Abstract
Brassinosteroids play an essential role in regulating various aspects of plant growth and development as well as adaptation to various environmental stresses. The present work provides an analysis of the response of various stress markers upon exogenous application of 28-homobrassinolide (HBL) on Pusa Basmati-1, a commercially important rice variety, under salt and pesticide (Chlorpyrifos and Imidacloprid) stress. Rice seeds treated with HBL were analyzed for various growth parameters, protein, proline and malondialdehyde content (MDA), antioxidant enzyme activities, and their gene expression analysis (Cu/Zn-SOD, Fe-SOD, Mn-SOD, APX, CAT, and GR) in the presence or absence of salt and pesticide stress. Stress-induced reduction in growth, protein, and chlorophyll content and enhancement of proline and MDA content of seedlings was observed. The exogenous application of HBL resulted in the improvement of growth parameters as well as protein and proline content. MDA content decreased significantly under the effect of HBL treatment both under stress and control conditions. HBL treatment also enhanced the activity of various antioxidant enzymes which corroborated with the reduced accumulation of O ⋅-2 and H2O2 under the effect of salt and pesticides. The differential response of various isoforms of SOD under the effect of HBL and stress treatments was observed under salt and among different pesticide treatments. From this study, the potent activity of HBL in stress mitigation in response to salt and pesticide treatment in rice is established.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice is an important staple crop which provides food security to many countries worldwide. The ever increasing global population has resulted in increased demand for rice cultivation. The demand to enhance its productivity is further aggravated due to shrinking cultivable land and adverse impacts of multiple stress factors. Soil salinity is the major abiotic stress encountered by rice which greatly reduces its productivity (Kumar and others 2013; Golldack and others 2014). It is particularly a major problem in coastal regions because of the intrusion of brackish water during the dry season and at the start of the wet season. Salt stress is also detrimental to inland areas owing to the buildup of salinity as a consequence of use of excessive irrigation water and/or the poor quality irrigation water (Ismail and others 2010). High salt content in the rhizosphere causes plants to undergo osmotic and ionic stress due to the accumulation of salts outside the roots and inside the plant cells, respectively. This generates a zone of low water potential creating hindrance for roots to uptake water and nutrients from the soil resulting in a physiological drought condition (Barkla and others 2013; Mahajan and others 2008). Thus, soil salinity is a complex phenotypic and physiological phenomenon causing ion disequilibration thereby disrupting the integrity of cellular membranes and adversely affecting the activities of various enzymes linked to plant metabolism, growth, and development. It has also serious bearing on plant nutrient acquisition and functioning of the photosynthetic apparatus (Kumar and others 2013; Barkla and others 2013).
Another growing concern in rice cultivation has been the superfluous use of pesticides. To counter the challenges posed by declining resources for crop cultivation and to increase crop productivity, the use of synthetic pesticides has been a routine practice in modern agriculture. Though pesticides help control crop pests, they pose several unwanted side effects to the environment and biodiversity (Sharma and others 2013a). Among several pesticides, chlorpyrifos (CPF) and imidacloprid (IMI) are the most commonly used insecticides worldwide. CPF is a broad spectrum systemic insecticide which has been used for more than a decade to control a number of important arthropod pests (Chen and others 2012; Farahat and others 2011). IMI, on the other hand, was the first commercialized neonicotinoid insecticide and enjoys the status of one of the largest selling insecticides possessing both contact and long-lasting systemic activity to control a wide range of domestic and agricultural pests (Starner and Goh 2012). Continuous application of pesticides to the crops results in disruption of various physiological and biochemical processes affecting the crop and its resistance to pests (Xia and others 2006; Sharma and others 2012). Further, its excessive application on crops raises concern toward safety and quality of the harvested products.
In the past, several efforts including genetic engineering, QTL mapping, and conventional breeding were undertaken to improve rice plants to withstand various abiotic stresses (Kumar and others 2013). However, in the era of sustainable agriculture, the major challenge is to evolve effective and acceptable crop stress management strategies. In this context, development of crops with an inherent capacity to withstand stresses has taken center stage. Recently, the potential of phytohormones for their anti-stress effects are being explored worldwide using rational scientific approaches (Dhaubhadel and others 1999, 2002; Zhang and others 2014). Brassinosteroids, a class of polyhydroxysteroids, are known for their versatile role in plants (Clouse and Sasse 1998). BRs are perceived by the cell surface receptor complex and the subsequent activation of downstream transcription factors and genes results in various cellular responses like stem elongation, vascular differentiation, the regulation of gene expression, nucleic acid, and protein synthesis and photosynthesis (Gruszka 2013). However, insights into the role of BRs in abiotic stress amelioration has recently gained momentum (Wang and others 2014; Zhang and others 2014; Fariduddin and others 2014). In continuation with these efforts, the present paper reports the behavior of various stress markers under the effect of salt (NaCl), pesticides (CPF and IMI), and HBL (28-homobrassinolide) treatment in a commercially important indica rice variety, Pusa Basmati-1. The present study also provides information on comparative study of two different types of stresses in rice employing physiological, biochemical, and molecular approaches.
Materials and Methods
Plant Material and Growth Conditions
Seed sterilization was performed as mentioned by Sharma and others (2013b). Based on previously published results (Sharma and others 2012, 2013b) and initial experiments conducted in the laboratory on HBL, 10−7 M HBL was found to be most effective in enhancing the growth of seedlings under stress and control conditions, and thus, in the present study, HBL treatment was given as a 10−7 M formulation. The stock solution (10−3 M) of HBL was prepared by dissolving HBL in ethanol (HPLC grade) and was stored at −20 °C. The working concentration of HBL was prepared by diluting the stock solution with double distilled water. The concentrations for treatment of pesticides and salt were chosen based on their IC50 values determined on the basis of rate of germination and seedling growth under a range of concentrations of pesticides and salt. Seeds were soaked for 8 h in distilled water (control) and in a solution of HBL (10−7 M). Afterward, seeds were sown in autoclaved sand moistened with a solution of distilled water/NaCl (100 mM)/CPF (0.04 %)/IMI (0.015 %) in plastic boxes. Seeds were kept for germination in each box under controlled conditions: 25 °C (day/night), 70–80 % RH (day/night), and 14 h photoperiod. Samples were collected after 12 days to assess the following parameters.
Harvesting of Samples
Seedlings were harvested after 12 days. They were removed from the boxes and were dipped in water to remove adhering sand particles. A representative lot of 15 seedlings was chosen for study of morphological parameters, whereas the remaining seedlings were flash frozen in liquid nitrogen and then stored at −80 °C for further analysis.
Study of Morphological Parameters
For morphological parameter analysis, root and shoot lengths were measured using a meter scale, and observations for fresh weight of seedlings were made. Root number for each of the seedlings was recorded. The seedlings were then placed in an oven at 70 °C until a constant weight was achieved, and then observations for dry weight were recorded. The experiment was repeated thrice with three biological replicates.
Analysis of Plant Stress Indices
Chlorophyll content was estimated following the standard method given by Arnon (1949). Proline content in the seedlings was determined by the method suggested by Bates and others (1973). Lipid peroxidation in the samples was determined according to the method of Hodges and others (1999) by quantification of malondialdehyde (MDA) content which is an end-product of lipid peroxidation. The activity of antioxidative enzymes superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), guaiacol peroxidase (GPX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase, (DHAR) and glutathione reductase (GR) was determined by the standard methods as mentioned in Sharma and others (2013b).
Histological Determination of H2O2 and Superoxide Radical
Superoxide radicals (O ⋅-2 ) were visually detected in rice leaves according to the method suggested by Wu and others (2010). Plants were excised at the base of stems with a razor blade and supplied with 10 mM Na-citrate buffer (pH 6.0) containing 6 mM NBT for 8 h under light at 25 °C. Pale yellow NBT reacts with superoxide radicals and forms a dark-blue insoluble formazan compound. The leaves were then decolorized by immersing them in boiling ethanol (95 %) for 10 min to remove the green background of leaves except for the dark-blue insoluble formazan deposits produced by the reaction of NBT with O ⋅-2 . After cooling, leaves were observed using a stereomicroscope and photographed.
Hydrogen peroxide (H2O2) production in rice leaves was visualized histochemically using 3,3-diaminobenzidine (DAB) as a substrate (Wu and others 2010). Plants were excised at the base of stems with a razor blade, and through the cut stems, 1 mg ml−1 solution of DAB (pH 3.8) was supplied under light at 25 °C for 6 h. The leaves were eventually immersed in boiling ethanol (95 %) for 10 min to remove the green background. The deep brown polymerization product formed after reaction of DAB with H2O2 was clearly visualized under a stereomicroscope and photographed.
Statistical Analysis
Data were subjected to one-way analysis of variance (ANOVA) for studying the interaction of stress (NaCl, CPF, and IMI) with HBL and expressed as mean ± SE of three independent replicates. The Fisher’s LSD test was applied for multiple comparisons using Sigmastat version 3.5, and significance of difference between stress and HBL treatment was set at p ≤ 0.05.
Expression Analysis of Key Antioxidant Genes
Semi-quantitative reverse transcriptase-polymerase chain reaction (semi-qRT-PCR) was performed to study the expression profile of selected genes (Fe-SOD, Cu–Zn-SOD, Mn-SOD, CAT, GR, APX) in response to various treatments. Total RNA was extracted using Trizol reagent (Invitrogen; www.invitrogen.com) according to the manufacturer’s instructions. 5 μg of total RNA was reverse transcribed using the Super Script First Strand Synthesis System for RT-PCR (Invitrogen). The cDNA synthesized from mRNA of different samples was used as a template for PCR using gene specific primers (Table 1). The rice elongation factor 1α (EF1α) gene was used as a reference. All PCRs were repeated using at least three biological samples.
Results and Discussion
The adverse effects of salt and pesticides on morphological parameters such as shoot and root lengths, fresh and dry weight, and root number were clearly observed (Table 2). However, such physiological disturbances were substantially prevented by chemical pretreatment with HBL. In comparison to control (13.25 ± 0.24), the shoot length (cm) decreased to 38 % (8.16 ± 0.61), 51 % (6.43 ± 0.60), and 42 % (7.79 ± 0.17) in seedlings growing in soil solutions of NaCl, CPF, and IMI, respectively. On pre-soaking of seedlings with HBL, there was a significant enhancement in the shoot length in seedlings growing in distilled water as well as in NaCl, CPF, and IMI. An enhancement of 19 % (9.66 ± 0.38), 28 % (8.72 ± 0.43), and 34 % (10.43 ± 0.66) was observed in samples pre-treated with HBL and then grown in NaCl, CPF, and IMI, respectively. A similar decrease in root length was also observed in seedlings growing in stress conditions. However, pretreatment with HBL to the seedlings followed by application of NaCl, CPF, and IMI stress, resulted in an enhancement in root number by 34, 33, and 38 %, respectively, as compared to only stress conditions. Induction of stress conditions also led to a similar decrease in root number, fresh weight (g seedling−1), and dry weight (g seedling−1). However, co-treatment of seedlings with HBL resulted in significant enhancement in growth parameters under all the stress conditions. HBL-induced reversal of growth inhibition under stress and control conditions may be associated with the ability of BRs to enhance cell elongation, cell cycle progression, and enhancement of mRNA transcripts for cellulose biosynthesis and cell wall modifying enzymes contributing to plant growth and enhanced photosynthesis (Zhang and others 2014; Jin and others 2014; Xie and others 2011; Dobrikova and others 2014). Moreover, BRs augment the activity and expression of pesticide metabolism and detoxification enzymes which degrade and reduce their residual level in plants resulting in promotion of growth of seedlings under pesticide stress (Xia and others 2009).
Salt and pesticide stress resulted in a significant decline in the chlorophyll content (mg gFW−1) (Table 3). However, a significant enhancement of 21 % (1.61 ± 0.06), 22 % (0.66 ± 0.01), and 26 % (2.27 ± 0.04) was observed in chlorophyll a, chlorophyll b, and total chlorophyll content, respectively, in seedlings treated with salt and HBL as compared to salt treated samples only. Similarly, a significant elevation in the chlorophyll content was observed in seedlings supplemented with HBL and then treated with pesticides as compared to pesticide treatment alone. The enhancement in chlorophyll content could be due to BR-mediated regulation of transcription and/or translation of genes responsible for synthesis of chlorophyll pigments (Ahammed and others 2013) or due to the their role in reducing the degradation of chlorophyll (Honnerova and others 2010). Increased pigment content under the effect of HBL may be the result of enhanced photosynthetic efficiency leading to improved growth of seedlings (Xia and others 2009).
Under various stress conditions, protein content (mg gFW−1) of rice seedlings decreased as compared to control (31.74 ± 1.84) (Table 3). It decreased to 43 % (18.01 ± 0.77), 31 % (21.91 ± 2.07), and 43 % (18.11 ± 1.25) under NaCl, CPF, and IMI stress as compared to control conditions (Table 4). However, pretreatment with HBL improved the protein content of seedlings. BRs are known to enhance the expression of some components of translational machinery, stimulate protein synthesis, and inhibit protein degradation (Dhaubhadel and others 2002; Chris and others 2011). On the other hand, stress treatment resulted in an enhanced proline content (µmoles gFW−1) (Table 4). Proline content (µmoles gFW−1) showed a marked increase under the effect of various stress conditions. An increase of proline content to 63 % (0.39 ± 0.014) in NaCl, 183 % (0.68 ± 0.01) in CPF, and 229 % (0.79 ± 0.07) in IMI stress conditions was observed compared to controls (0.24 ± 0.012). Treatment with HBL further elevated the level of proline content by 26 % (0.49 ± 0.007) under NaCl, 32 % (0.90 ± 0.02) under CPF, and 42 % (1.12 ± 0.04) under IMI stress as compared to seedlings growing in respective stress conditions only. Proline is a multifunctional molecule involved in osmoregulation, ROS scavanging, and serves as a source for carbon and nitrogen which is known to accumulate in cells under environmental stress (Hayat and others 2014). Elevation in proline content has been reported under salt and pesticide stress (Sharma and others 2013a, b; Wu and others 2010) which is further enhanced by HBL application indicating that proline accumulation might prevent salt and pesticide-induced production of ROS and protect plants from the oxidative damage. Salt- and pesticide-induced stresses were further manifested by the increase in the level of lipid peroxidation which is usually considered to be the initial step toward cellular membrane damage by pesticides and salts (Parween and others 2012; Sharma and others 2013b). In Table 4, the level of lipid peroxidation increased to 40 % in NaCl, 164 % in CPF, and 151 % in IMI-treated samples as compared to control plants which, however, decreased under the effect of HBL application. MDA content was found to be significantly reduced to 48 % (1.08 ± 0.05) under NaCl, 55 % (1.95 ± 0.04) under CPF, 39 % (2.15 ± 0.12) under IMI stress and HBL treatment, as compared to seedlings growing in stress conditions alone. This could be regarded as evidence for HBL-induced efficient scavenging of reactive oxygen species (ROS) (Hayat and others 2014; Fariduddin and others 2014). Thus, the increased pigment content, proline and protein content, and reduced membrane damage could be contributing to the enhanced growth of seedlings under control as well as under stress conditions.
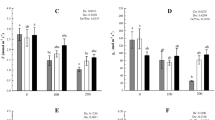
To monitor the production of ROS under the effect of stress conditions, histological determination of H2O2 and superoxide ions was done (Fig. 1). Figure 1 shows that in comparison to control leaves, leaves from the plants treated with stress showed a higher intensity of blue color pigment revealing superoxide ion accumulation. However, plants co-treated with stress and HBL showed less accumulation of superoxide ions revealed by a lesser intensity of blue color as compared to those treated with stress alone. Samples treated with HBL alone had only a slight visual difference from that of control plants. Similarly, H2O2 levels were also observed to be elevated in stressed plants as compared to the control. Seeds supplemented with HBL and then grown in stress conditions showed less coloration as compared to plants treated with stress alone, showing the alleviation of H2O2 levels due to HBL treatment as compared to the stress condition alone. The reduced accumulation of ROS may be due to the role of BRs in enhancing the process of ROS scavenging (Fariduddin and others 2014). Interestingly, treatment with HBL alone resulted in a slightly enhanced accumulation of H2O2 levels as compared to controls. A similar indication of a BR-induced transient oxidative burst with H2O2 accumulation has been reported recently (Xia and others 2009; Jiang and others 2012a, b). This clearly indicates a possible link between H2O2 and BR signaling.
O ⋅−2 and H2O2 accumulation in leaves of rice exposed to different stress conditions (NaCl, CPF and IMI) and HBL for 12 days. Plants were excised at the base of stems and supplied through the cut stems with nitroblue tetrazolium (NBT) solution for 8 h (a) and 3, 3diaminobenzidine (DAB) solution for 6 h (b). Afterward, the leaves were photographed
Modulation of the antioxidant system is believed to play a critical role in BR-mediated stress amelioration (Fariduddin and others 2014). In the present experiment, different stress conditions resulted in an alteration in the activity of various antioxidant enzymes. There was an enhancement in the activity of SOD under the effect of various stress conditions (Table 5). SOD activity increased to 41 % under NaCl (38.9 ± 1.2), 51 % under CPF (41.7 ± 1.0), and 37 % under IMI stress (37.73 ± 0.7) as compared to control samples (27.6 ± 0.7). Co-treatment with HBL led to a significant elevation of 13 % under NaCl (43.8 ± 0.5), 8 % under CPF (45.4 ± 1.2), 9 % under IMI (41.05 ± 0.7) stress in SOD activity as compared to respective stress conditions alone. Similar results were obtained with other enzymes too. For efficient scavenging of ROS, the activity of APX and SOD should be high so as to remove H2O2 produced by superoxide ion dismutation (Pospíšil 2012). Similar to SOD activity, APX activity increased not only under stress conditions but HBL treatment further augmented the activity by 17.4 % (33.38 ± 0.51) under NaCl, 22 % (53.4 ± 1.4) under CPF, and 24 % (35.8 ± 1.2) under IMI stress as compared to respective stress conditions alone. Interestingly in the case of CAT, of all the stresses, only CPF treatment induced the activity as compared to control. Such a result could be hinting toward the CPF-led targeting of peroxisomes resulting in activation of CAT activity for scavenging ROS. Like APX, GR plays an essential role in the protection of chloroplasts against oxidative damage by maintaining a high reduced/oxidized glutathione (GSH/GSSG) ratio (Parween and others 2012). GR activity elevated to 29 % (3.49 ± 0.06) under CPF and 31 % (4.03 ± 0.13) under IMI stress as compared to control revealing pesticide-induced toxicity of chloroplasts. HBL treatment further boosted the activity of GR for enhanced ROS mitigation as well as inactivation of pesticides by GSH-conjugate formation (Xia and others 2009; Parween and others 2012). In the case of GPX, HBL application elevated the activity under salt and IMI stress as compared to stress conditions alone. DHAR and MDHAR activity either decreased or did not show any change under stress conditions, though a slight increase in DHAR activity under the effect of HBL supplementation with CPF and NaCl was observed as compared to stress alone. Overall, BR application boosted the antioxidant defense system to protect plants against an oxidative burst. BR-induced changes in antioxidant enzyme activities could be due to enhanced protein synthesis or changed kinetic properties of the enzymes (Thao and Tran 2012).
To understand the transcriptional regulation of antioxidant genes under HBL and stress conditions, expression analysis of the key antioxidant genes was done (Fig. 2). The differential response of antioxidant genes under the effect of stress alone as well as in combination with HBL merits some attention. Treatment with salt stress led to the upregulation of all the SOD isoforms whereas CPF stress resulted in major upregulation in Cu/Zn-SOD and Mn-SOD and seedlings treated with IMI stress manifested a pronounced enhancement in the Fe-SOD transcript level. Samples co-treated with HBL and different stresses resulted in an enhanced expression of SOD isoforms, though to different levels. Cu/Zn-SOD expression increased to 1.7-fold in HBL + IMI samples as compared to only IMI-treated sample. In the case of Fe-SOD, HBL application resulted in an enhancement in the expression to 1.3-, 2.3-, and 1.3-fold under the NaCl, CPF and IMI stress conditions, respectively, as compared to respective stress conditions alone. Expression of Mn-SOD increased to 1.3- and 1.2-fold under the combined treatment of HBL with NaCl and with CPF, respectively, in comparison to stress conditions alone. The differential regulation of certain antioxidant enzymes of the seedlings in response to different types of stresses and hormone treatment could be linked to their cellular localization and targeted effect on specific cell organelles (Gomez and others 2004; Mylona and others 2007). Similarly, in the case of APX, the combined treatment of HBL and stress resulted in a selective upregulation (1.5 fold) under the effect of HBL and IMI as compared to samples treated with IMI alone. The expression of GR and CAT was enhanced under the effect of stress, whereas the combined treatment of stress with HBL did not result in a significant change in expression. The expression profile for most of the genes corroborated with the activity of the antioxidant enzymes except for CAT expression in samples treated with salt and HBL and GR expression, which either decreased or remained unchanged, though a significant increase in the activity was shown. These data indicate that the expression of these genes involves both transcriptional and post-translational regulation. Increased activity and expression of chloroplastic genes APX, GR, and Cu/Zn-SOD under various stresses points toward enhanced ROS generation in chloroplasts under these stresses. However, CPF stress showed marked enhancement in the expression of mitochondrial Mn-SOD revealing that CPF may have a more deleterious effect on mitochondrial function. Previously it has been reported that pesticides as well as their adjuvants affect chloroplastic membrane fluidity, inhibition of photosynthetic rate, and photosynthetic electron transport which can be connected to the damage caused by increased ROS production (Caux and Weingerger 1993; Xia and others 2009; Chris and others 2011). The diverse effects of the different stress conditions on mRNA abundance of various genes thus provide an insight into the mode of action of each treatment and their effect on the different subcellular compartments (Gomez and others 2004; Mylona and others 2007).
An ethidium bromide–stained agarose gel harboring products from reverse transcriptase–PCR of 12-day-old rice seedlings exposed to distilled water (control, C), NaCl (S), chlorpyrifos (CPF), imidacloprid (IMI), and their combination with 10−7 M HBL (H), NaCl and HBL (S + H), CPF and HBL (CPF + H), IMI and HBL (IMI + H) for various key antioxidant genes. The gel shows the PCR products after 35 cycles of PCR
Conclusion
Based on the present study, it is concluded that HBL helps restore the homeostasis of rice plants under different stress conditions and makes the plant better equipped to fight stress conditions. Stress amelioration is facilitated by BR impacts on various regulatory mechanisms like accumulation of osmolytes, improved pigment content, reduction in lipid peroxidation, reduced accumulation of ROS and enhanced activity, and expression of antioxidative defense genes. All these factors contribute to the array of protective mechanism employed by plants for combating the cytotoxic levels of various stresses including salt and pesticides. In conclusion, this study showed potent activity of BRs in stress response which is important not only for understanding the essential role of this plant growth regulator to a variety of stresses but also for its futuristic application in agriculture.
References
Ahammed GJ, Choudhary SP, Chen S, Xia X, Shi K, Zhou Y, Yu J (2013) Role of brassinosteroids in alleviation of phenanthrene-cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J Exp Bot 64:199–213
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant physiol 24:1–15
Barkla BJ, Castellanos-Cervantes T, Diaz de León JL, Matros A, Mock HP, Perez- Alfocea F, Salekdeh GH, Witzel K, Zörb C (2013) Elucidation of salt stress defense and tolerance mechanisms of crop plants using proteomics—current achievements and perspectives. Proteomic 13:12–13
Bates LS, Waldeen RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Caux PY, Weingerger P (1993) Efects of pesticide adjuvants on membrane lipid compositon and fluidity in Lemna minor. Can J Bot 71:1291–1297
Chen S, Liu C, Peng C, Liu C, Hu M, Zhong G (2012) Biodegradation of chlorpyrifos and its hydrolysis product 3,5,6-trichloro-2-pyridinol by a new fungal strain Cladosporium cladosporioides Hu-01. PLoS ONE 7:e47205
Chris C, Luxmisha G, Masih J, Abraham G (2011) Growth, photosynthetic pigments and antioxidant responses of Azolla filiculoides to monocrotophos toxicity. J Chem Pharm Res 3:381–388
Clouse SD, Sasse JM (1998) Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol 49:427–451
Dhaubhadel S, Chaudhary S, Dobinson KF, Krishna P (1999) Treatment with 24-epibrassinolide, a brassinosteroid, increases the basic thermotolerance of Brassica napus and tomato seedlings. Plant Mol Biol 40:333–342
Dhaubhadel S, Browning KS, Gallie DR, Krishna P (2002) Brassinosteroid functions to protect the translational machinery and heat-shock protein synthesis following thermal stress. Plant J 29:681–691
Dobrikova AG, Vladkova RS, Rashkov GD, Todinova SJ, Krumova SB, Apostolova EL (2014) Effects of exogenous 24-epibrassinolide on the photosynthetic membranes under non- stress conditions. Plant Physiol Biochem 80:75–82
Farahat FM, Ellison CA, Bonner MR, McGarrigle BP, Crane AL, Fenske RA, Lasarev MR, Rohlman DS, Anger WK, Lein PJ, Olson JR (2011) Biomarkers of chlorpyrifos exposure and effect in Egyptian cotton field workers. Environ Health Perspect 119:801–806
Fariduddin Q, Yusuf M, Ahmad I, Ahmad M (2014) Brassinosteroids and their role in response of plants to abiotic stresses. Biol Plant 58:9–17
Golldack D, Li C, Mohan H, Probst N (2014) Tolerance to drought and salt stress in plants: unraveling the signaling networks. Front Plant Sci 22:151–176
Gomez JM, Jimenez A, Olmos E, Sevilla F (2004) Location and effects of long-term NaCl stress on superoxide dismutase and ascorbate peroxidase isoenzymes of pea (Pisum sativum cv. Puget) chloroplasts. J Exp Bot 55:119–130
Gruszka D (2013) The brassinosteroid signaling pathway-new key players and interconnections with other signaling networks crucial for plant development and stress tolerance. Int J Mol Sci 14:8740–8874
Hayat S, Khalique G, Wani AS, Alyemeni MN, Ahmad A (2014) Protection of growth in response to 28-homobrassinolide under the stress of cadmium and salinity in wheat. Int J Biol Macromol 64:130–136
Hodges MD, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid- reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Honnerova J, Rothova O, Hola D, Kocova M, Kohout L, Kvasnica M (2010) The exogenous application of Brassinosteroids to Zea mays (L.) stressed by long term chilling does not affect the activities of photosystem 1 or 2. J Plant Growth Regul 29:500–505
Ismail AM, Thomson MJ, Vergara GV, Rahman MA, Singh RK, Gregorio GB (2010) Designing resilient rice varieties for coastal deltas using modern breeding tools. In: Hoanh CT, Szuster BW, Pheng KS, Ismail AM, Nobel AD (eds) Tropical Deltas and coastal zones: food production, communities and environment at the land-water interface. CAB, Wallingford, pp 154–165
Jiang YP, Cheng F, Zhou YH, Xia XJ, Mao WH, Shi S, Chen Z, Yu JQ (2012a) Cellular glutathione redox homeostasis plays an important role in the brassinosteroid-induced increase in CO2 assimilation in Cucumis sativus. New Phytol 194:932–943
Jiang YP, Cheng F, Zhou YH, Xia XJ, Mao WH, Shi K, Chen ZX, Yu JQ (2012b) Hydrogen peroxide functions as a secondary messenger for brassinosteroids-induced CO2 assimilation and carbohydrate metabolism in Cucumis sativus. Journal of Zhej Univ Sci 13:811–823
Jin H, Do J, Shin SJ, Choi JW, Choi YI, Kim W, Kwon M (2014) Exogenously applied 24-epi brassinolide reduces lignification and alters cell wall carbohydrate biosynthesis in the secondary xylem of Liriodendron tulipifera. Phytochem 101:40–51
Kumar K, Kumar M, Kim SR, Ryu H, Cho YG (2013) Insights into genomics of salt stress response in rice. Rice 6:27–38
Mahajan S, Pandey GK, Tuteja N (2008) Calcium- and salt-stress signaling in plants: shedding light on SOS pathway. Arch Biochem Biophys 471:146–158
Mylona PV, Polidoros AN, Scandalios JG (2007) Antioxidant gene responses to ROS-generating xenobiotics in developing and germinated scutella of maize. J Exp Bot 58:1301–1312
Parween T, Jan S, Mahmooduzzafar S, Fatma T (2012) Evaluation of oxidative stress in Vigna radiata L. in response to chlorpyrifos. Int J Environ Sci Technol 9:605–612
Pospíšil P (2012) Molecular mechanisms of production and scavenging of reactive oxygen species by photosystem II. Biochim Biophys Acta 1817:218–231
Sharma I, Bhardwaj R, Pati PK (2012) Mitigation of adverse effects of chlorpyrifos by 24- epibrassinolide and analysis of stress markers in a rice variety Pusa Basmati-1. Ecotoxicol Environ Saf 85:72–81
Sharma I, Bhardwaj R, Pati PK (2013a) Stress modulation response of 24-epibrassinolide against imidacloprid in an elite indica rice variety Pusa Basmati-1. Pest Biochem Physiol 105:144–153
Sharma I, Ching E, Saini S, Bhardwaj R, Pati PK (2013b) Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol Biochem 69:17–26
Starner K, Goh KS (2012) Detections of the neonicotinoid insecticide imidacloprid in surface waters of three agricultural regions of California, USA, 2010-2011. Bull Environ Contam Toxicol 88:316–321
Thao NP, Tran LS (2012) Potentials toward genetic engineering of drought-tolerant soybean. Crit Rev Biotech 32:349–362
Wang X, Chen J, Xie Z, Liu S, Nolan T, Ye H, Zhang M, Guo H, Schnable PS, Li Z, Yin Y (2014) Histone lysine methyltransferase SDG8 is involved in brassinosteroid regulated gene expression in Arabidopsis thaliana. Mol Plant. doi:10.1093/mp/ssu056
Wu GL, Cui J, Tao L, Yang H (2010) Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa). Ecotoxicol 19:124–132
Xia XJ, Huang YY, Wang L, Huang LF, Yu YL, Zhou YH, Yu JQ (2006) Pesticides induced depression of photosynthesis was alleviated by 24-epibrassinolide pre-treatment in Cucumis sativus L. Pest Biochem Physiol 86:42–48
Xia XJ, Wang YJ, Zhou YH, Tao Y, Mao WH, Shi K, Asami T, Chen ZX, Yu JQ (2009) Reactive oxygen species are involved in brassinosteroid—induced stress tolerance in cucumber. Plant Physiol 150:801–814
Xie L, Yang C, Wang X (2011) Brassinosteroids can regulate cellulose biosynthesis by controlling the expression of CESA genes in arabidopsis. J Exp Bot 62:4495–4506
Zhang C, Bai MY, Chong K (2014) Brassinosteroid-mediated regulation of agronomic traits in rice. Plant Cell Rep 33:683–696
Acknowledgments
University Grants Commission (UGC), New Delhi is duly acknowledged for funding the proposed work under major research Project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, I., Bhardwaj, R. & Pati, P.K. Exogenous Application of 28-Homobrassinolide Modulates the Dynamics of Salt and Pesticides Induced Stress Responses in an Elite Rice Variety Pusa Basmati-1. J Plant Growth Regul 34, 509–518 (2015). https://doi.org/10.1007/s00344-015-9486-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-015-9486-9