Abstract
Mid-infrared cavity ring-down spectroscopy (CRDS) using an external cavity, widely tunable pulsed quantum cascade laser operating at 3.8 μm, was employed for simultaneous detections of ethanol, ether and acetone in this paper. The experiments were performed with a maximum cavity mirror reflectivity of 99.915 % between the wave number 2614 and 2634 cm−1, leading to an effective optical path length of 588 m. The absorption spectra of ethanol, ether and acetone were measured with high spectral resolution in the range of 2614–2634 cm−1, and the spectroscopic analysis of the mixture of ethanol, ether and acetone with overlapping absorption spectra was demonstrated. The experimentally achieved detection limits (\(3\sigma\), or three times of standard deviation) for ethanol, ether and acetone were 157, 60 and 280 ppb, respectively. The simultaneously measured concentration results were in good agreement with the results with the standard gravimetric method, indicated that the mid-infrared CRDS has the potential for multi-component trace gas detection as well as for spectroscopic measurements of multi-broadband absorbers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ethanol, ether and acetone, three important volatile organic compounds (VOCs), play a significant role in chemical, pharmaceutical and gas industries, etc. [1, 2]. They are common solvents for waxes, fats, perfumes, alkaloids and gums, etc., and also common detergents typically for cleaning purposes in various laboratories. However, continuous exposure to these VOCs may lead to a wide variety of short-term and long-term adverse health effects. Due to their pungent smell and anesthetic effect, at sufficiently high concentrations, they produce irritation in the eyes and upper respiratory airways, even lead to unconsciousness and respiratory failure [3–5]. Besides, they are highly flammable and can form explosive mixture with air at room temperature. The standards for occupational exposure limits for hazardous agents in the workplace have been set in American conference of governmental industrial hygienists (ACGIH) [6], which are present in Table 1. Hence, the real-time monitoring is essential to reduce their impact on the health of people with exposure to these VOCs and to avoid explosive risks.
Many technical solutions have been developed in the past for the detections of ethanol, ether and acetone. Among them, gas chromatography–mass spectrometry (GC–MS) is one of the most widely used techniques. However, GC–MS is not suitable for real-time and on-site analysis [7]. Open-path Fourier transform infrared spectrometry (OP/FT-IR) is another traditional detection method which is capable of real-time monitoring. However, OP/FT-IR suffered from a low spectral resolution [8]. The electrochemical sensor, semiconductor sensor, as well as surface acoustic wave quartz crystal sensor can also be used to detect gases like ether, acetone, etc., but with limited sensitivity and poor selectivity [9–11]. The cataluminescence (CLT)-based sensors have been developed with higher selectivity. CTL is a kind of chemiluminescence emitted during catalytic oxidation reaction of organic molecules on solid catalyst surface. Recently, Shi et al. [12] reported the development of a CLT sensor, which achieved a detection limit of 6.7 ppm for ether at the operation temperature of 310 °C. However, the dependence of CLT sensor’s performance on the high operation temperature limited its application. On the other hand, infrared laser spectroscopy, especially the cavity ring-down spectroscopy (CRDS), provided a powerful access to trace gas analysis with extremely high sensitivity and selectivity. Since introduced in 1988, CRDS was developed into a highly sensitive absorption spectroscopy technique widely used for trace gas detection [13–16]. The detection limit of CRDS is highly enhanced due to a very long effective optical path length achieved by a compact, high-finesse optical cavity. As CRDS measures the rate of intensity decay rather than the absolute intensity of light, CRDS measurements are immune to the intensity fluctuations of the laser sources, leading to further improvement of the detection sensitivity.
Concerned with VOCs detection in real applications, high sensitivity alone is still far from enough. The simultaneous detection of multi-VOCs is required in many applications such as agro-industrial activities, energy production and national security. Although the spectroscopic detection technique has been widely used in multi-component gas detection, most of these reported applications are focusing on the detection of small molecules with narrow, resolved vibrational–rotational lines in the near-infrared region [17]. Of particular importance for VOC monitoring is often the detection of gas molecules with complicated quantum structures and broadband absorption spectra in the mid-infrared region [18]. In this case, a laser source tunable over the required spectral range is the key to the successful detections of broadband absorbers. Recent advances in semiconductor laser technology, particularly the quantum cascade lasers (QCLs), provided an effective way for highly sensitive and selective detection of trace gases [19–22]. The major advantage of QCLs is their ability to operate over a wide spectral range, providing an opportunity for simultaneous detection of mixed gases with broad absorption bands.
In this paper, the work is concentrated on pulsed CRDS since the experimental setup can be reduced to a small size in real applications that require portability. CRDS is combined with a pulsed QCL covered a wide spectral range in the mid-infrared and thus multiple broadband absorbers can be simultaneously measured with high sensitivity and selectivity. With pulsed CRDS, the absorption spectra of ethanol, ether and acetone in the spectral range of 2614–2634 cm−1 are first measured with high spectral resolution. The simultaneous detection of ethanol, ether and acetone is demonstrated.
2 Experimental procedures
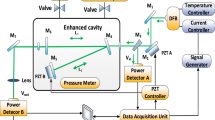
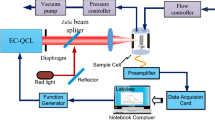
The pulsed QCL-based CRDS experimental setup is schematically shown in Fig. 1. An external cavity QCL (Model TLS-41038, Daylight Solutions) is used as the optical source, which is tunable in the spectral range of 2610–2720 cm−1 with a maximum output power of 250 mW. With pulsed operation, the spectral line-width (FWHM) is approximately 0.7 cm−1. The laser pulse repetition rate is 50 kHz, and pulse duration is 0.5 μs. The high-finesse ring-down cavity consists of a pair of highly reflective, plane concave mirrors with diameter of an inch and radius of curvature of −1 m attached to the two ends of a 50-cm-long stainless steel tube (CRD Optics) (the sample cell) with two inlets/outlets for gas exchange. In the spectral range of 2614–2634 cm−1 used in the CRDS measurements, the reflectivity of the cavity mirrors is between 99.915 and 99.913 %, resulting in an effective optical path length of approximately 588 m. A He–Ne laser, which is visible at 632.8 nm, is employed to align the high-reflectivity cavity mirrors. An optical isolator with central wavelength of 3.8 μm and isolation ratio of >30 dB is used to block the back-reflection of the laser beam by the ring-down cavity optics from reentering the QCL resonator and destabilizing the output spectrum and pulse energy. The light that transmitted through the ring-down cavity is focused by a focusing lens (f = 50 mm), placed closely behind the rear cavity mirror, into a TE-cooled, high-speed IR photovoltaic detector (model PVI-4ET-4, Vigo, Poland) for detecting the ring-down signal. The CRDS signal is triggered by the rising edge of a synchronizing signal of the laser pulse with an appropriate time delay. The decay is fitted after 0.75 μs relative to the trigger point, when the CRDS signal is around its maximum. A fitting program based on the Levenberg–Marquardt algorithm is applied to fit the detected ring-down signal to an exponential decay function to determine the ring-down time τ. By tuning the wavelength of QCL, the dependence of the ring-down time on the wavelength over the required spectral range is obtained. The absorption coefficient α of the gas sample within the ring-down cavity (the sample cell) is determined from the ring-down time τ using the equation \(\alpha (\lambda ) = \frac{1}{c}\left( {\frac{1}{\tau } - \frac{1}{{\tau_{0} }}} \right)\), where c is the speed of light, \(\lambda\) is the laser wavelength, and \(\tau_{0}\) is the ring-down time of an empty cavity (without absorbing sample inside the sample cell. In our case, the sample cell is filled with high-purity nitrogen). To improve the signal-to-noise ratio of the measurements, at each wavelength, 256 ring-down signals are recorded and 256 corresponding fitted ring-down times are averaged. This process for one wavelength takes less than 6 s. The whole process for single spectral acquisition is completed in approximately 10 min.
The gas samples used in the measurements are prepared by the standard gravimetric method (GM) [23] using high-purity nitrogen (N 2 > 99.999 %) as the buffer gas. The compounds ethanol, ether and acetone supplied by Chuandong Chemical Co. Ltd are with purities of 99.8 %. Three different kinds of gas samples are prepared. The first kind is individual compound in high-purity nitrogen, with three samples for the three compounds (one sample for each compound). The nominal concentrations of ethanol, ether and acetone for each sample are 190.9 ± 3.8, 217 ± 4.3 and 1021.1 ± 20.4 ppm, respectively. The second kind is a mixture of 14.7 ± 0.3 ppm ethanol and 6.55 ± 0.13 ppm ether in high-purity nitrogen. The third kind is a mixture of the three compounds ethanol, ether, acetone and a small amount of laboratory air mixed in high-purity nitrogen to simulate the laboratory environment. The detail of this third-kind sample is presented in the next section.
3 Results and discussion
Before performing the trace compound measurements, the wavelength-dependent total loss (or the ring-down time \(\tau_{0}\)) of the empty cavity (sample cell) has to be determined. As nitrogen has negligible absorption in the spectral region of interest, the cavity containing only high-purity nitrogen (N 2 > 99.999 %) is deemed as the “empty” cavity and its ring-down time \(\tau_{0}\) is measured as a function of wave number (wavelength). The results are presented in Fig. 2. The ring-down time varies between 1.92 and 1.97 μs across the spectral range from 2614 to 2634 cm−1 due to the spectral dependence of the cavity mirror reflectivity. Taking into consideration the fact that the cavity mirror reflectivity should be a smooth function of the wave number (wavelength), a smooth cubic function is used to fit the measured ring-down time data by a least-square fitting method. The determined cubic function is then used to calculate the \(\tau_{0}\) value for each wave number. The residual between the measured and calculated \(\tau_{0}\) values as a function of the wave number is also shown in Fig. 2. The standard deviation of the residual is 1.23 × 10−3 μs.
The minimum detectable absorption coefficient is estimated from the measurement error of the cavity ring-down time and can be calculated as
where \({{\Delta \tau } \mathord{\left/ {\vphantom {{\Delta \tau } {\tau_{0} }}} \right. \kern-0pt} {\tau_{0} }}\) is the relative error of the measured ring-down time. If three times standard deviation (3σ) is taken, from Fig. 2 \(\alpha_{\hbox{min} }\) is estimated to be 3.2 × 10−8 cm−1 for our experimental setup. From Eq. (1), the detection sensitivity can be improved by increasing the ring-down time \(\tau_{0}\) of the empty cavity. As \(\tau_{0} = {L \mathord{\left/ {\vphantom {L {c\left( {1 - R} \right)}}} \right. \kern-0pt} {c\left( {1 - R} \right)}}\), with R the mirror reflectivity and L the cavity length, the use of higher mirror reflectivity and longer cavity length would lead to improved detection sensitivity.
3.1 Detection of ethanol, ether and acetone in nitrogen
Figure 3 shows the broadband absorption of ethanol, ether and acetone in 2610–2720 cm−1 spectral region at 1 atm pressure and 296 K temperature, taken from the Pacific Northwest National Laboratory (PNNL) data base [24]. The unit of the absorption intensity in the PNNL library is ppm−1m−1, corresponding to the absorbance for a sample concentration of one part-per million (ppm) over an optical path length of one meter (m). From Fig. 3, the absorption peaks are observed for ether and ethanol in the spectral region between 2614 and 2634 cm−1 (marked with the rectangular box). Also from Fig. 2, the maximum mirror reflectivity (therefore the highest detection sensitivity) occurs at the short wave number region. Taking into consideration these two facts, the spectral region between 2614 and 2634 cm−1 is selected for the detection of ether, ethanol and acetone in our experiments.
The measured absorption spectra of ethanol, ether and acetone in high-purity nitrogen are presented in Fig. 4. The samples described in “Experimental procedures” section are used in the measurements. The concentrations of ethanol, ether and acetone are 190.9 ± 3.8, 217 ± 4.3 and 1021.1 ± 20.4 ppm, respectively. Since ethanol, ether and acetone all have broad absorption bands, which are much larger than the line-width of the QCL, the effects of the laser line-width on the detection could be ignored [25]. For comparison, the PNNL spectra which were measured by a Bruker-66 V FT-IR instrument are also presented. The CRDS measured spectra of the gas samples are directly compared with the absorption spectra with the same concentrations based on PNNL data base. Clearly, the spectra measured by CRDS have higher SNRs (signal-to-noise ratio). From Fig. 4, for ether the agreement between the CRDS measured spectrum and the PNNL spectrum is excellent (the standard deviation of the difference \(D = (\alpha_{\text{CRDS}} - \alpha_{\text{PNNL}} )/\alpha_{\text{CRDS}}\) is 0.0055), while for ethanol the agreement is reasonably good (the standard deviation of the difference is 0.010). On the other hand, for acetone the agreement is relatively poor (the standard deviation of the difference is 0.035). It is reasonable to believe that our measured spectra of ether, ethanol and acetone between 2614 and 2634 cm−1 should be more sensitive than the PNNL data due to higher SNR. The CRDS spectra presented in Fig. 4 are then used for the concentration determination in the following mixture measurements in this paper.
3.2 Simultaneous detection of ethanol and ether in nitrogen
The mixture of ethanol and ether in high-purity nitrogen is then measured with the CRDS experimental setup to demonstrate the simultaneous detection capacity. The nominal concentrations of ethanol and ether in the mixture are 14.7 ± 0.3 and 6.55 ± 0.13 ppm, respectively. The measured spectrum of the mixture in the spectral range of 2614–2634 cm−1 is presented in Fig. 5. The measured spectrum is fitted to the following equation
where \(a_{\text{ethanol}}\) and \(a_{\text{ether}}\) represent the absorption spectra of ethanol and ether measured with the samples of known concentrations (determined with the GM method), that is, the CRDS spectra presented in Fig. 4. PNNL spectrum data are not used as the references for the reason mentioned above. \(c_{1}\) and \(c_{2}\) are the concentration ratios between the measured ethanol and ether and the known-concentration samples. From Eq. (2), the concentrations of ethanol and ether in the mixture are determined to be 14.75 and 6.65 ppm, respectively, in excellent agreement with the nominal concentrations.
3.3 Simultaneous detection of ethanol, ether and acetone in mixed air and nitrogen
In the mid-infrared spectral region, most gas molecules exhibit strong absorption lines arising from the fundamental vibrational–rotational transitions. The molecules common in air, like CO2, H2O, also have absorption spectra in this spectral region and usually act to mask the species of interest. Figure 6 shows the spectral line intensities of H2O and CO2 at 1 atm pressure, 296 K temperature in the mid-infrared region based on HITRAN 2012 [26]. The logarithmic scale is the integrated intensity of line on a per-molecule basis. As shown in Fig. 6 that the spectral region around 3.8 μm wavelength (marked with the blue rectangular box), known as the “atmospheric window,” is an excellent spectral region for trace VOC detection in air, as in this region the spectral line intensities of moisture and carbon dioxide are relatively low and the interference caused by air is therefore relatively weak.
However, even the air absorption lines-induced interference in the measured spectral range is weak, it is not negligible and has to be accounted for in VOC detection. Therefore, the absorption spectrum of laboratory air in the spectral range of 2614–2634 cm−1 is measured and the result is presented in Fig. 7. For comparison, the measured absorption spectrum of 400 ppm moisture in high-purity nitrogen and the water vapor’s HITRAN spectrum taking into account the laser line profile-induced broadening are also presented. The measured and HITRAN spectra are in good agreement, indicating that in the measured spectral range the moisture is the predominant interference species for the VOC detection, which can also be seem from Fig. 6. It is noticed that except the moisture absorption, an elevated baseline of approximately 2 × 10−7 cm−1 is also presented in the measured spectrum of laboratory air. This baseline is believed to be related to various VOCs existing in in-door air. These compounds originate from sources located inside the building, such as adhesives, carpeting, wood products and cleaning products. The out-door air that enters the laboratory room can also contribute to the in-door VOCs, especially traffic-related VOCs like benzene, toluene, ethylbenzene and isomers of xylene (BTEX) [27].
CDRS is used to measure the mixture of ethanol, ether and acetone. As the preparation of mixture of ethanol, ether and acetone directly in laboratory air is difficult, a certain amount of laboratory air is added to the mixture of ethanol, ether and acetone in high-purity nitrogen to simulate the laboratory or factory environment. This is done by partially opening the inlet of the sample cell to laboratory air for an hour. Figure 8 shows the measured and fitted spectra of the mixture at 296 K temperature and 1 atm pressure. The concentrations of ethanol, ether and acetone are calculated by fitting the measured spectrum to the following equation
where \(a_{\text{ethanol}}\), \(a_{\text{ether}}\), \(a_{\text{acetone}}\) represent the absorption spectra of ethanol, ether and acetone measured with the samples of known concentrations (determined with the GM method), that is, the CRDS spectra presented in Fig. 4, and \(a_{\text{air}}\) represents the measured absorption spectrum of laboratory air (Fig. 7). The values of c 1, c 2, c 3 and c 4 are determined to be 0.0837, 0.0281, 0.028 and 0.049, respectively, corresponding to 15.98 ppm ethanol, 6.1 ppm ether, 28.6 ppm acetone, and approximately 460 ppm moisture in the mixture. During the measurement, the moisture concentration of the laboratory air is 0.94 %, given by an in-door temperature/humidity meter (608-H2, Testo).
The residual between the measured and fitted spectra is also presented in Fig. 8. The standard deviation of the residual is 1.1 × 10−8 cm−1. Correspondingly, the SNR of the measured signal is approximately 101.9. From Fig. 8, the detection limits are 157, 60 and 280 ppb for ethanol, ether and acetone, respectively. On the other hand, in our experiment the maximum detectable concentrations for ethanol, ether and acetone are estimated to be approximately 1280, 360 and 8900 ppm, respectively. The corresponding dynamic range of CRDS measurements is approximately four orders of magnitude of the detection limits. Compared to OP/FT-IR, CRDS shows much improved detection limits for VOC monitoring [28], while compared to GC–MS [29], CRDS has the potential for continuously and noninvasively real-time monitoring of VOCs. Compared to semiconductor gas sensors, CRDS has faster response time and lower operation temperature. Further, CRDS and semiconductor gas sensor-based equipments can be reduced to portable sizes in real applications. The detection limits of CRDS could be further improved by using cavity mirrors with higher reflectivity. In practical applications, the dynamic range of CRDS measurements can also be adjusted by appropriately selecting the cavity mirror reflectivity. In case a higher concentration needs to be measured, a lower mirror reflectivity should be used in the measurement.
Worthy mentioning that in practical applications of VOC monitoring, care has to be taken to deal with the influence of other VOCs also presented in the real environment (presented as the elevated baseline of the absorption spectrum of air shown in Fig. 7) on the simultaneous detection. In this paper, this influence is measured and eliminated from the measurements. However, in case such influence cannot be measured and accounted for, efforts have to be taken to identify these VOCs. It is a practical difficulty in field measurements for all spectroscopic techniques to selectively detect the target VOCs from abundant unidentified broadband absorbers in the selected wavelength region. In order to perform sensitive and selective spectroscopic detection, the appropriate spectral range must be carefully selected. The influence of sticky VOC on mirror reflectivity is also an important limiting factor. The three VOCs used in this work are common solvents typically for cleaning purposes in laboratories. Their influence on the mirror reflectivity is not noticeable in our experiment. For detecting VOCs that could cause mirror reflectivity degradation, gas (nitrogen or clean air) purging to the mirror surface can be employed to protect the mirrors from VOC contaminations [30, 31]. In addition, the influence of adsorption and memory effects can be decreased by measuring the baseline regularly
4 Conclusions
In this paper, we have particularly focused on the simultaneous detection of trace heavy molecules with overlapping rotational and vibrational transitions. The QCL, tunable over a wide spectral range, has been combined with CRDS in favor for gas monitoring applications of broadband absorbers and multi-species. We have demonstrated the application of a room-temperature pulse-QCL-based CRDS to determine simultaneously the concentrations of ethanol, ether and acetone. The detection limits of 157 ppb for ethanol, 60 ppb for ether and 280 ppb for acetone have been achieved. Mid-infrared CRDS has showed great potential in monitoring various solvents commonly used in laboratories and VOCs both in-door and out-door.
References
E. Rahardjo, in International Congress Series, vol 1242 (2002), pp. 51–55
M.Z.F. Kamarudin, S.K. Kamarudin, M.S. Masdar, W.R.W. Daud, Int. J. Hydrog. Energy 38, 22 (2013)
P. Dalton, C.J. Wysocki, M.J. Brody, H.J. Lawley, Am. J. Ind. Med. 31, 5 (1997)
H. Guo, S.C. Lee, L.Y. Chan, W.M. Li, Environ. Res. 94, 1 (2004)
J.C.M. Brust, Int. J. Environ. Res. Public Health 7, 4 (2010)
American Conference of Governmental Industrial Hygienists (ACGIH), http://www.acgih.org/
J. Rudnicka, M. Walczak, T. Kowalkowski, T. Jezierski, B. Buszewski, Sens. Actuators B Chem. 202, 31 (2014)
B.K. Hart, P.R. Grifths, Environ. Sci. Technol. 34, 7 (2000)
C.C. Wang, Y.C. Weng, T.C. Chou, Sens. Actuators B Chem. 122, 2 (2007)
L. Wang, A. Teleki, S. Pratsinis, P. Gouma, Chem. Mater. 20, 15 (2008)
H.B. Lin, J.S. Shih, Sens. Actuators. B Chem. 92, 3 (2003)
G.L. Shi, B. Sun, Z. Jin, J.H. Liu, M.Q. Li, Sens. Actuators B Chem. 171–172, 699–704 (2012)
A. O’Keefe, D.A.G. Deacon, Rev. Sci. Instrum. 59, 2544 (1988)
D. Marchenko, J. Mandon, S.M. Cristescu, F.J.M. Harren, Appl. Phys. B 111, 3 (2013)
Y.B. He, C.J. Jin, R.F. Kan, J.G. Liu, W.Q. Liu, J.L. Hill, I.M. Jamie, B.J. Orr, Opt. Express 22, 11 (2014)
F.M. Schmidt, O. Vaittinen, M. Metsälä, M. Lehto, C. Forsblom, P.H. Groop, J. Breath Res. 7, 1 (2013)
M. Scotoni, A. Rossi, D. Bassi, R. Buffa, S. Iannotta, A. Boschetti, Appl. Phys. B 82, 3 (2006)
C.C. Harb, T.K. Boyson, A.G. Kallapur, I.R. Petersen, M.E. Calzada, T.G. Spence, K.P. Kirkbride, D.S. Moore, Opt. Express 20, 14 (2012)
R. Maulini, A. Mohan, M. Giovannini, J. Faist, Appl. Phys. Lett. 88, 201113 (2006)
T.K. Boyson, D.R. Rittman, T.G. Spence, M.E. Calzada, A.G. Kallapur, I.R. Petersen, K.P. Kirkbride, D.S. Moore, C.C. Harb, Opt. Express 22, 9 (2014)
M.C. Phillips, M.S. Taubman, B.E. Bernacki, B.D. Cannon, R.D. Stahl, J.T. Schiffern, T.L. Myers, Analyst 139, 2047–2056 (2014)
S. Welzel, R. Engeln, J. Röpcke, Cavity-Enhanced Spectroscopy and Sensing, Chapter 3 (Springer, Berlin, 2014)
ISO 6142-2001
S.W. Sharpe, T.J. Johnson, R.L. Sams, P.M. Chu, G.C. Rhoderick, P.A. Johnson, Appl. Spectrosc. 58, 12 (2004)
P. Zalicki, R.N. Zare, J. Chem. Phys. 102, 2708 (1995)
L.S. Rothman, I.E. Gordon, Y. Babikov, A. Barbe, D. Chris Benner, P. F. Bernath, M. Birk, L. Bizzocchi, V. Boundon, L.R. Brown, A. Campargue, K. Chance, E.A. Cohen, L.H. Coudert, V.M. Devi, B.J. Drouin, A. Fayt, J.-M. Flaud, R.R. Gamache, J.J. Harrison, J.-M. Hartmann, C. Hill, J.T. Hodges, D. Jacquemart, A. Jolly, J. Lamouroux, R.J. Le Roy, G. Li, D.A. Long, O.M. Lyulin, C.J. Mackie, S.T. Massie, S. Mikhailenko, H.S.P. Muller, O.V. Naumenko, A.V. Nikitin, J. Orphal, V. Perevalov, A. Perrin, E.R. Polovtseva, C. Richard, M.A.H. Smith, E. Starikova, K. Sung, S. Tashkun, J. Tennyson, G.C. Toon, Vl.G. Tyuterev, G. Wagner, J. Quant. Spectrosc. Radiat. Transfer 130, 4–50 (2013)
X.L. Han, P.N. Luke, Environ. Int. 32, 1 (2006)
Z. Bacsik, V. Komlósi, T. Ollár, J. Mink, Appl. Spectrosc. Rev. 41, 1 (2006)
M.R. Ras-Mallorquí, R.M. Marcé-Recasens, F. Borrull-Ballarín, Talanta 72, 3 (2007)
H. Sumizawa, H. Yamada, K. Tonokura, Appl. Phys. B 100, 4 (2010)
C.L. Hagen, B.C. Lee, I.S. Franka, J.L. Rath, T.C. VandeBoer, J.M. Roberts, S.S. Brown, A.P. Yalin, Atmos. Meas. Tech. 7, 345 (2014)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, S., Han, Y. & Li, B. Simultaneous detection of ethanol, ether and acetone by mid-infrared cavity ring-down spectroscopy at 3.8 μm. Appl. Phys. B 122, 187 (2016). https://doi.org/10.1007/s00340-016-6465-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-016-6465-1