Abstract
This study employed atmospheric plasma (AP) pretreatment to enhance the hydrophobicity of polytetrafluoroethylene (PTFE) thin films. When a PTFE thin film was deposited on the substrate pretreated with AP, the deposition followed the Frank-van der Merwe mode at hydrophilic regions and the Volmer–Weber mode at hydrophobic regions, thereby causing the PTFE thin film to have continuous bumps. In accordance with the Wenzel and Cassie equation, increasing the surface roughness of hydrophobic materials increased their hydrophobicity. The water contact angle increased from 100° to 110° after deposited PTFE thin film on substrate pretreated with AP. Finally, the water contact angle further increased to 115° after annealed in nitrogen gas. A salt spray test demonstrated that the hydrophobic PTFE thin film had excellent corrosion resistance and stability. This hydrophobic film preparation technology can be used for the protective film of electronic device, sensor, solar cell and so on.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Water contact angle can be hydrophilic or hydrophobic. Contact angles of less than 10°, 10°–90°, 90°–120°, and greater than 120° are super hydrophilic, hydrophilic, hydrophobic, and superhydrophobic, respectively. Hydrophobic surfaces are extensively applied in glass, stainless steel and metals to repel water and prevent corrosion or staining [1,2,3,4,5]. The hydrophobicity of a surface can be increased by the following two methods. The first method involves coating the surface with a thin film containing CF or CH functional groups, ensuring low surface tension. This method is simple and can be performed quickly. However, it yields only a small range of hydrophobic water contact angles. Common examples include polystyrene, polymethyl methacrylate, polylactic acid, and polytetrafluoroethylene (PTFE) [6, 7]. The second method involves roughening the surface of thin films. For example, E. Fadeeva et al. mimicked the surface texture of lotus leaves and used laser etching to engrave a titanium surface to prepare a dual-layer micro nano superhydrophobic structure [8]. The downside of this approach is that laser etching is expensive. Another technique, which involves the use of photolithography or electron beam lithography, produces excellent hydrophobic results but is not suitable for large surfaces or mass production [9, 10]. Therefore, the aforementioned two methods must be integrated to achieve superhydrophobic thin films.
PTFE is a synthetic fluoropolymer that exhibits excellent chemical stability because of its multiple stable [–(CF2 )–]n bonds (485 kJ/mol) [11]. PTFE thin films, which are hydrophobic and have anti-icing properties, can be found in sensor, solar cell, electronics, wires, cables and textiles [12,13,14,15,16]. PTFE’s hydrophobic characteristics have been extensively studied. Hydrophobic PTFE technology is mature and stable. The present study prepared PTFE thin films with hydrophobic surfaces. A substrate is pretreated with atmospheric plasma (AP) which does not require a vacuum chamber for reactions. Furthermore, AP is environmentally-friendly and low-cost because the main reactants of AP are inert gases, such as nitrogen and helium, thereby minimizing the generation of additional pollution during preparation. AP is primarily used to efficiently graft functional groups onto substrates. Functional groups can be generated from the plasmas of normal air, nitrogen, argon, oxygen, and helium, eliminating the need for conventional wet chemical reactions [17]. This again makes AP an environmentally-friendly and convenient technique.
This study investigated whether AP could increase the hydrophobicity of a PTFE thin film. Because N2 and O2, which are readily available from the air, are used as reactants, this method is deemed suitable for mass production. A substrate is pretreated with a metal shadow mask that is holed at regular intervals so that uncovered areas of the substrate could be grafted with hydrophilic OH functional groups through AP surface modification. When the PTFE film is deposited, it will show continuous periodic bumps. The change of the surface morphology increases the surface roughness and reduces the surface energy. Few studies have explored the effects of intermittent AP pretreatment of a substrate surface on the performance of a hydrophobic PTFE thin film. Therefore, this study investigated the surface morphology, surface roughness, and water contact angle of the PTFE thin film deposited on substrates intermittently pretreated with AP. The effects of different shadow mask opening rates and PTFE annealing processes are also explored.
2 Experiment
First, this study designed a rectangular stainless steel shadow mask (1 cm\(\:\:\times\:\:\)1 cm) with intermittent openings. The spacing between the openings was set at 0.02 cm, and the opening rates were 20% (Mask 1), 30% (Mask 2), 40% (Mask 3), and 50% (Mask 4). Next, the study used a solution containing deionized water, alcohol, and acetone to wash a glass substrate (Corning Eagle XG; area: 1 cm\(\:\:\times\:\:\)1 cm, thickness: 0.7 mm, mean visible light transmittance: 90%) in an ultrasonic cleaner for 10 min. The study then used nitrogen gas (purity: 99.99%) to dry the glass substrate. Afterward, the metal shadow mask was attached to the glass substrate for AP pretreatment with current and inlet pressure of 5 A and 1.5 MPa, respectively. The study used a track to move a nozzle back and forth 1 cm/s along the x-axis 10 times for surface AP pretreatment, which lasted 20 s. Afterwards, the pretreated glass substrate was placed in a radio-frequency (13.56 MHz) sputtering system that had a background pressure of 5 µtorr. A two-inch PTFE (99.99 wt%) block was used as the sputtering target. The temperature, pressure, and argon flow parameters during sputtering were 30 °C, 5 mtorr, and 30 sccm, respectively. Various sputtering powers (140, 160, 180, and 200 W) were used to deposit hydrophobic PTFE thin films. The thickness of the hydrophobic thin films was maintained at 20 nm. Finally, the PTFE thin films were annealed in nitrogen gas at different temperatures (100, 200, 250, and 300 °C) for 1 h and then cooled in nitrogen gas until they were 50 °C.
In this study, the surface morphologies of the PTFE films were analyzed using field-emission scanning electron microscopy (FE-SEM; JEOL JSM-7000 F) at an accelerating voltage of 15 kV. The surface roughness and thickness of the PTFE films were examined using surface profilometer (EZSTEP; force precision instrument, scan speed 100 μm/sec). The PTFE film water contact angles was measured at room temperature using a contact angle meter by the sessile drop method (FIBRO System, TQC Sheen Fibro PGX + Pocket Goniometer). The functional group of PTFE films were identified by Fourier transform infrared spectroscopy (FTIR, Spectrum one, Perkin-Elmer, USA). The salt spray test standard was based on the ASTM B-117 specification of the American Institute of Materials Standards. The solution was used 5% salt water solution with pH value in the range of 6.5 to 7.2 and keep the test temperature at 50oC for 3 days.
3 Results and discussion
Figure 1 shows the deposition mechanism of PTFE thin film deposited on the AP pretreated substrate. Areas pretreated with AP became super hydrophilic (Fig. 1(b)), whereas areas not pretreated with AP remained hydrophobic (Fig. 1(a)), causing the substrate to become partially hydrophobic and partially hydrophilic (Fig. 1(c)). If a surface has tiny bumps, hydrophilic surfaces become more hydrophilic, whereas hydrophobic surfaces become more hydrophobic [18]. When a PTFE thin film is deposited on the pretreated substrate, the deposition at hydrophilic regions follows the Frank-van der Merwe (FM) mode and the surface becomes continuous and flat (Fig. 1(b)) [19]. Whereas the thin film deposition at hydrophobic regions follows the Volmer–Weber (VW) mode and has an island-like nucleation mechanism that makes the surface coarser (Fig. 1(a)) [20]. The result is continuous bumps across the surface when a PTFE thin film is deposited on the substrate.
Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) images of the PTFE thin films deposited at different sputtering powers (140, 160, 180, and 200 W) are shown in Fig. 2. At 140 W, the content of carbon and fluorine in the PTFE thin film were 75.9% and 24.1%, respectively (Fig. 2a). This indicates that the fluorine content is lower when the sputtering power is lower, causing the PTFE thin film to have less hydrophobic C-F functional groups. At 160 W, the content of carbon and fluorine in the PTFE thin film were 43.8% and 56.2%, respectively (Fig. 2b). PTFE is a non-conductive material, large power is required to effectively deposit the material during sputtering. The main elements of PTFE are carbon and fluorine. During sputtering, carbon has a lower atomic number and is easier to be deposited by sputtering, while fluorine has a larger atomic number and requires greater energy for sputtering. The fluorine content increased considerably, suggesting that the PTFE thin film is more hydrophobic because it had more hydrophobic C-F functional groups. The water contact angles of the PTFE thin films deposited at 140, 160, 180, and 200 W are 48°, 76°, 100°, and 91°, respectively (Fig. 3). When the sputtering power is 140 W, the PTFE thin film had less hydrophobic C-F functional groups and weaker hydrophobicity. Because fluorine atoms are not easily to deposited at low power by sputtering. When the sputtering power is 180 W, the PTFE thin film is more hydrophobic and had more C-F functional groups because the content of fluorine increased from 24.1 to 56.2%. Therefore, this study speculated that the fluorine content is a key factor affecting the hydrophobicity of PTFE thin films.
SEM and EDS images of the PTFE thin films deposited on substrates pretreated with AP under different shadow masks are shown in Fig. 4. The power, temperature, pressure, and argon flow parameters during sputtering are 180 W, 30 °C, 5 mtorr, and 30 sccm, respectively. The content of carbon and fluorine dose not vary greatly after different shadow masks are used for AP pretreatment. The contents of carbon and fluorine are 42.5–43.8% and 56.2–57.5%, respectively. Therefore, after AP plasma pretreatment, the partially hydrophilic and partially hydrophobic glass substrate does not affect the chemical composition of the PTFE thin film. SEM analysis of the surface of the thin films revealed that as the opening rate changed from 20 to 50%, the surface of the thin films formed crystals of different sizes because after AP pretreatment, the density of hydrophilic regions and hydrophobic regions on the glass substrate increased, thereby increasing the density of the deposition following the FM and VW modes on the PTFE thin films.
The roughness of the PTFE thin films deposited on substrates pretreated with AP through shadow masks with different opening rates by surface profilometer analysis shown in Fig. 5. The initial surface roughness of the PTFE thin films is 0.8 nm. After AP pretreatment under shadow masks with opening rates of 20%, 30%, 40%, and 50%, the surface roughness values Ra of the PTFE thin films are 10.9, 13.5, 14.4, and 11.2 nm, respectively. The surface roughness values Rq of the PTFE thin films are 13.8, 18.3, 18.6, 15.0 nm, respectively. The results demonstrated that the surface roughness of the PTFE thin film increased as the opening rate increased. The Wenzel and Cassie equation is as follows [18]:
cosθ = t (r cosθ0 + 1) -1
where θ0 is the initial water contact angle of the material surface, r is the surface roughness, and t is the material surface area. A hydrophobic material will become more hydrophobic if its surface becomes coarser, whereas a hydrophilic material will become more hydrophilic if its surface becomes coarser. This study referenced the aforementioned principle and discovered that the glass substrate pretreated with AP changed the deposition mechanism of the PTFE thin film, thereby changing the roughness of the thin film and facilitating increased hydrophobicity.
The water contact angles of the PTFE thin films deposited on substrates pretreated with AP under different shadow masks are shown in Fig. 6. The black and white regions in the figure are areas not pretreated and pretreated with AP, respectively. When Mask 1, Mask 2, Mask 3, and Mask 4 are used, the water contact angles of the PTFE thin films are 94°, 102°, 110°, and 81°, respectively. Without AP pretreatment, the water contact angle of the PTFE thin film is 100°. The area pretreated with AP under Mask 1 was 20%, so the density of hydrophilic and hydrophobic regions on the glass substrate is low. Although the surface roughness increased from 0.8 nm to 10.86 nm, the material surface area (t) is too great and caused the water contact angle to decrease from 100° to 94°. When Mask 2 and Mask 3 are used, the water contact angle increased as the area pretreated with AP increased. The density of hydrophilic and hydrophobic regions increased, thereby decreasing the material surface area (t). Furthermore, the surface roughness of the hydrophobic thin film increased from 0.8 nm to 13.5 nm (Mask 2) and 14.4 nm (Mask 3). As the material surface area decreased and the surface roughness increased, the hydrophobicity increased. However, when the opening rate pretreated with AP increased to 50%, the water contact angle decreased greatly. Although the surface roughness (r) increased from 0.8 nm to 11.2 nm, the material surface area decreased greatly and caused the hydrophobicity of the PTFE thin film to decrease. In order to facilitate observation a cross section image of the PTFE thin film with 150 nm is deposited on glass substrate with AP pretreatment under Mask 3 is shown in Fig. 7. The pretreated area is hydrophilic and caused the deposition of the PTFE thin film to follow the FM mode; the thin film is continuous and flat. The untreated area is hydrophobic and caused the deposition to follow the VW mode. Because island-like nucleation occurred, the surface of the thin film is coarse. Therefore, AP pretreatment of substrates changes the deposition of the hydrophobic thin film and further increases the hydrophobicity of the thin films.
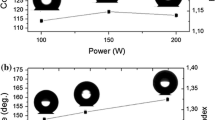
The water contact angles of the PTFE thin films deposited on glass substrates pretreated with AP under Mask 3 and annealed in nitrogen gas are shown in Fig. 8. When the annealing temperatures are 100, 200, 250, and 300 °C, the water contact angles are 110°, 112°, 115°, and 98°, respectively. The results demonstrated that the water contact angle of the PTFE thin film increased as the annealing temperature increased, possibly because the annealing process caused the thin film to become denser and have more consistent crystals, which are both conducive for enhanced hydrophobicity. However, when the annealing temperature is 300 °C, the water contact angle decreased from 115° to 98°. This study used a surface profiler and discovered that the roughness before and after annealing at 300 °C are 14.4 nm and 11.2 nm, respectively. This result indicated that the higher annealing temperature damaged the FM and VW deposition of the thin film, decreased the surface roughness of the thin film, and increased the surface area, resulting in poorer hydrophobicity.
The FTIR spectroscopy of PTFE thin films as a function of annealing temperature are shown in Fig. 9. The peaks at 1217 and 1365 cm− 1 are originate from CF2 bonds [21]. The peak at 1738 cm− 1 belong to C = O bond [22]. The weak peaks at 2970 and 3455 cm− 1 are related to N–H bonds and O–H stretching, respectively [21]. The CF2 bonds are the key to affecting hydrophobicity and the strong CF2 bonds peak of PTFE film is annealing at 250 °C. However, when the annealing temperature is 300 °C, the CF2 bonds peak decrease and C = O bond peak increase. In this way, the water contact angle is decrease to 98° because the CF2 bonds peak decrease. The C = O bond is increase with temperature but does not help increase hydrophobicity. The N-H bond is produced due to nitrogen annealing. The results indicated that the water contact angle of PTFE thin films are proportional to the surface roughness and CF2 bonds. The mean visible light transmittance and water contact angle of a PTFE thin film under a salt spray test at 50 °C for 3 days are shown in Fig. 10. The light transmittance of the hydrophobic thin film remained virtually unchanged at 78% ± 0.07%, and the water contact angle remained at 115° ± 0.584°. These results further demonstrated that the PTFE thin films developed in this study had excellent hydrophobicity and stability.
4 Conclusions
This study used AP pretreatment to increase the hydrophobicity of PTFE thin films by designing a shadow mask that opened intermittently so that the glass substrate is partially hydrophobic and partially hydrophilic. When a hydrophobic PTFE thin film is deposited on the glass substrate pretreated with AP, the deposition of the thin film followed the FM mode at hydrophilic regions and followed the VW mode at hydrophobic regions, resulting in continuous bumps on the surface of the PTFE thin film, thereby increasing hydrophobicity. The water contact angles of the PTFE thin films when the sputtering power values were 140, 160, 180, and 200 W are 48°, 76°, 100°, and 91°, respectively. When the sputtering power values are 140 and 180 W, the proportions of fluorine are 33.5% and 56.18%, respectively. Therefore, this study speculated that fluorine content is a key factor affecting the hydrophobicity of the PTFE thin film. The surface roughness of the PTFE thin films deposited on substrates pretreated with AP under shadow masks with opening rates of 20%, 30%, 40% and 50% are 10.9, 13.5, 14.4 and 11.2 nm, respectively. This result demonstrated that the surface became coarser as the opening rate increased. By measuring the water contact angle, this study found that an opening rate of 40% yielded an optimal water contact angle of 110°. When the PTFE thin film was annealed in nitrogen gas, the water contact angle further increased to 115°. Finally, this study performed a salt spray test and discovered that the mean visible light transmittance and water contact angle of the PTFE thin film remained at 78% ± 0.07% and 115° ± 0.584°, respectively. The results demonstrated that intermittent AP pretreatment increases the hydrophobicity and reliability of the PTFE thin film, making it suitable as protective coating for electronic device, optical lenses and precision machinery.
Data availability
Data will be made available on request.
References
O. Sharifahmadian, A. Pakseresht, S. Mirzaei, M. Eliáš, D. Galusek, Diam. Relat. Mater. 138, 110252 (2023)
M.B. Esfahani, A. Eshaghi, S.R. Bakhshi, J. Non Cryst. Solids. 583, 121479 (2022)
M.O.A. Ferreira, F.E. Ariani, N.B. Leite, R.V. Gelamo, I.V. Aoki, A. Siervo, H.C. Pinto, J.A. Moreto, Mater. Chem. Phys. 312, 128610 (2024)
A. Flehan, L. Jinna, M. Tabish, A. Kumar, Y.A.Y.A. Mohammed, M. Mubeen, M.M. Makhlouf, G. Yasin, J. Alloys Compd. 968, 172139 (2023)
C.E. Caballero-Güereca, M.R. Alfaro Cruz, E. Luévano-Hipólito, L.M. Torres-Martínez, Surf. Interfaces. 37, 102705 (2023)
X. Xie, J. Yan, Q. Wu, T. Wang, F. Chu, S. Yang, J. Hazard. Mater. 434, 128898 (2022)
H. Younas, Y. Zhou, X. Li, X. Li, Q. Sun, Z. Cui, Z. Wang, Polymer. 179, 121593 (2019)
E. Fadeeva, S. Schlie-Wolter, B.N. Chichkov, G. Paasche, T. Lenarz, Laser Surface Modification of Biomaterials (Woodhead Publishing, United Kingdom, 2016)
H. Zhang, Q. Yan, Q. Xu, C. Xiao, X. Liang, Sci. Rep. 7, 3983 (2017)
P. Nuchuay, T. Chaikeeree, M. Horprathum, N. Mungkung, N. Kasayapanand, C. Oros, S. Limwichean, N. Nuntawong, C. Chananonnawathorn, V. Patthanasettakul, P. Muthitamongkol, B. Samransuksamer, S. Denchitcharoen, A. Klamchuen, C. Thanachayanont, P. Eiamchai, Curr. Appl. Phys. 17, 222–229 (2017)
W.V. Venner, G.J. Puts, P.L. Crouse, J. Fluor. Chem. 217, 63–71 (2019)
Q. Guo, Y. Huang, M. Xu, Q. Huang, J. Cheng, S. Yu, Y. Zhang, C. Xiao, J. Membr. Sci. 664, 121115 (2022)
V. Singh, A.K. Singl, A. Bansal, Surf. Coat. Technol. 470, 129858 (2023)
K.A. Kuptsov, M.N. Antonyuk, A.N. Sheveyko, D.V. Shtansky, Surf. Coat. Technol. 465, 129621 (2023)
M.A. Saffar, A. Eshaghi, M.R. Dehnavi, Mater. Chem. Phys. 259, 124085 (2021)
F. Tao, J. Zheng, L. Wang, Y. Yuan, F. Wan, W. Xu, Z. Huang, S. Wang, Y. Huang, J. Alloys Compd. 866, 158827 (2021)
Y. Xie, J. Zhu, L. Fu, W. Yang, D. Li, L. Zhou, Appl. Surf. Sci. 654, 159509 (2024)
A.B.D. Cassie, S. Baxter, Trans. Faraday Soc. 40, 546 (1944)
F.C. Frank, J.H. Merwe, Phys. Eng. Sci. 198, 205–216 (1949)
M. Volmer, A. Weber, Z. Phys. Chem. (N F). 119, 277–301 (1926)
J. Piwowarczyk, R. Jedrzejewski, D. Moszy´nski, K. Kwiatkowski, A. Niemczyk, J. Baranowska Polym. 11, 1629 (2019)
K. Yamauchi, Y. Yao, T. Ochiai, M. Sakai, Y. Kubota, G. Yamauchi, J. Nanotechnol. 7, 1–7 (2011)
Acknowledgements
The authors thank the National Science and Technology Council of Taiwan, for financially supporting this research under Contract No. 112-2221-E-212-003, No. 111-2622-E-212 -005 and SBIR-112A024.
Author information
Authors and Affiliations
Contributions
Y. S. Cho: Methodology, Formal analysis, Validation, Data curation. S. C. Lin: Methodology, Formal analysis. C. C. Wang: Methodology, Formal analysis. Y. T. Yang: Investigation, Data curation. Y. R. Ho: Investigation, Formal analysis, Writing – original draft. J. J. Huang: Conceptualization, Methodology, Writing – review & editing, Project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cho, YS., Lin, SC., Wang, CC. et al. Periodic atmospheric plasma substrate treatment to improve the hydrophobicity of sputtering PTFE films. Appl. Phys. A 130, 663 (2024). https://doi.org/10.1007/s00339-024-07838-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-024-07838-4