Abstract
Confocal micro-Raman spectroscopy is employed to monitor the damage to haemoglobin from mid-ultraviolet (UVB) radiation. We obtained the Raman spectra of an erythrocyte, which indicated that a peroxidation reaction occurs after UVB radiation. Further, the surface enhanced Raman scattering (SERS) spectra of isolated haemoglobin show that the intensities of the 1375 and 1399 cm−1 bands, which are markers of haem aggregation, obviously increase with prolonged UVB irradiation. This increase reveals that haem aggregation occurs in the peroxidation of erythrocytes. The UV–Vis spectra of isolated haemoglobin indicate that the Soret band, which is indicative of excitonic interactions in the aggregated haems, has a redshift (~12 nm) after 30 min of UVB irradiation of erythrocytes. It can be deduced that an excitonic interaction occurs in the aggregated haems, which is caused by haemoglobin denaturation following UVB irradiation. In addition, the changes of the Raman marker bands during aggregation primarily originate from excitonic interactions. Throughout the process, a higher UVB radiation dose causes greater damage to haemoglobin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The ultraviolet (UV) region of solar radiation is a critical factor in the initiation and development of several tissue and blood diseases [1]. UV radiation damages not only the skin that is directly exposed to the sunlight but also “non-skin” tissues. With the depletion of ozone in recent years, more and more ultraviolet radiation has reached the ground. Early reports have indicated that, after UVB exposure, the concentrations of reactive oxygen species (ROS) (free radicals that contained the oxygen atom) and hydrogen peroxide (H2O2) could increase dramatically within cells, which would significantly affect cell structures [2]. More recently, the damage from UV irradiation of platelets [3], lymphocytes [4] and erythrocytes [5] has been studied. However, none of these studies investigated the damage done to haemoglobin, which is very important to erythrocytes. Erythrocyte plays a crucial role in the human body due to its ability to transport oxygen and carbon dioxide to different tissues. To perform such an important function, its intracellular haemoglobin should be properly oxygenated [6]. An important question that can be addressed is whether the haemoglobin experiences structural changes in the oxidative stresses of erythrocytes when they are subject to excessive UVB radiation.
Raman spectroscopy, an ideal tool for detecting the haem groups within single cell, has been used extensively to monitor the molecular dynamics of the conformational changes in haemoglobins. In contrast with other technologies [7,8,9], this investigative tool is non-destructive and free from water interference, providing molecular structure information for in vivo studies [10]. Reports have demonstrated the sensitivity of this technique in the detection of haem perturbations resulting from Fe displacements on the porphyrin plane [11, 12]. Further, Bayden R. Wood and Larissa Hammer found that the exposure of erythrocytes to high temperatures (>42 °C) or a prolonged laser treatment would result in a general increase in the signal-to-noise ratio as well as an enhancement of several bands [13]. In another study, the spectra of erythrocytes orientated either horizontally or vertically with respect to the plane of the Petri dish were recorded using both parallel and perpendicularly scattered light [14]. Moreover, surface enhanced Raman scattering (SERS), an effective method used to increase the Raman cross section of the small aromatic molecules, has also been used to detect isolated haemoglobins and get optimized measurements [15,16,17].
In this study, in order to investigate the damage to haemoglobin from UVB radiation, we built an oxidative stress erythrocytes model forced by UVB radiation. Confocal micro-Raman spectroscopy is employed to obtain the spectra of the erythrocytes. Then, in order to eliminate the impact of the erythrocytic membrane and directly investigate the damage done by UVB irradiation of haemoglobin, SERS is performed to find the spectra of the isolated haemoglobin solution, which is extracted from the corresponding irradiated erythrocyte specimens. We hypothesise that an excitonic interaction occurs in the aggregated haem, which is caused by haemoglobin denaturation after UVB radiation; UV–Vis spectroscopy is then employed to verify this hypothesis.
2 Materials and methods
2.1 Sample preparation
Fresh blood (5 mL) is obtained by venepuncture of healthy adults and is placed in glass tubes, which contain acid citrate dextrose, which serves as an anticoagulant. The blood is centrifuged (at 3000 rpm, 5 min, 4 °C) to produce a buffy coat. Then, the erythrocytes are found at the bottom of the tube and are washed with an isotonic phosphate-buffered saline (PBS) three times.
The erythrocytes are placed in a culture dish with a 3 cm diameter. The thickness of the erythrocyte specimens in the culture dish is approximately 2 mm, and the distance between the UVB lamp and specimen is adjusted to 16.3 cm, at which distance the UVB radiation dose rate is 8.27 × 10−4 W cm−2. The UVB lamp in this study was purchased from the PHILIPS Company and was a PL-S 9 W/12 type lamp with a voltage of 60 V. Different radiation dose rates are obtained by adjusting the distance between the specimen and UVB lamp or by prolonging the exposure time. The specimens are irradiated for 10, 20 and 30 min to make sure the UVB radiation doses reach 0.4962, 0.9924 and 1.4886 J cm−2, respectively. In the process of irradiating human skin, approximately 90% of the UVB light was absorbed by the skin, while 10% penetrated the skin and affected the erythrocytes into the subcutaneous tissue. In southern China, the average UVB radiation dose in the summer is approximately 11.484 J cm−2 from an exposure to sunlight of 1–2 h [18, 19]; as such, the UVB radiation dose in this study is selected as described above. The irradiated erythrocytes are divided into two groups: one is used for Raman detection, and the other is used for extracting the isolated haemoglobin.
The isolated haemoglobin in this study is prepared by adding the irradiated erythrocytes into double distilled water for a volume ratio of 1:1; the haemolytic erythrocytes are then centrifuged for 30 min (3000 rpm, 4 °C), which brings isolated haemoglobin to the top of the tube for SERS detecting. For all the experiments mentioned above, we obtained the approval of an ethics committee.
AgNPs are synthesized for SERS detection based on a previously published method [20]. In short, 0.53 mL of 0.1 M AgNO3 is added to double distilled water (50 mL) at 45 °C and is heated rapidly until it boils. Then, 1 mL of 1% sodium citrate dihydrate is injected while vigorously stirring, and the resulting solution is held at boiling for 45 min.
2.2 Spectroscopy measurements and data pre-processing
The Raman spectra in this study are recorded using a Renishaw system (inVia, New Mills, UK) with a spectrum resolution of 1 cm−1; this system is equipped with a 514.5 nm laser. The iron porphyrins in the haemoglobin have strong electronic transitions; using the aforementioned laser as the excitation source allowed a selective probing, which could avoid interference from other substances in the haemoglobin [21]. The spectra are collected in a back-scattered geometry using a Leica DM2500 microscope, which was produced in Germany. The laser beam is focused on the sample surface (~0.15 mW) with a 50 × objective (NA = 0.75). Each spectrum is collected with at a 3 s exposure time and using 5 accumulations, with a recording range from 500 to 1800 cm−1. To conduct the statistical analysis, five spectra are recorded at random in each erythrocyte. The peak frequencies are calibrated with silicon at 520 cm−1 [22]. All the data are collected under the same conditions.
The SERS spectra of the isolated haemoglobin are also collected with the same system mentioned above but with a 20 × objective (NA = 0.4). Before SERS scanning, each extracted isolated haemoglobin specimen is incubated with AgNPs for approximately 1 h to ensure an efficient adsorption. The volume ratio of [AgNPs]:[Hb] is 2:3. After incubating, the haemoglobin is placed in a glass capillary tube for the SERS experiment. The measurement parameters for the SERS are identical to the Raman spectra of erythrocytes.
To gain further information about the changes of the haemoglobin conformation, UV–Vis spectroscopy (NanoDrop, ND-1000) is also performed to detect the excitonic interactions of the isolated haemoglobin within the range of 500–700 nm.
The Raman and SERS spectra are baseline corrected using the software R 2.8.1, which is provided by Renishaw, and are smoothed, normalized and averaged using Origin Pro 8.0 (OriginLab Corporation, Northampton, MA, USA). The UV–Vis Spectra are baseline adjusted and vector normalized by ND-1000 spectroscopic software, which is provided by NanoDrop.
3 Results
3.1 Raman spectra of living erythrocytes
The erythrocytes are placed in a culture dish with a thickness of 2 mm for the Raman detection experiments. Figure 1 shows the averaged spectrum of the control and irradiated erythrocytes, along with the calculated standard deviation (SD) spectrum for the Raman peaks. Raman peak at 676 cm−1 (v 7) is used to normalize all the Raman spectra because its intensity remains the same in both oxygenated and deoxygenated states in living cells. The precise Raman bands identified based on the control erythrocytes are shown in Table 1 [23, 24].
Compared with those of the controlled erythrocytes, the spectra of the irradiated erythrocytes are significantly different. The Raman bands that were enhanced after irradiation include those at 1223, 1586 and 1639 cm−1, which are indicative of oxygenated erythrocytes, while the bands at 1210 and 1605 cm−1, which are indicative of deoxygenated erythrocytes, tended to decrease [21]. The bands at 1586, 1605 and 1639 cm−1 are associated with the C–C vibrations in the porphyrin skeleton; the bands at 1210 and 1223 cm−1 are associated with methane deformation modes. For erythrocytes, the dramatical enhancements of the 1639, 1586 and 1223 cm−1 bands are indicative of the appearance of oxygenated erythrocytes. The 1639 cm−1 band, which belongs to ν10, is especially linked to the O2 concentration marker in erythrocytes. In addition, the decreasing trends of the Raman peaks at 1210 and 1605 cm−1 are related to the decrease in deoxygenated erythrocytes [21, 23, 24].
3.2 SERS spectra of isolated haemoglobin
To eliminate the impact of the erythrocyte membrane and directly investigate the UVB radiation damage to the haemoglobin, the isolated haemoglobin was extracted from the corresponding irradiated erythrocytes specimens for high-quality SERS detecting. The SERS spectra of the control haemoglobin (CH) and irradiated haemoglobin (RH) are shown in Fig. 2, which indicates an extreme ordering of the haems within the cells. Figure 2 shows that there is an obvious enhancement of the Raman bands at 1375 (ν4), 1586 (v 37) and 1639 cm−1 (v 10) after UVB irradiation. However, the bands at 1358(ν4), 1538 (v 11) and 1604 cm−1 (v 19) are diminished with a prolonged radiation time. Meanwhile, there is a slight blueshift (~2 nm) in the RH spectra. It is important to note that the bands at 1538 and 1604 cm−1 are enhanced in deoxygenated haemoglobin, while the bands at 1586 and 1639 cm−1 are increased in oxygenated haemoglobin; these results are consistent with the previous studies [25]. However, the band at 1358 cm−1, which remains the same in the living cells in the previous reports [21], is dramatically reduced in our experiment. In addition to the obvious changes in these peaks, the Raman spectra intensity at 1399 cm−1 has also slightly increased.
4 Discussion
Raman and SERS provide precise “finger” spectra of the erythrocytes after UV radiation. In this section, we analyse the peroxidation reaction of the erythrocytes caused by UV radiation, quantitatively depict the aggregation state of the haem, and then discuss the reasons for these changes.
First, I1639 is a marker of oxy-erythrocyte and the increase in the intensity of this peak indicates a higher amount of oxygenated erythrocytes. Meanwhile, I 1605 is regarded as a marker of deoxygenated erythrocyte [13, 21]. I 1639/I 1605 is used for the quantitative analysis of the relative Raman intensity ratio between the controlled and irradiated erythrocytes, and the result is shown in Fig. 3. After 30 min of UVB radiation, the band at 1605 cm−1, which is a deoxygenation marker, disappeared. With prolonged UVB radiation exposure time, the ratio of I 1639/I 1605 is dramatically enhanced, which indicates that the erythrocytes have a peroxidation reaction after UVB irradiation and a higher UVB radiation dose causes a higher peroxidation level in erythrocytes. It also suggests that our radiation model was successful.
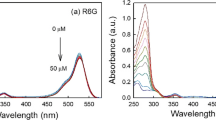
Second, we quantitatively depicted the aggregation state of the haem. The intensity change of the Raman band at 1399 cm−1 (v 20), which is one of the markers of haem aggregation, is shown in Fig. 4. In addition, as noted before, the other haem aggregation marker band, at 1375 cm−1, is obviously enhanced. We hypothesize that the enhancement of these two bands in the RH spectra results from the strong excitonic interactions in the aggregated haem moieties [13, 21], which are the result of haemoglobin denaturation after UVB irradiation. We also suggest that the bands at 1375 and 1399 cm−1 are markers of haem aggregation with prolonged UVB radiation exposure time. To test this hypothesis, UV–Vis spectroscopic analysis is performed on the CH and RH (30 min). Both the CH and RH UV–Vis spectra are shown in Fig. 5. The spectral changes in this figure tell us there is a redshift (~12 nm) of the Soret band (510–580 nm) in the RH spectrum. Such a shift had been considered as indicative of the excitonic interactions resulting from porphyrin aggregation, as has been interpreted in previous studies [21, 26].
We know that haemoglobin catalyses a variety of vital redox reactions in biological systems. This reaction largely depends on the iron centre, which can take on a variety of oxidation and spin states [27]. The structure of haemoglobin consists of a porphyrin macrocycle with an extended π conjugation. This porphyrin is surrounded by an iron atom, which is connected with four nitrogen atoms. In a deoxygenated state, the ferrous ion combines with a H2O molecule below the porphyrin plane. However, in an oxygenated state, O2 replaces the H2O molecule, and the ferrous ion transforms to ferric ion. In this study, the Raman spectra of a single erythrocyte clearly present the transition from a deoxygenated to oxygenated state [28]. Figure 3 suggests that a peroxidation reaction occurs in erythrocytes after UVB irradiation. ROS, H2O2 and hydroxyl radicals are produced during the peroxidation process. These oxidization products will attack the haemoglobin bonds; as a result, a denaturation reaction occurs in the haemoglobin. The intermolecular distances between the haem groups dramatically decrease because of the denatured haemoglobin, which facilitates energy migration in the form of electron transitions through the porphyrin network [13]. Previous studies have demonstrated that the aggregation of molecules enhances Raman scattering [29, 30]. We deduce that the enhancement of the 1375 and 1399 cm−1 bands, which are the haem aggregation markers, is primarily derived from the excitonic interactions in the haem groups. The band at 1358 cm−1 (v 4), which decreased, also confirms the electronic transition theory. The Raman intensity at ν4 is correlated with the number of electrons on the π* orbitals, which lie within the porphyrin ring. This is supported by the Weiss model [31]. In the process of an interaction between an oxygen molecule and ferrous ion, the former binds to a deoxygenated haemoglobin and is converted to superoxide (O2 −) [32]. The latter loses an electron and is converted to a low spin ferric ion. As to the theory of electron transition, our results could depict that the π orbital ferrous ion, which lies within the porphyrin ring, radiates electrons in a back-scattered geometry, and the number of electrons in the π* orbital increases, which caused changes at the 1358 cm−1 peak [25].
5 Conclusions
In this study, Micro-Raman spectroscopy provides a highly sensitive way to characterize the changes of erythrocytes and haemoglobin after UVB irradiation. Our results confirm that (I) a peroxidation reaction occurs in erythrocytes after UVB irradiation, which is marked by the increased Raman bands at 1639, 1586 and 1223 cm−1; (II) more and more high oxidation products are produced with the prolonging of the UVB irradiation time; haemoglobin is attacked and deformed by these products and as a result of haem aggregation, which can be indicated by the 1375 and 1399 cm−1 Raman bands. Meanwhile, a π–π* electron transition occurs during the process of haem aggregation, which causes a decrease of the 1358 cm−1 band. Finally, (III) during the whole process, increased UVB radiation doses cause greater damage to erythrocytes and haemoglobin. This experiment may be useful to help understand the basic mechanisms of haemoglobin alterations at a molecular level.
References
A.R. Svobodová, A. Galandáková, J. Šianská, D. Doležal, J. Ulrichová, J. Vostálová, Acute exposure to solar simulated ultraviolet radiation affects oxidative stress-related biomarkers in skin, liver and blood of hairless mice. Biol. Pharm. Bull. 34(4), 471–479 (2011)
E. Zachariah, A. Bankapur, C. Santhosh, M. Valiathan, D. Mathur, Probing oxidative stress in single erythrocytes with Raman Tweezers. J. Phtotchem. Photobiol. B. 100(3), 113–116 (2010)
P. Chen, Q. Tian, S.J. Baek, X.L. Shang, A. Park, Z.C. Liu, X.Q. Yao, J.Z. Wang, X.H. Wang, Y. Cheng, J. Peng, A.G. Shen, J.M. Hu, Laser Raman detection of platelet as a non-invasive approach for early and differential diagnosis of Alzheimer’s disease. Laser Phys. Lett. 8(7), 547–552 (2011)
C.W. Spellman, J.G. Woodward, R.A. Daynes, RA: Modification of immunological potential by ultraviolet radiation. I. Immune status of short-term UV-irradiated mice. Transplantation 24, 112–119 (1977)
M. Mulero, E. Rodríguez-Yanes, M.R. Nogués, M. Giralt, M. Romeu, S. González, J. Mallol, Polypodium leucotomos extract inhibits glutathione oxidation and prevents Langerhans cell depletion induced by UVB/UVA radiation in a hairless rat model. Exp. Dermatol. 17(8), 653–658 (2008)
Y. Wu, Y.X. Huang, L.L. Kang, Z.J. Wu, L. Man, Effect of pH on molecular constitution and distribution of hemoglobin in living erythrocyte. Biopolymers 93(4), 348–354 (2009)
S. Zhuo, J. Chen, S. Xie, L. Fan, L. Zheng, X. Zhu, X. Jiang, Monitoring dermal wound healing after mesenchymal stem cell transplantation using nonlinear optical microscopy. Tissue Eng. C. 16(5), 1107–1110 (2010)
S. Zhuo, J. Chen, G. Wu, S. Xie, L. Zheng, X. Jiang, X. Zhu, Quantitatively linking collagen alteration and epithelial tumor progression by second harmonic generation microscopy. Appl. Phys. Lett. 96(21), 213704 (2010)
Z.F. Zhuang, H.P. Liu, Z.Y. Guo, S.M. Zhuo, B.Y. Yu, X.Y. Deng, Second-harmonic generation as a DNA malignancy indicator of prostate glandular epithelial cells. Chin. Phys. B 19(4), 049501 (2010)
J. Lademann, P.J. Caspers, A. Van Der Pol, H. Richter, A. Patzelt, L. Zastrow, M. Darvin, W. Sterry, J.W. Fluhr, In vivo Raman spectroscopy detects increased epidermal antioxidative potential with topically applied carotenoids. Laser Phys. Lett. 6(1), 76–79 (2009)
T.G. Spiro, T.C. Streakas, Resonance Raman spectra of heme proteins. Effects of oxidation and spin state. J. Am. Chem. Soc. 96(2), 338–345 (1974)
H. Brunner, A. Mayer, H. Sussner, Resonance Raman scattering on the haem group of oxy- and deoxyhaemoglobin. J. Mol. Biol. 70, 153–156 (1972)
B.R. Wood, L. Hammer, L. Davis, D. McNaughton, Raman microspectroscopy and imaging provides insights into heme aggregation and denaturation within human erythrocytes. J. Biomed. Opt. 10(1), 014005 (2005)
B.R. Wood, L. Hammer, D. McNaughton, Resonance Raman spectroscopy of erythrocytes. Vib. Spectrosc. 38, 71–78 (2005)
W.Z. Yin, Z.Y. Guo, Z.F. Zhuanga, S.H. Liu, K. Xiong, S.J. Chen, Application of surface_enhanced Raman in skin cancer by Plasma1. Laser Phys. 22(5), 996–1001 (2012)
H.X. Xu, E.J. Bjerneld, M. Käll, L. Börjesson, Spectroscopy of single hemoglobin molecules by surface enhanced Raman scattering. Phys. Rev. Lett. 83(21), 4357–4360 (1999)
N.A. Brazhe, A.R. Brazhe, O.V. Sosnovtseva, S. Abdali, Novel chiroptical analysis of hemoglobin by surface enhanced resonance Raman optical activity spectroscopy. Chirality 21(1E), E307–312 (2009)
Y.F. Liao, W.Y. Wang, L. Zhang, L.S. Yang, Distribution of biologically effective solar ultraviolet radiation intensity on the ground in China. Geogr. Res. 26(4), 821–827 (2007)
L.N. Luo, X.P. Yang, H.Y. Zhang, Q.H. Li, H.Y. He, Discussion about ultraviolet and forecast method of its radiation intensity. Shanxi Meteorol. Q. 3(9), 12–14 (2001)
W. Ren, Y. Fang, E. Wang, A binary functional substrate for enrichment and ultrasensitive SERS spectroscopic detection of folic acid using graphene Oxide/Ag nanoparticle hybrids. ACS Nano 5(8), 6425–6433 (2011)
B.R. Wood, B. Tait, D. McNaughton, Micro-Raman characterization of the R to T state transition of haemoglobin within a single living erythrocyte. Biochem. Biophys. Acta 1539, 58–70 (2001)
Z. Zhuang, M. Zhu, Z. Guo, K. Xiong, N. Li, S. Chen, Study of molecule variation in various stages of human nuclear cataracts by micro-Raman Spectroscopy. Appl. Phys. Lett. 101(17), 173701 (2012)
B.R. Wood, D. McNaughton, Raman excitation wavelength investigation of single red blood cells in vivo. J. Raman Spectrosc. 33(7), 517–523 (2002)
B.R. Wood, P. Caspers, G.J. Puppels, S. Pandiancherri, D. McNaughton, Resonance Raman spectroscopy of red blood cells using near-infrared laser excitation. Anal. Bioanal. Chem. 387(5), 1691–1703 (2007)
Y.H. Kim, D.H. Jeong, D. Kim, S.C. Jeoung, H.S. Cho, S.K. Kim, N. Aratani, A. Osuka, ‘Photophysical properties of long rodlike meso-meso-linked Zinc (II) porphyrins investigated by time-resolved laser spectroscopic methods. J. Am. Chem. Soc. 123(1), 76–86 (2001)
A.A. Bhuiyan, J. Seth, N. Yoshida, A. Osuka, D.F. Bocian, ‘Resonance Raman characterisation of excitonically coupled meso, meso-linked porphyrin arrays. J. Phys. Chem. B 104(46), 10757–10764 (2000)
M. Casella, A. Lucotti, M. Tommasini, M. Bedoni, E. Forvi, F. Gramatica, G. Zerbibi, Raman and SERS recognition of β-carotene and haemoglobin fingerprints in human whole blood. Spectrochim. Acta Part A. 79(5), 915–919 (2011)
T.G. Spiro, Iron Porphyrins (Addison-Wesley, London, 1983)
D.L. Akins, ‘Theory of Raman scattering by aggregated molecules’. J. Phys. Chem. 90(8), 1530–1534 (1986)
D.L. Akins, J.W. Macklin, Dependence of Raman scattering by aggregated 2,2-cyanine on pH and excitation wavelength’. J. Phys. Chem. 93(16), 5999–6007 (1989)
J.J. Weiss, Nature of the iron-oxygen bond in oxyhaemoglobin. Nature 202(11), 83–84 (1964)
C. Balagopalakrishna, O.O. Abugo, P.T. Manoharan, J. Horsky, P.T. Manoharan, E. Nagababu, J.M. Rifkind, Superoxide produced in the heme pocket of the β-chain of hemoglobin reacts with the β-93 cysteine to produce a thiyl radical. Biochemistry 37(38), 13194–13202 (1998)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (nos. 11404116, 61335011, 61275187) and the Young Teachers Nurturing Fund of South China Normal University (2012KJ020) and the Horizontal Topic Research Project of SENSING.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, Y.Y., Li, N., Zhou, S.N. et al. Study of hemoglobin response to mid-ultraviolet (UVB) radiation using micro-Raman spectroscopy. Appl. Phys. B 123, 237 (2017). https://doi.org/10.1007/s00340-017-6800-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-017-6800-1