Abstract
Laser-induced fluorescence (LIF) technique has been widely used to measure the polycyclic aromatic hydrocarbons (PAHs) in sooting flames, but the assignment of the LIF signals measured in flames to specific PAH species is hard to obtain. Investigation on the LIF spectra of gas-phase PAH mixtures could be helpful to analyze LIF spectra measured in flames for more detailed PAH information. In this study, the LIF spectra of gas-phase PAH mixtures containing naphthalene, phenanthrene, pyrene, fluoranthene were experimentally investigated in an optical cell at 673 K and 1073 K. The experimental results indicated that the integral fluorescence intensity of each PAH was in direct proportion to its mole fraction. Moreover, the LIF spectra of PAH mixtures were linearly superposed from the spectra of their components weighted using mole fractions. The component concentrations of the PAH mixtures were able to be fitted from the LIF spectra using multiple linear regression method, and most of the relative errors were less than 5%. In the light of these results from gas-phase PAHs, the LIF spectra of flame-formed PAHs measured in a laminar premixed ethylene flame were discussed. Considering the temperature effects on LIF spectra and simulated PAH concentrations in the flame, the lighter PAHs will contribute to the fluorescence signals shorter than 450 nm measured in the flame. Using the multiple linear regression method established in this study, the relative ratios of naphthalene, phenanthrene and pyrene can be fitted from the LIF spectrum of the flame. In this way, more selective information of these lighter PAHs can be obtained from the LIF spectrum measured in flames. On the other hand, fluorescence signals in the flame longer than 450 nm are possibly emitted from the PAHs with five-membered-ring structures or (and) nanoparticles and nascent soot particles, which are still ambiguous and needs to be further investigated in future works.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Polycyclic aromatic hydrocarbons (PAHs) are usually formed in the incomplete combustion processes of fossil fuels and have received great attentions due to their adverse health and environment effects [1,2,3,4]. PAHs are considered as important precursors of soot, and extensive studies have focused on the PAH and soot formation mechanisms [5,6,7,8,9,10]. Therefore, it is important to measure the lighter PAHs to understand the earliest growth processes of aromatic molecule and soot in the combustion process [11, 12]. Laser-induced fluorescence (LIF) technique is a non-intrusive and on-line method capable of capturing the temporally and spatially resolved information of PAHs [13], and was widely used to measure the PAH information in sooting flames [14,15,16,17,18,19]. The size of the PAH molecules can be approximatively distinguished according to the fluorescence wavelength [20,21,22]. Choi et al. [23, 24] investigated the PAH distribution in counterflow diffusion flames using several bandpass filters of 330, 400, 450, and 500 nm to differentiate relative size groups of PAHs. However, in this way, it is difficult to identify the specific PAH species mainly due to the overlap of the LIF spectra emitted from different PAHs.
The PAHs formed in sooting flames are complicated mixtures, therefore, considering that the overlap of LIF spectra from PAHs is inevitable, it is necessary to understand the spectrum superposition regulation of PAH mixtures. Some studies focusing on the LIF spectra of gas-phase PAH mixtures have been conducted. Allain et al. [25] had collected the fluorescence spectra of benzo[a]pyrene, pyrene, anthracene, and phenanthrene vapors as well as their mixtures at 300 °C, and they found that the spectra of mixtures were in accordance with the spectra calculated by linearly summing the standard PAH spectra weighted using compositions. Sun et al. [26] conducted a research on the LIF measurement for PAHs contained in tar from gasification and pyrolysis of biomass. They investigated the LIF spectra gas-phase PAH mixtures at 300 °C, and confirmed that the fluorescence signals of PAHs obey the principle of linear superposition. However, the existing studies of LIF spectra of gas-phase PAH mixtures are at relatively low temperatures, which are inadequate for analysis of LIF spectra measured in flames. To further distinguish the composition of a complex PAH mixture, an analytic method which can calculate the component concentrations from the LIF spectra of PAH mixtures needs to be established at a wider temperature range.
In the current study, the LIF spectra of gas-phase PAH mixtures containing four components (naphthalene, phenanthrene, pyrene, fluoranthene) were systematically investigated in an optical cell at 673 K and 1073 K. These PAHs are extensively produced in sooting flames and they are the interested targets of in-flame measurement. The relationship between fluorescence intensity and mole fraction of each individual PAH, as well as the spectrum superposition regulations of PAH mixtures, were experimentally studied at both temperatures. Then, with these results, the component concentrations were fitted from the LIF spectra of PAH mixtures on the basis of multiple linear regression method. In our previous study [27], the temperature effects on the fluorescence spectra and photophysical properties of these gas-phase PAHs have been investigated at the temperature range of 673–1373 K. Based on the temperature effects and the analytic method used for gas-phase PAH mixtures, subsequently, the LIF spectra of flame-formed PAHs were measured in a laminar premixed ethylene flame, and the assignment of the LIF spectra to specific PAH species was analyzed.
2 Experimental and computational methods
2.1 LIF diagnostic system
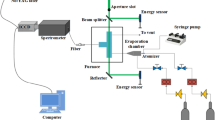
The LIF diagnostic system used in this study, as shown in Fig. 1, is the same as the one used in our previous paper [27] and is only introduced here briefly. The excitation light source was generated by fourth harmonic (266 nm) of a pulsed Nd:YAG laser (Quantel Q-smart 850). The incident laser energy was around 15 mJ/cm2 per pulse. This energy density should be kept at a relatively low value for weak linear excitation, in the meantime the laser should have enough energy to ensure the reasonable signal to noise ratios. A 4-mm aperture slot was placed at the central portion of the laser beam and directed to the fluorescence probe region. A spectrometer (Princeton SP-2-500i) with a 1200 g/mm 500 nm blazed grating was used to obtain spectral resolved LIF signals which were conveyed by an optical fiber placed at a right angle with respect to the laser propagation. The spectrometer was calibrated by a standard tungsten lamp. An intensified charge-coupled device (ICCD, Princeton PI-MAX3) camera was linked to the exit of the spectrometer to record the LIF signals. The gate width of ICCD was set at 50 ns without delay, and triggered by the laser. For each measurement, fluorescence signals were averaged more than 200 laser shots. The spectral resolution of the detection system is 0.05 nm, and the original spectrum data were smoothed by an adjacent-average filter with a window size of 8 nm to reduce the fluctuation of the spectrum. This processing method reduced spatial resolution but increased signal qualities and certainties. The laser, spectrometer and ICCD were controlled by a personal computer.
2.2 PAH gasification system
Naphthalene, phenanthrene, pyrene and fluoranthene were selected for analysis of PAH mixtures due to their importance in sooting flames. Their basic information was listed in Table 1. The analytical standard substances of investigated PAHs were purchased from suppliers and used without further purification. The solid-state PAH substances were dissolved in n-hexane firstly, then these solutions were injected to a quartz atomizer by a syringe pump (LSP01-1A). The PAHs/n-hexane solution was crushed by argon at 0.2 MPa in the atomizer and sprayed into an evaporation chamber preheated at 300 °C by electric heating tapes. In the evaporation chamber, the atomized solutions were diluted by argon and conveyed to the optical cell. The LIF signals were detected in an optical cell placed in a tube furnace. To control the mole fractions of each component in PAH mixtures, the masses of PAHs in solutions were adjusted. The optical cell was evacuated by argon before measurement until no LIF signal can be detected, and each set of measurement was conducted at steady conditions.
2.3 Burner and flames
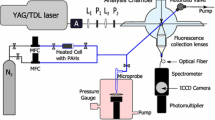
A laminar premixed flame was generated from a water-cooled porous plug burner, as shown in Fig. 2. In the measurement of flame-formed PAHs, the optical cell and tube furnace were replaced by the burner. Ethylene, oxygen and argon were premixed in a 5-cm diameter cylindrical mixing chamber and the shield argon flowed from an outer coaxial annulus to isolate the flame from the ambient air. A water-cooled stagnation plate was placed above the burner at 20 mm height to stabilize the flame. The equivalence ratio was 2.1, the dilution ratio defined as \(\left( {Q_{\text{AR}} } \right)/\left( {Q_{{{\text{C}}_{2} {\text{H}}_{4} }} + Q_{{{\text{O}}_{2} }} + Q_{\text{AR}} } \right)\) was 60%, and the total flow was 5.3 L/min (C2H4/O2/Ar = 0.873/1.247/3.180). The flame was at atmospheric pressure.
To obtain the mole fractions of flame-formed PAHs, the laminar premixed flame was simulated using a premixed burner stagnation flame model in Chemkin-Pro Software package. A PAH growth mechanism (KAUST PAH Mechanism 2, KM2) [28] which includes 202 species and 1351 reactions was used in this model. KM2 mechanism is able to calculate PAH concentrations up to seven-aromatic-ring species (coronene) and has been validated with existing experimental data on premixed flames [28, 29] and counterflow diffusion flames [7, 30, 31]. The temperature profiles of the flame were measured by an S-type thermocouple, and the detailed methods were described in our previous paper [14]. Then, the gas components, pressure and measured temperature profiles were the input parameters of the model. The allowed maximum number of grid points was set as 500 to get grid-independent solutions. The curvature between grid points and allowed maximum gradient were 0.5 and 0.2, respectively, while the other parameters were set as default values.
3 Results of gas-phase PAH LIF spectra
3.1 LIF spectra of gas-phase PAHs with different mole fractions
Naphthalene, phenanthrene, pyrene and fluoranthene extensively exist in sooting flames and they are the interested species in LIF measurement. To investigate the spectral characteristics of these PAHs, the LIF spectra of these four gas-phase PAH were measured in the optical cell at 673 K and 1073 K, and the mole fractions of each PAH were all controlled at 1.5 × 10−5. The results of LIF spectra showed that the fluorescence wavelengths of these PAHs ranged from ultraviolet to visible region as shown in Fig. 3. The second feature was that the spectra of different PAHs overlapped with each other, causing the complexity to analyze the LIF spectra of PAH mixtures. As the temperature increased from 673 to 1073 K, the fluorescence intensities of these four PAHs decreased observably and their descent degrees were quite different, which was described in detail in our previous paper [27].
For weak excitation, the relation of the integral fluorescence intensity S and PAH concentration can be expressed by the following Eq. (1):
where ηopt is the optical efficiency of the system, Elaser is the laser energy, h is Planck constant, c is the speed of light, λ is the excitation wavelength, dVc is the detection volume, X is the mole fraction of excited species, k is Boltzmann constant, σ is the absorption cross section, and ϕfl is the fluorescence quantum yield. This equation indicates that the fluorescence intensity S (i.e., the integral area underneath the curves of fluorescence spectrum) is directly proportional to the mole fraction, if the temperature, pressure and experiment system are kept unchanged. The direct proportional relationship between fluorescence intensity and PAH concentration is the foundation of the in-flame PAH measurement [13, 32]. To verify this relationship described by Eq. (1) and obtain the proportionality coefficients of each PAH, fluorescence spectra and integral intensities of these PAHs as a function of their mole fractions were further investigated at 673 K and 1073 K.
The results of the concentration dependence of fluorescence spectra and integral intensities at 673 K and 1073 K are shown in Figs. 4 and 5, respectively. The detected spectral shapes and emission wavelengths (shown in the left column) were not sensitive to the mole fractions of PAHs. The right column of the figures showed that the fluorescence intensities of these PAHs were in direct proportion to the mole fractions at both 673 K and 1073 K. The linear fitting equations are also shown in the right column of Figs. 4 and 5, and the proportionality coefficients of each PAH were different from each other. More importantly, the proportionality coefficients were different at these two temperatures, as described in the Eq. (1). It is notable that when the fluorescence intensities are used to represent the relative concentrations of flame-formed PAHs, the temperature gradient of the flame needs to be considered. The proportionality coefficients presented in Figs. 4 and 5 can only be used to analyze the component concentrations of gas-phase PAH mixtures at 673 K and 1073 K, but for other temperatures, the proportionality coefficients should be calculated by the relationships presented in our previous paper [27].
3.2 LIF spectrum superposition of PAH mixtures
Based on the linear relationship between fluorescence intensities and PAH concentrations, the component concentrations of individual PAH are potential to be calculated from the LIF spectra of PAH mixtures. Here, the linear superposition principle of PAH mixtures was firstly checked at 673 K and 1073 K. Four groups of PAH mixtures with different mixing ratios were investigated and the mole fractions of each component are listed in Table 2. On the basis of the linear superposition principle, the superposed spectra of the mixtures can be calculated by Eq. (2):
In this equation, Fsuperposed, f1, f2, f3 and f4 are the fluorescence intensity vectors of dimension n, where n is the number of points (wavelengths) of the spectrum. Fsuperposed represents the superposed fluorescence intensities, and f1–f4 represent the reference fluorescence intensities of naphthalene, phenanthrene, pyrene and fluoranthene, respectively, when the mole fractions were all kept at 1.5 × 10−5 as shown in Fig. 3. The superposition coefficients R1–R4 are the ratios of the mole fractions of each component (in Table 2) and the mole fractions of the reference PAHs (1.5 × 10−5). The comparisons of the measured LIF spectra (black solid line) and superposed LIF spectra (red dot line) at 673 K and 1073 K are shown in Figs. 6 and 7, respectively. The spectra beneath the superposed spectrum were calculated by the product of Ri and fi, representing each item in Eq. (2). A good agreement between the superposed spectra and the measured spectra of PAH mixtures was obtained at both 673 K and 1073 K. This result indicated that the linear superposition principle could be used to analyze the LIF spectra of PAH mixtures at different temperatures.
Based on the proven linear superposition principle, a quantitative analysis would be conducted to estimate the concentrations of the components in PAH mixtures. Firstly, the superposition coefficients R1–R4 would be fitted according to a minimization procedure of a deviation vector. The deviation vector was expressed by ɛ = Fmeasured − Fsuperposed, where Fmeasured was the measured fluorescence intensity vectors of PAH mixtures spectra, and Fsuperposed was calculated by Eq. (2). In this study, multiple linear regression on the basis of a least square method was applied to calculate the superposition coefficients R1–R4 when the deviation vector ɛ was optimized to the minimum value. The calculated superposition coefficients R1–R4 and their relative errors (in the bracket) at 673 K and 1073 K were listed in Tables 3 and 4, respectively. The results in Table 3 showed that the calculated R1–R4 at 673 K were very close to the actual values and most of the relative errors were less than 5%. However, at 1073 K, the relative errors of R4 for fluoranthene in mixture 1 and mixture 3 were greater than 10%, because the fluorescence intensities (f4) of fluoranthene in mixture 1 and mixture 3 were very weak compared with other species, as shown in Fig. 7a, c. This suggested that the reduction of fluorescence intensities of each component with increasing temperature needs to be considered.
In the next step, after obtaining the superposition coefficients R1–R4, the measured LIF spectrum of PAH mixture can be decomposed into four spectra of each component which can be expressed by the product of Ri and fi. Then, based on the relationship of the integral LIF intensities and PAH concentrations described in Figs. 4 and 5, the mole fractions of each component can be calculated from the integral LIF intensities of decomposed spectra. The calculated mole fractions and their relative errors of each component in these mixtures were shown in Tables 5 and 6. Compared with the actual mole fractions shown in Table 2, the calculated results were reasonable and most of the relative errors were less than 5%. Considering the high linearity between the integral LIF intensities and PAH concentrations shown in Figs. 4 and 5, the errors of the calculated mole fractions of each component were mainly from the multiple linear regression process to obtain R1–R4. In conclusion, this analytic method can obtain the concentration information of each component in the PAH mixtures quantitatively at different temperatures.
4 Implication to LIF spectra of flame-formed PAHs
As described in the above sections, the LIF spectra of the gas-phase PAH mixtures can be analyzed to obtain their component concentrations. PAHs formed in combustion process are gas-phase mixtures which can be induced by UV-lasers and emit fluorescence, although the flame environment is pretty complicated, especially soot particles in flames. In this section, LIF spectra measured in a laminar premixed ethylene flame were experimentally investigated. To avoid the LII signals emitted from soot disturbing the LIF spectra measured in flames, the incident laser energy was kept at 15 mJ/cm2 per pulse and the gatewidth of ICCD was set at 50 ns without delay. An attempt to interpret the LIF spectra of flame-formed PAHs was carried out based on the method used in gas-phase PAH mixtures.
Firstly, the LIF spectra of flame-formed PAHs were measured in a laminar premixed flame. According to the previous study of our group [15], the shapes of LIF spectra kept nearly unchanged along with different flame heights, therefore, in this study, the LIF spectrum of the flame was measured only at the 15-mm height above the burner where the mole fractions of PAHs had reached a stable value. The normalized LIF spectrum measured in the flame is shown in Fig. 8 (black thick line), and it was notable that the flame-formed PAHs emitted a broadband LIF spectrum mostly in the visible range and centered around 500 nm. This LIF spectrum was in accordance with the spectrum of a methane premixed flame measured by Bejaoui et al. [17, 33]. To analyze assignment of the LIF spectra measured in flames to specific PAH species, the LIF spectra of the four gas-phase PAHs at 673 K in Fig. 3a were normalized by their maximum intensity and are also shown in Fig. 8 (solid thin lines). The LIF spectra of naphthalene, phenanthrene, pyrene and fluoranthene at 673 K mainly located in UV and visible region ranging from 300 to 580 nm, which revealed significant differences compared with the LIF spectra of flame-formed PAHs.
The measurement conditions of the gas-phase PAHs and flame-formed PAHs were quite different, especially the temperatures. In this study, the temperature of the measuring point in the laminar premixed flame was around 1700 K [15] while the four gas-phase PAHs were measured at 673 K, which was likely to be the important reason of the significant differences. As temperature increases, the fluorescence intensity will decrease and the LIF spectra will shift to the red. Based on these two factors, therefore, the LIF spectra of four gas-phase PAHs at 673 K were transformed to their predicted spectra at 1700 K. According to the results in our previous study [27], the fluorescence intensities of naphthalene, phenanthrene, pyrene and fluoranthene decreased to 3.73%, 32.54%, 28.05% and 2.63% from 673 to 1700 K, respectively. The redshifts of LIF spectra are approximately 3–5 nm per 100 K [27, 34, 35], therefore, the redshift in this study was estimated to be 40 nm. Finally, the predicted spectra of the four gas-phase PAHs at 1700 K are shown in Fig. 8 (dashed thin lines).
To better understand the LIF spectra measured in flames, the mole fractions of main flame-formed PAHs were simulated using a laminar premixed stagnation flame model in Chemkin-Pro Software coupled with KM2 PAH formation mechanism. The calculated mole fractions of the seven most abundant lighter PAHs at the measuring height of this flame are shown in Fig. 9. The chemical formulas and structures of these seven PAH species are listed in Tables 1 and 7.
On the basis of the experimental data of LIF spectra and the simulation results of mole fractions, the four investigated PAHs (naphthalene, phenanthrene, pyrene and fluoranthene), which have high mole fractions in flames and high fluorescence quantum yields even at flame temperatures, will contribute to the fluorescence signals shorter than 450 nm measured in flames. However, the fluorescence signals longer than 450 nm in flames are still ambiguous. As shown in Fig. 9, acenaphthylene, acephenanthrylene and cyclopenta[cd]pyrene, with five-membered-ring structures added on naphthalene, phenanthrene and pyrene, respectively, have high mole fractions in the flame. And according to the quantum chemical calculation using time-dependent density functional theory (TD-DFT) [20], PAHs with five-membered-ring structures have high fluorescence quantum yields and are possible to emit the LIF signals longer than 450 nm. Unfortunately, the LIF spectra of acenaphthylene, acephenanthrylene, and cyclopenta[cd]pyrene can hardly be measured under gas phase conditions in the optical cell due to the absence of analytical standard substances, and their experimental spectrum data cannot be found in literature. In addition, D’Anna [2] detected the spectral behavior of PAHs between 290 and 600 nm in ethylene flames, and presented that the visible fluorescence in the yellow-orange region may be attributed to nanoparticles or nascent soot particles. Therefore, the fluorescence signals longer than 450 nm in flames are possibly emitted from PAHs with five-membered-ring structures or the nanoparticles and nascent soot particles.
On the hypothesis that the fluorescence signals shorter than 450 nm in flames are mainly emitted from the lighter PAHs (naphthalene, phenanthrene, pyrene and fluoranthene), the LIF spectrum of the flame was analyzed based on the multiple linear regression method used in Sect. 3.2. The predicted spectra of four gas-phase PAHs at 1700 K were the reference fluorescence spectra f1, f2, f3 and f4, and the fluorescence spectrum shorter than 450 nm measured in the flame was the measured spectrum Fmeasured. Then, the superposition coefficients R1–R4 could be fitted. Because the LIF spectrum of fluoranthene was very weak and covered by the strong fluorescence signals longer than 450 nm measured in the flame, the calculated R4 was not accurate enough and was discarded. The superposed spectrum was calculated from R1–R3 and f1–f3 using the Eq. (2), and is shown in Fig. 10 (orange dashed line). The rising edge of the superposed spectrum can well fit that of the spectrum measured in the flame, which indicating that the calculated superposition coefficients R1–R3 were reliable.
Considering that the different measurement conditions of the gas-phase PAHs and flame-formed PAHs such as soot in flames, the relationship between the fluorescence intensities and concentrations for gas-phase PAHs cannot be used directly to calculate the absolute concentrations of the flame-formed PAHs. Therefore, only relative ratios of naphthalene, phenanthrene and pyrene were able to be fitted from the LIF spectrum measured in the flame, as shown in Table 8. In addition, the simulated mole fractions of these four PAHs and their relative ratios are also shown in Table 8. It can be seen that the relative errors between the fitted and simulated results were within 20%. This confirmed that the fluorescence signals shorter than 450 nm measured in flames are mainly emitted from naphthalene, phenanthrene and pyrene, and based on this analytic method, more selective information of these lighter PAHs can be obtained from the LIF spectrum measured in flames.
5 Conclusion and outlook
Understanding of the LIF spectra of gas-phase PAHs and the spectrum superposition regulation is important to analyze the LIF spectra measured in flames. In this work, the LIF spectra of four gas-phase PAHs, naphthalene, phenanthrene, pyrene, fluoranthene and their mixtures were experimentally investigated at 673 K and 1073 K. What is more, the LIF spectrum measured in a laminar premixed ethylene flame was discussed with the assistance of experimental results of gas-phase PAHs. The following conclusions can be remarked.
The integral fluorescence intensities of these gas-phase PAHs were in direct proportion to the PAH mole fractions at both 673 K and 1073 K, and the proportionality coefficients of different PAHs were obtained. In addition, the relationship between the fluorescence intensity and mole fraction would be influenced by temperature, and this needs to be considered for analysis of LIF spectra in flames. The superposed LIF spectra were able to well fit the measured LIF spectra of PAH mixtures, which confirmed the linear superposition principle. The superposition coefficients could be fitted from the LIF spectra of the mixture using the multiple linear regression method. In the next step, the mole fractions of each component could be calculated in combination with the relationship of integral fluorescence intensities and PAH concentrations. Using this analytic method, the mole fractions of PAH component in mixtures could be obtained and most of the relative errors were less than 5%.
Based on the experimental results of gas-phase PAHs, the LIF spectra of flame-formed PAHs in a laminar premixed ethylene flame were further analyzed. The results showed that the flame-formed PAHs emitted a broadband LIF spectrum mainly in the visible range and centered around 500 nm. The fluorescence signals shorter than 450 nm are mainly emitted from the gas-phase lighter PAHs. Then, the relative ratios of naphthalene, phenanthrene and pyrene can be fitted from the LIF spectrum measured in the flame, according to the multiple linear regression method and the temperature effects on PAH photophysical properties. On the other hand, fluorescence signals in flames longer than 450 nm are possibly emitted from the PAHs with five-membered-ring structures or the unidentified heavier part of condensed species, but this is still ambiguous.
In future works, a more complete database of LIF spectra is necessary to be established, which includes large molecular PAHs, PAHs with branched chains and PAHs with five-membered-ring structures. What is more, the influences of nanoparticles or soot particles on the PAH LIF spectra need to be considered because the nanoparticles or soot particles are also fluorescent in the visible range. To verify LIF measurement for PAH mole fractions, the ex situ LIF and GC–MS are the effective methods to provide the quantitative information of PAHs in flames.
References
H. Wang, Formation of nascent soot and other condensed-phase materials in flames. Proc. Combust. Inst. 33, 41–67 (2011)
A. D’Anna, Combustion-formed nanoparticles. Proc. Combust. Inst. 32, 593–613 (2009)
C.S. Mcenally, L.D. Pfefferle, B. Atakan, K. Kohse-Höinghaus, Studies of aromatic hydrocarbon formation mechanisms in flames: progress towards closing the fuel gap. Prog. Energy Combust. Sci. 32, 247–294 (2006)
C.L. Friedman, Y. Zhang, N.E. Selin, Climate change and emissions impacts on atmospheric PAH transport to the Arctic. Environ. Sci. Technol. 48, 429 (2014)
G. Jia, M. Yao, H. Liu, P. Zhang, B. Chen, L. Wei, PAHs formation simulation in the premixed laminar flames of TRF with alcohol addition using a semi-detailed combustion mechanism. Fuel 155, 44–54 (2015)
F. Bisetti, G. Blanquart, M.E. Mueller, H. Pitsch, On the formation and early evolution of soot in turbulent nonpremixed flames. Combust. Flame 159, 317–335 (2012)
Y. Wang, A. Raj, S.H. Chung, Soot modeling of counterflow diffusion flames of ethylene-based binary mixture fuels. Combust. Flame 162, 586–596 (2015)
P. Liu, H. Lin, Y. Yang, C. Shao, C. Gu, Z. Huang, New insights into thermal decomposition of polycyclic aromatic hydrocarbon oxyradicals. J. Phys. Chem. A 118, 11337–11345 (2014)
L. Peng, L. He, Z. He, Y. Zhang, B. Guan, H. Zhen, The mechanism and kinetic analysis of C4H4 +C4H4 (but-1-ene-3-yne) reaction with features of H-transfer in combustion. Int. J. Hydrog. Energy 41, 3249–3258 (2016)
P. Liu, H. Lin, Y. Yang, C. Shao, B. Guan, Z. Huang, Investigating the role of CH2 radicals in the HACA mechanism. J. Phys. Chem. A 119, 3261–3268 (2015)
F. Ossler, T. Metz, M. Aldén, Picosecond laser-induced fluorescence from gas-phase polycyclic aromatic hydrocarbons at elevated temperatures. II. Flame-seeding measurements. Appl. Phys. B 72, 479–489 (2001)
P. Liu, Y. Zhang, Z. Li, A. Bennett, H. Lin, S.M. Sarathy, W.L. Roberts, Computational study of polycyclic aromatic hydrocarbons growth by vinylacetylene addition. Combust. Flame 202, 276–291 (2019)
P. Desgroux, X. Mercier, K.A. Thomson, Study of the formation of soot and its precursors in flames using optical diagnostics. Proc. Combust. Inst. 34, 1713–1738 (2013)
Y. Zhang, L. Wang, P. Liu, B. Guan, H. Ni, Z. Huang, H. Lin, Experimental and kinetic study of the effects of CO2 and H2O addition on PAH formation in laminar premixed C2H4/O2/Ar flames. Combust. Flame 192, 439–451 (2018)
P. Liu, Y. Zhang, L. Wang, B. Tian, B. Guan, D. Han, Z. Huang, H. Lin, The chemical mechanism of exhaust gas recirculation on polycyclic aromatic hydrocarbons formation based on LIF measurement. Energy Fuels 32(6), 7112–7124 (2018)
M. Sirignano, A. Collina, M. Commodo, P. Minutolo, A. D’Anna, Detection of aromatic hydrocarbons and incipient particles in an opposed-flow flame of ethylene by spectral and time-resolved laser induced emission spectroscopy. Combust. Flame 159, 1663–1669 (2012)
S. Bejaoui, X. Mercier, P. Desgroux, E. Therssen, Laser induced fluorescence spectroscopy of aromatic species produced in atmospheric sooting flames using UV and visible excitation wavelengths. Combust. Flame 161, 2479–2491 (2014)
H. Liu, P. Zhang, X. Liu, B. Chen, C. Geng, B. Li, H. Wang, Z. Li, M. Yao, Laser diagnostics and chemical kinetic analysis of PAHs and soot in co-flow partially premixed flames using diesel surrogate and oxygenated additives of n-butanol and DMF. Combust. Flame 188, 129–141 (2018)
B. Chen, X. Liu, H. Liu, H. Wang, D.C. Kyritsis, M. Yao, Soot reduction effects of the addition of four butanol isomers on partially premixed flames of diesel surrogates. Combust. Flame 177, 123–136 (2017)
P. Liu, Z. He, G.L. Hou, B. Guan, H. Lin, Z. Huang, The diagnostics of laser induced fluorescence (LIF) spectra of PAHs in flame with TD-DFT: special focus on 5-membered ring. J. Phys. Chem. A 119(52), 13009–13017 (2015)
S.S. Yoon, D.H. Ahn, S.H. Chung, Corrigendum to “synergistic effect of mixing dimethyl ether with methane, ethane, propane, and ethylene fuels on polycyclic aromatic hydrocarbon and soot formation” [Combust. Flame 154 (2008) 368–377]. Combust. Flame 154, 368–377 (2008)
Y. Kobayashi, T. Furuhata, K. Amagai, M. Arai, Soot precursor measurements in benzene and hexane diffusion flames. Combust. Flame 154, 346–355 (2008)
B.C. Choi, S.K. Choi, S.H. Chung, Soot formation characteristics of gasoline surrogate fuels in counterflow diffusion flames. Proc. Combust. Inst. 33, 609–616 (2011)
S.K. Choi, B.C. Choi, S.M. Lee, J.H. Choi, The effect of liquid fuel doping on PAH and soot formation in counterflow ethylene diffusion flames. Exp. Therm. Fluid Sci. 60, 123–131 (2015)
L.R. Allain, D.N. Stratis, B.M. Cullum, J. Mobley, M.R. Hajaligol, T. Vo-Dinh, Real-time detection of PAH mixtures in the vapor phase at high temperatures ☆. J. Anal. Appl. Pyrol. 66, 145–154 (2003)
R. Sun, N. Zobel, Y. Neubauer, C.C. Chavez, F. Behrendt, Analysis of gas-phase polycyclic aromatic hydrocarbon mixtures by laser-induced fluorescence. Opt. Lasers Eng. 48, 1231–1237 (2010)
Y. Zhang, L. Wang, P. Liu, Y. Li, R. Zhan, Z. Huang, H. Lin, Measurement and extrapolation modeling of PAH laser-induced fluorescence spectra at elevated temperatures. Appl. Phys. B 125, 6 (2019)
Y. Wang, A. Raj, S.H. Chung, A PAH growth mechanism and synergistic effect on PAH formation in counterflow diffusion flames. Combust. Flame 160, 1667–1676 (2013)
A. Bhargava, P.R. Westmoreland, Measured flame structure and kinetics in a fuel-rich ethylene flame. Combust. Flame 113, 333–347 (1998)
N. Olten, S. Senkan, Formation of polycyclic aromatic hydrocarbons in an atmospheric pressure ethylene diffusion flame. Combust. Flame 118, 500–507 (1999)
A.M. Vincitore, S.M. Senkan, Polycyclic aromatic hydrocarbon formation in opposed flow diffusion flames of ethane. Combust. Flame 114, 259–266 (1998)
C. Schulz, V. Sick, Tracer-LIF diagnostics: quantitative measurement of fuel concentration, temperature and fuel/air ratio in practical combustion systems. Prog. Energy Combust. Sci. 31, 75–121 (2005)
S. Bejaoui, R. Lemaire, E. Therssen, Analysis of laser-induced fluorescence spectra obtained in spray flames of diesel and rapeseed methyl ester using the multiple-excitation wavelength laser-induced incandescence technique with IR, UV, and visible excitations. Combust. Sci. Technol. 187, 906–924 (2015)
M. Orain, P. Baranger, B. Rossow, F. Grisch, Fluorescence spectroscopy of naphthalene at high temperatures and pressures: implications for fuel-concentration measurements. Appl. Phys. B 102, 163–172 (2011)
M. Orain, P. Baranger, B. Rossow, F. Grisch, Fluorescence spectroscopy of 1,2,4-trimethylbenzene at high temperatures and pressures: application to temperature measurements. Appl. Phys. B 100, 945–952 (2010)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (91441129, 51210010) and the National Key R&D Program of China (2016YFC0208000).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Y., Li, Y., Wang, L. et al. Investigation on the LIF spectrum superposition of gas-phase PAH mixtures at elevated temperatures: potential for the analysis of PAH LIF spectra in sooting flames. Appl. Phys. B 125, 72 (2019). https://doi.org/10.1007/s00340-019-7185-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-019-7185-0