492–500. doi:10.1111/j.1365-313X.2011.04885.x
Trevisan S, Manoli A, Quaggiotti S (2014) NO signaling is a key component of the root growth response to nitrate in Zea mays L. Plant Signal Behav 9:28290. doi:10.4161/psb.28290
Wang Y, Wu W (2013) Potassium transport and signaling in higher plants. Annu Rev Plant Biol 64:451–476. doi:10.1146/annurev-arplant-050312-120153
Wang Y, Wu W (2015) Genetic approaches for improvement of the crop potassium acquisition and utilization efficiency. Curr Opin Plant Biol 25:46–52. doi:10.1016/j.pbi.2015.04.007
Wang BL, Tang XY, Cheng LY et al (2010) Nitric oxide is involved in phosphorus deficiency-induced cluster-root development and citrate exudation in white lupin. New Phytol 187:1112–1123. doi:10.1111/j.1469-8137.2010.03323.x
Wang C, Chen H, Hao Q et al (2012) Transcript profile of the response of two soybean genotypes to potassium deficiency. PLoS ONE 7:e39856. doi:10.1371/journal.pone.0039856
Wilson ID, Neill SJ, Hancock JT (2008) Nitric oxide synthesis and signalling in plants. Plant Cell Environ 31:622–631. doi:10.1111/j.1365-3040.2007.01761.x
Xia J, Kong D, Xue S, Tian W, Li N, Bao F, Hu Y, Du J, Wang Y, Pan X, Wang L, Zhang X, Niu G, Feng X, Li L, He Y (2014) Nitric oxide negatively regulates AKT1-mediated potassium uptake through modulating vitamin B6 homeostasis in Arabidopsis. Proc Natl Acad Sci USA 111:16196–16201. doi:10.1073/pnas.1417473111
Xia X, Fan X, Wei J, Feng H, Qu H, Xie D, Miller AJ, Xu G (2015) Rice nitrate transporter OsNPF2.4 functions in low-affinity acquisition and long-distance transport. J Exp Bot 66:317–331. doi:10.1093/jxb/eru425
Xie Y, Mao Y, Lai D, Zhang W, Zheng T, Shen W (2013) Roles of NIA/NR/NOA1-dependent nitric oxide production and HY1 expression in the modulation of Arabidopsis salt tolerance. J Exp Bot 64:3045–3060. doi:10.1093/jxb/ert149
Yang T, Zhang S, Hu Y et al (2014) The role of a potassium transporter OsHAK5 in potassium acquisition and transport from roots to shoots in rice at low potassium supply levels. Plant Physiol 166:945–959. doi:10.1104/pp.114.246520
Zhang Z, Wang Q, Li Z, Duan L, Tian X (2009) Effects of potassium deficiency on root growth of cotton seedlings and its physiological mechanisms. Acta Agron Sin 35:718–723. doi:10.1016/S1875-2780(08)60079-6
Zhao D, Tian Q, Li L, Zhang W (2007) Nitric oxide is involved in nitrate-induced inhibition of root elongation in Zea mays. Ann Bot 100:497–503. doi:10.1093/aob/mcm142
Zhao M, Chen L, Zhang L, Zhang W (2009) Nitric reductase-dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis. Plant Physiol 151:755–767. doi:10.1104/pp.109.140996
Zhou B, Guo Z, Xing J, Huang B (2005) Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J Exp Bot 56:3223–3228. doi:10.1093/jxb/eri319
Acknowledgements
This work was funded by the National Nature Science Foundation of China (31672225, 31471936, and 3601818), The Agricultural Science and Technology Innovation Program (ASTIP-TRIC03), Science Foundation for Young Scholars of Tobacco Research Institute of Chinese Academy of Agricultural Sciences (No. 2016A03), Special Fund for Agro-scientific Research in the Public Interest (201203013), China Tobacco General Project: Soil Nutrient Changes and Recovery with Variable Fertilization after Land Reclamation (2013-149), and by the China Scholarship Council (CSC). The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/DNhvtC.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Wenjing Song and Ren Xue contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
344_2017_9711_MOESM1_ESM.docx
Supplementary table 1 The primer of genes for qRT-PCR. Supplementary Fig S1 Effect of NO donor SNP on elongation of root development and NO accumulation in cv. NC89. Supplementary Fig S2 Effect of NO donor SNP on elongation of root development and NO accumulation in cv. Yunyan1. Supplementary Fig S3 The expression of CYCB1;1 gene in tobacco roots. (DOCX 891 KB)
Rights and permissions
About this article
Cite this article
Song, W., Xue, R., Song, Y. et al. Differential Response of First-Order Lateral Root Elongation to Low Potassium Involves Nitric Oxide in Two Tobacco Cultivars. J Plant Growth Regul 37, 114–127 (2018). https://doi.org/10.1007/s00344-017-9711-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-017-9711-9