Abstract
A set of high-resolution absorption spectrometers based on TDLAS was used to determine the impact of combustion-relevant gases on the pressure shift and broadening of H2O, CO2, C2H2 and CH4 absorption lines in the near-infrared spectral region. In particular, self- and foreign-broadening coefficients induced by CO2, N2, O2, air, C2H2 and CH4 were measured. The absorption lines under investigation are suitable to measure the respective species in typical combustion environments via laser absorption spectroscopy. Additionally, species-dependent self- and foreign-induced pressure shift coefficients were measured and compared to the literature. The experiments were performed in two specifically designed absorption cells over a wide pressure range from 5 to 180 kPa. Different sources of uncertainty were identified and quantified to achieve relative measurement uncertainties of 0.7–1.5% for broadening coefficients and 0.6–1.6% for pressure shift coefficients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Approximately 80% of the global primary energy demand is satisfied by the combustion of fossil fuels. Even though the contribution of renewable energies to the power generation is on the rise, the combustion processes will continue to give an important contribution to the energy production [1]. In the automotive industry, despite the trend towards electrification of engines, internal combustion engines and engine–battery hybrids will continue to dominate the market over the coming decades [2]. To improve the efficiency of these processes and to reduce associated emissions of pollutants, detailed analysis of the underlying processes is crucial. Due to the high temperatures and pressures, low time scales and sensitivity to physical components within combustion processes, contact-free optical measurement techniques have proven versatile applicability and usefulness in the investigation and research [3,4,5,6]. Absorption spectroscopy, in particular tunable diode laser absorption spectroscopy (TDLAS), has been applied to study the gas temperature and species concentration in a multitude of applications [7,8,9,10,11,12], as it allows sample-free, rapid and quantitative measurements. A relatively low technical effort, as well as the ability of fiber-coupling make TDLAS well suited for large-scale applications and harsh industrial environments [12,13,14,15,16,17]. Goldenstein et al. [18] recently presented an excellent review of the technique and the application to combustion research. For TDLAS, the near-infrared is of special interest, as lasers in this spectral region are relatively low-cost, stable, spectrally narrow and can easily be fiber-coupled. On the other hand, the overtone transitions of the major combustion species in this region show a lower absorption line strength compared to the fundamentals in the mid-infrared. This is beneficial for measuring the major species (e.g., H2O, CO2, CH4) of large-scale applications under typical temperatures and species concentrations, as the absorption along the laser path should not exceed certain levels. For measuring the significantly lower concentrations of minor species (NO, NO2, CO), the mid-infrared is often preferable. Accurate spectroscopic line parameters, such as absorption line strength, line position, collision-induced pressure broadening coefficients and pressure shift coefficients are essential for a comprehensive description of absorption spectra. While the spectroscopic databases HITRAN [19] and HITEMP [20] provide relatively accurate data on position and line strength of major absorption lines, the pressure shift of the absorption line and the pressure broadening coefficients show large uncertainties. The databases are intended for environmental applications, and therefore, provide information on the broadening and shift caused by air as a foreign gas only. However, the gas composition of combustion processes, e.g., oxy-fuel processes [12, 21], can significantly differ from air. Furthermore, coefficients are often experimentally determined at low pressures in the range of kilopascal to avoid overlap of adjacent lines. For high-pressure applications such as internal combustion engines, a validation at higher pressures is necessary. Hence, a precise model description of the absorption lines cannot be given without additional data.
To close this gap, this work provides coefficients for the calculation of self- and foreign pressure broadening as well as pressure shift for selected absorption lines of major combustion species. This work focuses on absorption lines of the combustion products water vapor (H2O) and carbon dioxide (CO2), the fuel methane (CH4) and the soot precursor acetylene (C2H2), which are of high relevance for many applications [22, 23]. The absorption lines under investigation (summarized in Table 1) have already been utilized for TDLAS measurements within combustion systems in the literature, as lasers for the corresponding wavelengths are commercially available on stock. Two absorption cells with well-defined conditions have been designed to accurately measure the spectra over a broad pressure range (5–180 kPa). Whereas one cell was designed to measure the self-induced pressure broadening and shift, the other one was designed for accurate measurements of the foreign-induced broadening and shift. For the latter, the impact of CO2, N2, O2, C2H2, and CH4 as foreign gas—as well as air for a comparison—with the databases were measured, as all these gases are highly relevant in combustion environments.
In the following, the measurement technique and the spectral coefficients are introduced. Later, an uncertainty analysis followed by the results of the measurements is be provided. If possible, the results are compared to the literature values.
2 Experimental details
2.1 Spectroscopic principles and measurement method
When light of a certain frequency \(\nu\) propagates through a gas sample, a fraction of the light is absorbed due to resonant wavelength-dependent laser light losses by spectrally narrow absorption lines. These lines are generated by near-infrared vibrational–rotational transitions of the gas molecules, and are specific for each species. The absorbance \(\alpha (\nu ),\) which describes the logarithmic ratio of the incoming and attenuated light intensity \(~{I_0}\) and \(I,\) can be described by the Beer–Lambert relation [24]
Each transition \(~j\) of species \(i\) is described by a set of spectral parameters which can, if available, be taken from spectroscopic databases, such as HITRAN2016 [19] and HITEMP2010 [20], or from the specific literature on the chosen transition. The temperature-dependent line strength \({S_{i,j}}(T)\) at the center frequency \({\nu _{i,j}}\) is dependent on the transition probability between the corresponding energy levels. An increasing number density \({n_i}\) of species i leads to a stronger absorption. L is the absorption length within the gas sample. The shape of the absorption line is described by the area-normalized line-shape function \(\phi ,\) which provides a distribution of the absorbance around the center frequency \(~{\nu _{i,j}}.\) Due to elastic collisions among the molecules, the energy levels and thus the center frequency can be shifted. This effect is known as pressure shift and can be described by
with the vacuum transition frequency \({\nu _{0,i,j}},\) the self-induced pressure shift coefficient \({\delta _{\text{s}}},\) the pressure shift induced by foreign species \({\delta _{\text{f}}}\) and the partial pressures of the target and foreign species \({p_{\text{s}}}\) and \({p_{\text{f}}},\) respectively. While the vacuum frequency is usually well-known and the partial pressures is measured or estimated, the pressure shift coefficients, in particular of foreign gases, often have unsatisfactory uncertainties or are not available at all. The absorption lines are broadened by various effects: The natural line broadening, which originates from the uncertainty of the upper energy level, is too small to play a relevant role in technical applications. In contrast, the temperature-dependent Doppler broadening, which is a consequence of the thermal movement of the molecules, does have a significant impact. However, the width of the Doppler broadening \(\Delta {\upsilon _{\text{D}}}\) can be precisely calculated by \(\Delta {\upsilon _{\text{D}}}=\frac{{{\nu _{0,i,j}}}}{{2c}}~\sqrt {2~k~T\ln 2/m} ,\) with the speed of light c and Boltzmann’s constant k, the temperature of the gas sample T and the molecule mass m. The collisional broadening \(\Delta {\upsilon _{\text{C}}}\) originates from collisions with foreign molecules or those of the same species. It can be described by
The temperature coefficients of foreign gases \({n_{\text{f}}}\) and the target gas \({n_{\text{s}}}\) describe the influence of temperature and are usually available in the literature for air, or can be approximated. The pressure broadening coefficients \({\gamma _{\text{f}}}\) and \({\gamma _{\text{s}}},\) however, are often not available or need experimental validation. While the natural line broadening and the collisional broadening are modeled in a Lorentzian line profile, the Doppler broadening causes a profile which can be modeled as Gaussian. For technical applications, the two profiles have a width in the same order of magnitude, hence a convoluted Voigt profile [25] needs to be applied.
TDLAS utilizes infrared diode lasers with a narrow bandwidth to accurately resolve the absorption spectra by tuning the wavelength across the absorption lines and comparing the intensity of incoming and attenuated light. From Eq. 1, with the ideal gas law and integration over the spectral range, the gas concentration \({c_i}\) of species i can be calculated [15] via
while the coefficient \(E\) takes the influence of background emission from, e.g., hot walls into account, Tr describes the changes in transmissivity through broadband absorption. The laser tuning coefficient \(\partial \nu /\partial t\) is a characteristic parameter of the diode laser and can be measured via self-interference patterns (fringes) from a Fabry–Pérot Etalon [26].
2.2 Line selection
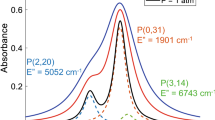
In the design of a TDLAS spectrometer, the expected species concentrations, pressures and temperatures in the process, the possible path length and desired detection limits have to be considered. Plenty of literature is available on the selection of optimal absorption lines [13, 15, 27,28,29,30,31], depending on the accuracy of the line data and the availability of corresponding lasers. For the target species H2O, the absorption bands ν1 + ν3 and ν2 + ν3 in the near-infrared around 1400 nm (7143 cm−1) and 1850 nm (5405 cm−1), respectively, have gained a lot of interest [8, 9, 30, 32, 33]. These absorption lines are relatively strong, well separated from each other and absorption lines of foreign species and have widely different temperature dependences of the line strength, due to a partial overlap with hot-bands (Fig. 1). This characteristic makes this region also interesting for temperature measurements [14, 30]. In particular, the 202 ⟵ 303 line at 7181.16 cm−1 and the 313 ⟵ 212 at 5393.65 cm−1 are very promising for water vapor measurements [8, 9, 33]. The same considerations hold for the ν1 + 2ν20 + ν3 band of CO2 around 2000 nm (5000 cm1), which has already been used for CO2 detection [14]. In this band, the R(18) line at 4991.26 cm−1 is of interest [34]. CH4 can easily be measured in the 2ν3 band at 1650 nm (6060 cm−1), where the R(3) line-triplet has been used for methane detection [31, 33, 34]. Hence, major suppliers of distributed-feedback (DFB), discrete-mode (DM) and vertical-cavity surface emitting diode lasers (VCSEL) offer sources specifically designed for operation around these absorption lines. C2H2 has rarely been measured in the near-infrared, as the line strengths are significantly lower than in the mid-infrared fundamental bands. However, the more complex experimental apparatus and a complicated fiber-coupling in the mid-infrared make the ν1 + ν3 band around 1540 nm (6494 cm−1) a more practical region for field measurements of acetylene [35]. The P(23) line at 6495.91 cm−1 is of interest [34] for combustion diagnostics, as it is sufficiently separated from H2O hot-bands.
The line parameters of the 202 ⟵ 303 water vapor line at 7181.16 cm−1 have been subject to versatile investigations. Due to the experiments of Durry et al. [36] and others [32, 37] using TDLAS and Fourier-transform infrared spectroscopy (FTIR), the absorption line strength is well-known. The foreign-pressure broadening coefficients, however, were measured for O2, N2 and air only [36, 38,39,40]. Experimental data on the pressure shift is only available for air [40]. For the 313 ⟵ 212 absorption line at 5393.65 cm−1, less line data is available. While the line strength was calculated [41] and measured using FTIR-spectroscopy [42, 43], and the self-induced pressure broadening experimentally measured using the same technique [43], no experimental data is available on foreign pressure broadening and pressure shift coefficients.
Various publications provide information on the line strength of the R(18) line of CO2 at 4991.26 cm−1 [44,45,46,47], but the self and O2-, N2- and air-induced pressure broadening coefficients have only been calculated [48, 49]. Régalia-Jarlo et al. [45] measured the self-induced pressure broadening using a FTIR-spectrometer. Similar to the H2O lines, no experimental data is available on the pressure shift. El Hachtouki and Auwera [50] measured and calculated the line strength of the P(23) line of C2H2. Self-, CO2-, N2-, air-, and O2-induced pressure shifts in combination with the corresponding pressure broadening coefficients were measured by Arteaga et al. [51] and Minutolo et al. [52]. However, the experiments were performed at relatively low pressures, and therefore need validation at higher pressures. The absorption line strengths of the R(3)-triplet of CH4 were measured precisely by Gharavi and Buckley [53] and others [54,55,56] using TDLAS, both for each line separately as well as for the combination. Additionally, pressure broadening coefficients by CO, N2, O2, H2O, CO2, air and CH4 itself were measured, as well as the pressure shifts by air and N2. Hence, these coefficients can be used for a comparison with the values determined in this work. However, the pressure broadening coefficients and in particular the shift in other gases are still highly absent.
2.3 Experimental setup and procedure
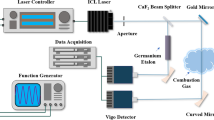
The experiments were conducted in two separate absorption cells. The SB cell is specifically designed to measure the self-induced pressure broadening and shift (Fig. 2). In pure target gas, the absorption is high, hence the cell is relatively short. Laser light is guided into the cell through a fiber, collimated inside and propagates 20 mm through the cell onto a photodiode. The second cell is designed to measure the foreign-induced pressure broadening and shift. For each experiment, the target gas is mixed with a single foreign gas. The second cell is designed to measure the foreign-induced pressure broadening and shift. For each experiment, the target gas is mixed with a single foreign gas in predominant concentration (typically 98–99.5%Vol.), leading to a negligible self-broadening compared to the foreign-broadening. Hence, the absorption of the target gas is very low. To account for this effect, the mixed gas cell is significantly longer than the pure gas cell. The laser light is fiber-coupled into the cell and divergently exiting the angled fiber tip. An adjustable off-axis parabolic mirror guides the light onto a detector, yielding an effective absorption length of 223 mm. For the fibers and detector wires, sealed feedthroughs were installed into the cell walls.
Before each measurement, the cells were evacuated to < 1 Pa using a HiPace turbomolecular pump in combination with a MVP-006-4 diaphragm pump (Pfeiffer Vacuum). The pressure was measured using a CMR 363 pressure sensor (Pfeiffer Vacuum, measurement uncertainty 0.2% of the measured value) at pressures < 1 kPa, a UNIK 5000 (GE, measurement uncertainty 0.07 kPa) for pressures between 1 and 35 kPa and a model 8262-5150 (Burster, measurement uncertainty 0.5 kPa) for higher pressures. The coefficients of all lines were determined at reference temperature (296 K) by stabilizing the cell temperature through air-conditioning. Multiple calibrated thermocouples (type K, 0.2 K measurement uncertainty) along the cells were used to measure the gas temperature and temperature homogeneity. The cells were filled with gas through a set of chambers and valves. Target and foreign gas were mixed within the cell and given a settling time to achieve a mixing homogenization and thermal equilibrium with the walls. The target-to-foreign gas ratio was adjusted by injecting the target gas up to a desired partial pressure and subsequently filling with foreign gas to the desired total pressure. The turbulence inside the cell introduced by this procedure ensures that the gases are well mixed. The target concentrations were double-checked using the TDLAS measurements. For water vapor as target gas, the foreign gas was moisturized using a bubble-seeder. The saturation pressure of water at ambient temperature (~ 3.5 kPa) impedes to use water vapor as foreign gas.
The laser light was provided by five diode lasers. To measure the coefficients of water vapor, a 1392 nm VCSEL (Vertilas) and a 1845 nm DM laser (Eblana Photonics) were used. The C2H2, CH4 and CO2 absorption lines were investigated using DFB lasers (NEL and nanoplus). The modulation rate of all lasers was set to 39 Hz to achieve a high spectral resolution in the range of 10−3 cm−1, allowing to sufficiently resolve the absorption lines in both the low (5 kPa) and high pressure range (180 kPa). Further information on the laser modulation scheme, the data acquisition setup and amplification as well as the data evaluation can be found in [33].
3 Results and discussion
3.1 Integration time and absorption line profiles
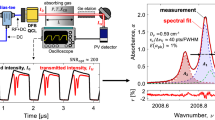
To determine broadening and shift coefficients, the absorption line profiles were measured at various pressures between 5 and 180 kPa. At each pressure, 1000 spectra were recorded and a model of the absorption line profile was fitted to the data. Within this model, the absorption line strength, the line center position and Lorentz-width of the absorption line were independently fitted. Due to measurement noise mainly from electrical white noise, each measured spectrum yields a slightly different position and width. Hence, averaging of the data is necessary. However, the averaging time should not exceed a certain number of spectra, as otherwise systematic are included. Here, the Allan–Werle variance, described by Werle et al. [57] to achieve a minimal detection limit by TDLAS spectrometry, was used to determine the optimal cluster size or integration time for averaging. Figure 3 shows the measured Allan–Werle variances of the five absorption lines for 50,000 consecutive spectra. During the measurements, the lines were broadened by N2 as foreign gas. The total pressure for the measurements was ~ 80 kPa and the concentration of the target species < 2%. Figure 3a shows the variance of collisional broadening, which is initially decreasing due to the elimination of white noise. For cluster sizes > 300 (integration time > 8 s) the variance is increasing, roughly independent of the spectrometer. Here, minor drifts come into play, probably a temperature or pressure drift within the cell, as the drift is roughly independent of the spectrometer used. Therefore, for the determination of broadening coefficients, 300 absorption profiles were averaged. The Allan–Werle variance of the line position, however, is dependent on the spectrometer. Figure 3b shows that a drift occurs significantly earlier for the 313 ⟵ 212 H2O spectrometer than for that of CO2. As a drift in the measured line position is effected by drifts in the diode laser temperature, these drifts may stem from fluctuations in the laser cooling. In fact, the DM laser in the 313 ⟵ 212 H2O spectrometer is the only one exhibiting an early drift, whereas the other spectrometers, which rely on DFB and VCSEL laser technology, drift at lower frequencies. For the DM laser, the variance is minimal for a cluster size of 20 (integration time 0.5 s), whereas it is minimal for a cluster size of 100 for the other spectrometers. Hence, to determine the shift coefficients, the integration times were chosen accordingly.
Figure 4 shows measured absorption line profiles for various pressures. In Fig. 4a, the foreign broadening of 313 ⟵ 212 H2O and adjacent absorption lines by N2 as foreign gas are shown for pressure levels between 5 and 180 kPa. With increasing pressure, the lines are spectrally broadened and begin to overlap. To separate the influence of the adjacent lines, the whole spectrum needs to be fitted. The residual shows the difference between the measured spectrum at 180 kPa and the model. Due to the comparably low variance of 4.2 × 10−4 and the high peak line strength of 0.23, the signal-to-noise ratio (SNR = αPeak/σResidual) lies in the range of 570, which is typical for this spectrum and results in a low fit uncertainty. Here, the residual is dominated by line profile deviations due to the complex structure of the spectrum, where some line positions and broadenings of the background are not known perfectly.
Figure 4b shows the absorption profile of the C2H2 P(23) line under self-broadening with pressure levels between 10 and 160 kPa. Here, the spectrum is dominated by one isolated absorption line and is, therefore, significantly less complex. Besides the broadening of the line, a small shift of the line peak towards lower wavelengths with increasing pressure is evident, which is the pressure shift. Due to the simple profile, the model and the experimental data agree better and the SNR exceeds 1000. Only minor deviations are evident, the residual is dominated by electrical noise, probably from the detector and amplifier. Due to the high SNR in both examples it can be concluded that a very good agreement between model and the experimental data is achieved and the uncertainties corresponding to errors in the profile are low.
3.2 Error analysis
Multiple sources of error affect the uncertainty of the broadening and shift coefficients, such as errors in pressure and temperature, purity of the gases, quality of the absorption profile fit, uncertainty of the laser tuning and the linear regression. The uncertainty in absorption path length, which is an important factor for many TDLAS sensors, does not influence the uncertainty of the broadening and shift coefficients, as both the line position and collisional width are independent of the absorption path length. Only the measurement of the target gas partial pressure using TDLAS is influenced by the path length. However, as the uncertainty of the length is small compared to the gas concentration uncertainty due to impurities of the gas, this effect is neglected.
While the relative error of the pressure stems from the pressure sensor uncertainty and is of the order of 0.5%rel., a temperature error of 2 K originates from temperature fluctuations within the cell and temperature inhomogeneities along the cell rather from the thermocouple uncertainty or the deviation of the mean cell temperature from the reference temperature. The laser tuning coefficient \(\partial \nu /\partial t\) is responsible for the accuracy of the spectral position and thus influences the broadening and shift coefficient. It is measured using a Fabry–Pérot Etalon [26]. The uncertainty of this factor is dominated by the uncertainty of the optical path length in the Etalon. Remaining impurities of the target and foreign gases affect both the broadening and shift coefficients. The purity of the gases ranges from 99.5% for CH4, O2, air and water vapor to 99.9999% for N2. While the uncertainty of the spectral fit through the SNR is described in the previous section, the error in the regression of the measured coefficients with pressure (Δm) will be discussed in the next section.
Figure 5 summarizes the relative contribution of the different sources of error to the uncertainty of all broadening and shift coefficients. The foreign broadening uncertainty of the H2O lines is dominated by the uncertainty of the pressure and temperature, due to a high fit quality of the spectra and linearity of the results. However, for some foreign broadening coefficients, the purity of the foreign gases plays a role. It was not possible to determine the C2H2 and CH4 broadening coefficients of the 202 ⟵ 303 water vapor absorption line, as minor absorption bands of the foreign species overlap with this absorption line. Here, the different spectra could not be isolated sufficiently due to a low quality of the available spectral data for the foreign species. For the foreign broadening of the CO2, C2H2 and CH4 absorption lines, the uncertainty of the fit plays a more important role, due to some remaining electrical noise. The impurities in the C2H2 and CH4 gas are relevant for the self-broadening of the respective lines but not for CO2, as this gas has a significantly higher purity. The foreign broadening and shift coefficients of C2H2 and CH4 in O2 and air were not measured due to an explosion hazard. For all broadening coefficients, the linearity of the line width with pressure is very high; therefore, the error in the regression is insignificant. This is different for the pressure shift coefficients (Fig. 5b). In particular, for the 313 ⟵ 212 water vapor absorption line and the self-broadening of C2H2 and CO2, the linearity is reduced, as can be seen in the next section. This reduction probably originates from the large width of the respective lines: As the lines are broad at high pressures, the central position cannot be calculated very well. Therefore, for these lines, a systematic error occurs which increases the uncertainty of the linear slope and hence the shift coefficient. However, the overall uncertainty remains small, because the pressure shift is insensitive to the temperature and thus the error is reduced. Similar to the pressure broadening coefficients, the uncertainty of the fit plays an important role in the total error of the CO2, C2H2 and CH4 absorption line pressure shift due to electrical noise in the spectra. In addition, the errors from the pressure sensors are significant for all shift coefficients. As for all coefficients multiple sources of uncertainty are important, the error cannot be significantly reduced by improving solely one factor. Hence, a further reduction of the uncertainty is complex. The uncertainties account for total errors in the range of 0.7–1.5%rel. for the pressure broadening coefficients and 0.6–1.6%rel. for the drift coefficients (Table 2).
3.3 Self- and foreign pressure broadening
Figure 6 exemplarily shows the Lorentz half-width of the R(18) CO2 absorption line for various pressures and collisional partners. For the broadening by foreign species, the self-broadening was subtracted to reduce systematic errors, even though the concentration and thus the broadening of the target species was low. Additionally, the partial pressure of the target gas was subtracted from the overall pressure. For these calculations, the target concentration measured by TDLAS was used. The fit was independent of the zero pressure/zero broadening point to check for offsets in the pressure sensors, which were below 0.8 kPa. This offset contributes to the pressure uncertainty by 0.4–4%. However, as the determination of the broadening coefficient depends on the slope only, this uncertainty is not included into the overall uncertainty analysis. For the R(18) CO2 absorption line, the Lorentz half-width is linearly increasing with increasing pressure. However, the slope is species dependent, as C2H2 is broadening the absorption with roughly twice the rate of O2. For a better comparison with the coefficient provided in HITRAN, the air-broadening coefficient is measured directly and not calculated from the N2 and O2 coefficients. The foreign broadening by water vapor is not measured due to the low saturation pressure at ambient temperature. The agreement between the measured data and a linear fit is very good with R2 values > 0.9999. Hence, the absolute uncertainty of the slope Δm, estimated by the standard deviation of the slope [58], is low. The self-broadening coefficient of the R(18) absorption line was determined to γself = (0.1011 ± 0.00073) cm−1/atm. The agreement with the value given in HITRAN2016 [19] (γself = (0.1 ± 0.002) cm−1/atm) is within the uncertainty. The experimentally measured coefficient by Régalia-Jarlo et al. [45] shows a deviation by − 5%, whereas the calculated coefficient by Rosenmann et al. [49] shows a deviation of + 5%. This makes the measurements prone to errors. Here, a significant improvement of the uncertainty was achieved compared to the literature. The measured air-broadening coefficient is consistent with the measured O2 and N2 broadenings, when taking the relative amounts of the gases in air into account. However, it slightly exceeds the uncertainty of the coefficient provided in HITRAN2016 (γAir = (0.0735 ± 0.0015) cm−1/atm) and Toth et al. [48]. The N2-broadening coefficient, slightly undercuts the error margins of the value provided by Rosenmann et al. [49], whereas the O2-coefficient is in perfect agreement with the value provided by the same publication. For the C2H2- and CH4-broadening coefficients, no literature was found.
Table 2 summarizes the pressure broadening coefficients for the five investigated absorption lines and provides the absolute and relative errors of the measurements and the R2 values of the linear fit. In addition, the available HITRAN2016 and most important literature values in combination with available uncertainties are given. As a general trend, the C2H2 broadening is always the strongest and the O2 broadening the weakest. In between, the order of the CO2, N2 and CH4 broadening coefficients depends on the target species. For the 202 ⟵ 303 absorption line of water vapor, low uncertainties of less than 1% are achieved. The influence of different foreign gases is similar to CO2 as target gas, with CO2 showing a strong broadening, whereas N2 and in particular O2 are weaker broadening partners. The air-broadening coefficient is again confirmed by the O2 and N2 coefficients and agrees well with the HITRAN2016 value and the ones given in [36, 39, 40]. However, the uncertainty is significantly reduced. The N2 and O2 coefficients agree well with the literature values, where unfortunately no uncertainties are provided. Similar to this H2O absorption line, measurements at the 313 ⟵ 212 line led to very low uncertainties. Here, only the HITRAN2016 [59] value for the air-broadening was found (γAir = (0.095 ± 0.005) cm−1/atm). The measured value agrees very well with this coefficient. For the other broadening coefficients of the 313 ⟵ 212 line, no literature values were found. The measured self-broadening of C2H2 agrees well with HITRAN2016 but has significantly lower uncertainties. In addition, the literature values for the CO2 and N2 broadening were reproduced. The absorption lines of the R(3) line-triplet of CH4 cannot be measured independently, as the lines are overlapping even for low pressure.
Hence, combined broadening coefficients and pressure shifts are measured. This is often done in the literature [53, 60], and can be motivated by the similarity of the three lines. The measured self-broadening is highly different from the HITRAN2016 coefficient. However, the measured value is still within the uncertainty of the HITRAN value, as the error of this quantity is very high. Compared to other literature values, the measured foreign-broadening is often outside the range of uncertainty, but the literature values disagree with each other as well. For example, the measured N2-broadening coefficient exceeds the value measured by Gharavi and Buckley [53] by 10%, but undercuts the value by Varansi et al. [60] by the same amount. For the C2H2 broadening of the CH4 line triplet, no literature values were found.
In general, the measured coefficients agree very well with the available literature values, but the uncertainties were often significantly reduced. In half of the cases, the coefficients were not known at all.
3.4 Self- and foreign pressure shift
Figure 7 exemplarily shows the relative line position (normalized with the origin of the linear fit) of the R(18) CO2 absorption line for various pressures and collisional partners. For measuring the pressure shift, in contrast to the line position, absolute knowledge of the laser frequency is not necessary. The absorption line linearly moves towards smaller wavenumbers with increasing pressure. However, the slope is dependent on the foreign species, as the absorption line moves nearly twice as far during collision with CH4 as with N2. The agreement between the measured line position and the linear fit is not as good as for the pressure broadening. The R2 values range from > 0.995 to > 0.99999 (Table 3) which, however, is still very high. Figure 7 shows that the residual is originating from two factors: scattering of the values around the mean and a systematic deviation from linearity. The scattering probably stems from the fitting of the absorption line to the spectral data. As the absorption lines, in particular for high pressures, are broad, the line center cannot be identified perfectly by the algorithm, as electrical noise on the detector signal leads to a reduced precision in line position. For the deviation from linearity, two reasons are possible: temperature fluctuations of the laser and errors from the fitting algorithm. If the laser temperature is not controlled perfectly, the central wavelength of the laser is slowly moving, and thus the absorption line seems to virtually move. As the temperature control is rather slow (~ min), this effect could lead to systematic deviation from linearity in consecutive measurement points. Additionally, in particular at high pressures, the laser baseline cannot be resolved perfectly due to the significantly broadened absorption lines in comparison with the laser tuning range. Then, an interaction between the fitting of the baseline and the line position may lead to small errors. One option to eliminate effects of temperature fluctuations is to perform a line-lock by splitting the laser into two beams, with the first one measuring within the cell under varying pressure and the second one measuring within a static absorption cell.
Then, slow drifts of the absorption line in the static cell are purely due to temperature fluctuations of the laser. These drifts could be removed by subtracting the laser-drift from the measurements using a line-lock, which was not done during the experiments and thus increases the overall uncertainty. However, the overall uncertainty of < 2% considers the drift with a fraction of 0.1–0.8%.
The literature values on the pressure shift are sparse, as this coefficient is usually too small to be resolved using FTIR spectrometers. Whereas HITRAN2016 provides the air-induced pressure shift of the R(18) CO2 line (δAir=-5.39) with a high uncertainty of 20%, Toth et al. [48] determined the value with a lower error of only 10%. The measured shift coefficient of δAir=-4.38 × 103 cm−1/atm is within the error margins of both values. It exceeds the coefficient by Toth et al. and undercuts the HITRAN value and has a significantly lower uncertainty of only 1%. For the other pressure shift coefficients of the R(18) absorption line of CO2 no literature values were found.
Table 3 lists the measured self- and foreign-induced pressure shifts of the five absorption lines and provides the absolute and relative errors of the measurements in combination with the R2 values of the linear fit. In addition, the available HITRAN2016 and most important literature values are given. The HITRAN2016 air-shift coefficient of the 202 ⟵ 303 absorption line of water vapor is relatively close to the measured value of δAir = − 13.43 × 10−3 cm−1/atm, considering the uncertainty of the HITRAN value (labeled: default). The measured value is very close to the one provided by Hunsman et al. [40] and has a significantly lower uncertainty of less than 1%. Similarly, to the 202 ⟵ 303 absorption line of water vapor, the air-shift of the 313 ⟵ 212 absorption line provided in HITRAN2016 δAir = − 2.99 × 103 cm−1/atm with unknown uncertainty is rather close to the measured value of δAir = − 3.41 × 103 cm−1/atm. The foreign-shift of the 202 ⟵ 303 absorption line is positive in some cases, which means the absorption line is moving towards larger wavenumbers with increasing pressure. The measured position of the 313 ⟵ 212 absorption line shows increased deviations from linearity, probably due to an increased temperature fluctuation of the laser central wavelength, as described above. Similarly to CO2, no information was found on the other measured pressure shift coefficients of H2O in the literature. Arteaga et al. [51] and Minutolo et al. [52] determined different pressure shift coefficients of C2H2. While the measured self- and N2-shifts are within the uncertainty of the values provided by Minutolo et al. and Arteaga et al., respectively, the latter is slightly outside the range of the coefficient determined by Minutolo et al. The CO2-shift measured by the same group is also far off the value measured in this work. For the R(3) line triplet of CH4, one combined pressure shift was determined. The N2-shift is in good agreement with the value provided by Dufour et al. [55].
Overall, it can be stated that the measured coefficients usually lie within the uncertainty of the available literature values, where available, as this uncertainty is usually very high. Here, the uncertainty was reduced and the majority of the shift coefficients were reported for the first time.
4 Summary
To measure the pressure broadening and shift of five absorption lines that are highly relevant for combustion measurements, two accurate absorption cells operating between 5 and 180 kPa were designed. The self- and foreign pressure broadening and individual shift coefficients of the absorption lines of the target species H2O, CO2, C2H2 and CH4, which are of high relevance for combustion processes, were measured separately within CO2, N2, O2, air, C2H2 and CH4 atmospheres. For the investigations, the cells were used in combination with five TDLAS-spectrometers operating in the near-infrared spectral region. First, an analysis of the different sources of error and their relative contribution to the uncertainty were given. The measured coefficients were compared to the literature values with a very good agreement in most cases. The uncertainty was either significantly improved, or the coefficient was determined experimentally for the first time. Relative uncertainties in the range of 0.7–1.5% for broadening coefficients and 0.6–1.6% for pressure shift coefficients were achieved. The results will be useful in the design and improvement of future TDLAS spectrometer for the investigation of combustion processes.
References
IEA, World energy outlook (2016). https://doi.org/10.1787/weo-2016-en (ISBN: 9789264264946)
H. Hao, Y. Geng, J. Sarkis, Energy 101, 121 (2016)
R.K. Hanson, Proc. Combust. Inst. 33, 1, (2011)
B. Peterson, E. Baum, B. Böhm, V. Sick, A. Dreizler, Proc. Combust. Inst. 35, 2923 (2015)
D. Zabrodiec, L. Becker, J. Hees, A. Maßmeyer, M. Habermehl, O. Hatzfeld, A. Dreizler, R. Kneer, Combust. Sci. Technol. 189, 1751 (2017)
E. Baum, B. Peterson, B. Böhm, A. Dreizler, Flow Turbul Combust 92, 269 (2014)
L.A. Kranendonk, X. An, A.W. Caswell, R.E. Herold, S.T. Sanders, R. Huber, J.G. Fujimoto, Y. Okura, Y. Urata, Opt. Express 15, 15115 (2007)
O. Witzel, A. Klein, C. Meffert, S. Wagner, S. Kaiser, C. Schulz, V. Ebert, Opt. Express 21, 19951 (2013)
S. Bürkle, L. Biondo, C.-P. Ding, R. Honza, V. Ebert, B. Böhm, S. Wagner, In-Cylinder Temperature Measurements in a Motored IC Engine using TDLAS. Flow Turbul. Combust. (2018). https://doi.org/10.1007/s10494-017-9886-y
M.E. Webber, J. Wang, S.T. Sanders, D.S. Baer, R.K. Hanson, Proc. Combust. Inst. 28, 407 (2000)
I.A. Schultz, C.S. Goldenstein, R. Mitchell Spearrin, J.B. Jeffries, R.K. Hanson, R.D. Rockwell, C.P. Goyne, J. Propuls. Power 30, 1595 (2014)
S. Balusamy, M.M. Kamal, S.M. Lowe, B. Tian, Y. Gao, S. Hochgreb, Exp. Fluids 56, 1 (2015)
A. Sepman, Y. Ögren, M. Gullberg, H. Wiinikka, Appl. Phys. B 122, 89 (2016)
K. Sun, R. Sur, X. Chao, J.B. Jeffries, R.K. Hanson, R.J. Pummill, K.J. Whitty, Proc. Combust. Inst. 34, 3593 (2013)
H. Teichert, T. Fernholz, V. Ebert, Appl. Opt. 42, 2043 (2003)
K.L. Mackay, A. Chanda, G. Mackay, J.T. Pisano, T.D. Durbin, K. Crabbe, T. Smith, J. Appl. Spectrosc. 83, 627 (2016)
S. Balusamy, A. Schmidt, S. Hochgreb, Exp. Fluids 54, 159 (2013)
C.S. Goldenstein, R.M. Spearrin, J.B. Jeffries, R.K. Hanson, Prog. Energy Combust. Sci. 60, 132 (2017)
I.E. Gordon, L.S. Rothman, C. Hill, R.V. Kochanov, Y. Tan, P.F. Bernath, M. Birk, V. Boudon, A. Campargue, K.V. Chance, B.J. Drouin, J.-M. Flaud, R.R. Gamache, J.T. Hodges, D. Jacquemart, V.I. Perevalov, A. Perrin, K.P. Shine, M.-A.H. Smith, J. Tennyson, G.C. Toon, H. Tran, V.G. Tyuterev, A. Barbe, A.G. Császár, V.M. Devi, T. Furtenbacher, J.J. Harrison, J.-M. Hartmann, A. Jolly, T.J. Johnson, T. Karman, I. Kleiner, A.A. Kyuberis, J. Loos, O.M. Lyulin, S.T. Massie, S.N. Mikhailenko, N. Moazzen-Ahmadi, H.S.P. Müller, O.V. Naumenko, A.V. Nikitin, O.L. Polyansky, M. Rey, M. Rotger, S.W. Sharpe, K. Sung, E. Starikova, S.A. Tashkun, J.V. Auwera, G. Wagner, J. Wilzewski, P. Wcisło, S. Yu, E.J. Zak, J. Quant. Spectrosc. Radiat. Transf. 203, 3 (2017)
L.S. Rothman, I.E. Gordon, R.J. Barber, H. Dothe, R.R. Gamache, A. Goldman, V.I. Perevalov, S.A. Tashkun, J. Tennyson, J. Quant. Spectrosc. Radiat. Transf. 111, 2139 (2010)
J. Hees, D. Zabrodiec, A. Massmeyer, S. Pielsticker, B. Gövert, M. Habermehl, O. Hatzfeld, R. Kneer, Combust. Flame 172, 289 (2016)
I. Glassman, Symp. (Int.) Combust. 22, 295 (1989)
P. Lindstedt, Symp. (Int.) Combust. 27, 269 (1998)
F. Stritzke, S. van der Kley, A. Feiling, A. Dreizler, S. Wagner, Opt. Express 25, 8180 (2017)
J. Humlíček, J. Quant. Spectrosc. Radiat. Transf. 21, 309 (1979)
A. Pogány, S. Wagner, O. Werhahn, V. Ebert, Appl. Spectrosc. 69, 257 (2015)
R.S.M. Chrystie, E.F. Nasir, A. Farooq, Appl. Phys. B 120, 317 (2015)
O. Diemel, J. Pareja, A. Dreizler, S. Wagner, Appl. Phys. B 123, 545 (2017)
U. KC, E.F. Nasir, A. Farooq, Appl. Phys. B 120, 223 (2015)
X. Zhou, J.B. Jeffries, R.K. Hanson, Appl. Phys. B 81, 711 (2005)
S. Schäfer, M. Mashni, J. Sneider, A. Miklós, P. Hess, H. Pitz, K.-U. Pleban, V. Ebert, Appl. Phys. B Lasers Opt. 66, 511 (1998)
A. Pogány, A. Klein, V. Ebert, J. Quant. Spectrosc. Radiat. Transf. 165, 108 (2015)
S. Bürkle, A. Dreizler, V. Ebert, S. Wagner, Fuel 212, 302 (2018)
S. Bürkle, L.G. Becker, A. Dreizler, S. Wagner, Experimental investigation of the flue gas thermochemical composition of an oxy-fuel swirl burner. Fuel 231, 61–72 (2018). https://doi.org/10.1016/j.fuel.2018.05.039
S. Wagner, B.T. Fisher, J.W. Fleming, V. Ebert, Proc. Combust. Inst. 32, 839 (2009)
G. Durry, V. Zeninari, B. Parvitte, T. Le barbu, F. Lefevre, J. Ovarlez, R.R. Gamache, J. Quant. Spectrosc. Radiat. Transf. 94, 387 (2005)
R.A. Toth, Appl. Opt. 33, 4851 (1994)
S. Moretti, Gianfrani, Ciurylo, J. Mol. Spectrosc. 205, 20 (2001)
O. Witzel, V. Ebert, In-situ-Laserabsorptionsspektroskopie zur µs-schnellen Bestimmung von Spezieskonzentrationen und Temperaturen in Verbrennungsmotoren (Darmstadt 2013)
S. Hunsmann, S. Wagner, H. Saathoff, O. Möhler, U. Schurath, V. Ebert, Messung der Temperaturabhängigkeit der Linienstärken und Druckverbreiterungskoeffizienten von H2O-Absorptionslinien im 1.4 μm-Band. VDI Berichte 1959, 149–164 (2006)
C. Camy-Peyret, J.M. Flaud, R.A. Toth, J. Mol. Spectrosc. 67, 117 (1977)
R.A. Toth, J. Quant. Spectrosc. Radiat. Transf. 94, 51 (2005)
I.V. Ptashnik, K.M. Smith, K.P. Shine, J. Mol. Spectrosc. 232, 186 (2005)
R.A. Toth, L.R. Brown, C.E. Miller, V.M. Devi, D.C. Benner, J. Mol. Spectrosc. 239, 221 (2006)
L. Régalia-Jarlot, V. Zéninari, B. Parvitte, A. Grossel, X. Thomas, P. von der Heyden, G. Durry, J. Quant. Spectrosc. Radiat. Transf. 101, 325 (2006)
G. Casa, D.A. Parretta, A. Castrillo, R. Wehr, L. Gianfrani, J. Chem. Phys. 127, 84311 (2007)
O.L. Polyansky, K. Bielska, M. Ghysels, L. Lodi, N.F. Zobov, J.T. Hodges, J. Tennyson, Phys. Rev. Lett. 114, 243001 (2015)
R.A. Toth, C.E. Miller, V. Malathy Devi, D.C. Benner, L.R. Brown, J. Mol. Spectrosc. 246, 133 (2007)
L. Rosenmann, J.M. Hartmann, M.Y. Perrin, J. Taine, Appl. Opt. 27, 3902 (1988)
R. El Hachtouki, J.V. Auwera, J. Mol. Spectrosc. 216, 355 (2002)
S.W. Arteaga, C.M. Bejger, J.L. Gerecke, J.L. Hardwick, Z.T. Martin, J. Mayo, E.A. McIlhattan, J.-M.F. Moreau, M.J. Pilkenton, M.J. Polston, B.T. Robertson, E.N. Wolf, J. Mol. Spectrosc. 243, 253 (2007)
P. Minutolo, C. Corsi, F. D’Amato, M. de Rosa, Eur. Phys. J. D 17, 175 (2001)
M. Gharavi, S.G. Buckley, J. Mol. Spectrosc. 229, 78 (2005)
V. Zéninari, B. Parvitte, D. Courtois, V.A. Kapitanov, Y.N. Ponomarev, Appl. Phys. B 72, 953 (2001)
G. Dufour, D. Hurtmans, A. Henry, A. Valentin, M. Lepère, J. Mol. Spectrosc. 221, 80 (2003)
A.V. Nikitin, O.M. Lyulin, S.N. Mikhailenko, V.I. Perevalov, N.N. Filippov, I.M. Grigoriev, I. Morino, T. Yokota, R. Kumazawa, T. Watanabe, J. Quant. Spectrosc. Radiat. Transf. 111, 2211 (2010)
P. Werle, R. Mücke, F. Slemr, Appl. Phys. B 57, 131 (1993)
W. Walcher, M. Elbel, Praktikum der Physik. Mit 88 Versuchen, 14 Tabellen im Text, einem Tabellenanhang und einem ausklappbaren Periodensystem der Elemente (Vieweg + Teubner, Wiesbaden, 2009)
L.S. Rothman, I.E. Gordon, Y. Babikov, A. Barbe, D. Chris Benner, P.F. Bernath, M. Birk, L. Bizzocchi, V. Boudon, L.R. Brown, A. Campargue, K. Chance, E.A. Cohen, L.H. Coudert, V.M. Devi, B.J. Drouin, A. Fayt, J.-M. Flaud, R.R. Gamache, J.J. Harrison, J.-M. Hartmann, C. Hill, J.T. Hodges, D. Jacquemart, A. Jolly, J. Lamouroux, R.J. Le Roy, G. Li, D.A. Long, O.M. Lyulin, C.J. Mackie, S.T. Massie, S. Mikhailenko, H.S.P. Müller, O.V. Naumenko, A.V. Nikitin, J. Orphal, V. Perevalov, A. Perrin, E.R. Polovtseva, C. Richard, M.A.H. Smith, E. Starikova, K. Sung, S. Tashkun, J. Tennyson, G.C. Toon, V.G. Tyuterev, G. Wagner, J. Quant. Spectrosc. Radiat. Transf. 130, 4 (2013)
P. Varanasi, J. Quant. Spectrosc. Radiat. Transf. 11, 1711 (1971)
Acknowledgements
The authors thank the Deutsche Forschungsgemeinschaft for its support through CRC/Transregio 150 “Turbulent, chemically reactive multi-phase flows near walls”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bürkle, S., Walter, N. & Wagner, S. Laser-based measurements of pressure broadening and pressure shift coefficients of combustion-relevant absorption lines in the near-infrared region. Appl. Phys. B 124, 121 (2018). https://doi.org/10.1007/s00340-018-6993-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-018-6993-y