Abstract
The dielectric characteristics of double perovskite La2Ni1 − xFexMnO6; (x = 0, 0.2, 0.5, 0.8 & 1.0) developed via sol-gel route, were studied through analysis of B-site cation substitution. La2Ni0.8Fe0.2MnO6 attained a higher dielectric constant than pure La2NiMnO6 owing to comparatively higher B-site cationic disorder. The crystallite size and strain were determined using Williamson-Hall analysis, and strain in samples decreased with increasing Fe substitution. High-resolution transmission electron microscopy (HRTEM) authenticated the crystalline nature and selected area electron diffraction patterns (SAED) confirmed the purity of the sample. X-ray photoelectron spectroscopy (XPS) confirmed the co-occurrence of different oxidation states. Dielectric constant and tangent loss of overall composition decreased with frequency, while AC conductivity increased. AC conductivity decreased upon Fe-substitution and was strongly affected by the degree of B-site cationic disorder. Cole-cole plots and combined variation of Z″ & M″ with frequency suggested non-Debye relaxation in developed compositions. The tuned dielectric parameters suggest the possible applicability of developed double perovskite materials for fabricating energy storage devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent times, double perovskite (DP) oxides based on the general formula A2BB′O6 have gained much attention due to their fascinating multifunctional properties and promising applications [1]. These compounds have widespread utility in fabricating new-age electronic devices like resonators, pyroelectric & piezoelectric transducers, capacitors, microwave devices, and energy storage devices [2]. DP structure is derived from a simple perovskite structure (ABO3) which is well-known for its attractive properties. The compositional and structural adaptability of perovskite structure permits the accommodation of nearly all periodic table elements. The cation substitution is the popular way to further tune the properties of perovskites [3]. The occupancy of localized 3d and relatively delocalized 4d/5d transition metal cations at the B & B′ site leads to DP structures where physical properties are strongly affected by the amount of B/B′ cations ordering, ionic radii, and oxidation states [4]. A-site is generally filled by trivalent rare-earth or divalent alkaline-earth cation [5]. DPs have been known for their multi-functions like multiferroic, magnetoresistive, magnetocapacitive, and magnetodielectric [6,7,8,9]. At low temperatures, the structure of La2NiMnO6 (LNMO) has an ordered monoclinic or a disordered orthorhombic phase which at high temperatures transforms to a disordered rhombohedral phase [10]. At near room temperatures, all three phases coexist in the LNMO structure resulting in anti-site disorder at Ni/Mn sites [11,12,13]. To tune the magnetodielectric response of a DP material, its dielectric constant is an important factor [14, 15]. The anti-site disorder at the B/B′ site strongly influences the dielectric constant. The large dielectric constant of LNMO results in colossal magnetodielectric coupling at near-room temperatures [9, 15]. Bulk LNMO has a large dielectric constant with relaxor-type behavior which is attributed to the ordering of cations (Ni2+ and Mn4+) [16]. Tang et al. have reported a giant tunability effect of dielectric constant in bulk LNMO at near room temperature [17]. Lin et al. reported that B-site ordering of Ni2+ and Mn4+ leads to better relaxor-like dielectric behavior in both LNMO ceramic and film at higher temperatures [16, 18]. Recently, another combination of Fe and Mn at the B-site (La2FeMnO6) has also been reported as a good dielectric material with a dielectric constant of 7 × 104 at 1 Hz and an activation energy of 0.37 [19]. In contrast, LNMO was reported with an activation energy of 0.17 eV [2]. In another study, Fe substitution in La2CoMnO6 has changed the conduction mechanism from variable range hopping to thermal activation [6]. Thus, substituting Fe at the B site in LNMO may disturb the cationic ordering and lead to tailoring the dielectric performance and conductivity.
The current research focuses on explaining the effect of Fe-substitution on the dielectric response of La2NiMnO6 double perovskite in detail. Fe being a transition metal may become an important factor in tuning the dielectric behavior. La2Ni1 − xFexMnO6 synthesized in our research includes three different transition metal ions at the B/B′-site which may provide new-fangled breakthroughs in scrutinizing the relations among oxidation states, cation ordering and dielectric relaxation. The aforesaid characteristics of double perovskites are generally dependent on the preparation settings and surroundings, hence best efforts have been made to attain a similar environment during the synthesis process for each composition to study even the minute variation at B-site after Fe substitution.
2 Experimental
2.1 Materials and methods
The chemicals of La(NO3)3.6H2O (Lanthanum-Nitrate), NiCl2.6H2O (Nickel-Chloride), MnCl2.4H2O (Manganese-Chloride), and Fe(NO3)3.9H2O (Iron-Nitrate) from Sigma-Aldrich with 99.5% purity were weighed as per proportion for the desired composition. Sol-gel (wet-chemical) procedure was deployed to develop DP samples. The de-ionized water was utilized for preparing various solutions during the synthesis process.
2.2 Sample preparation and characterization
Double perovskite samples La2Ni1 − xFexMnO6; (x = 0, 0.2, 0.5, 0.8 & 1.0) named LNMO, LNFMO1, LNFMO2, LNFMO3, LFMO in the sequence of composition, were synthesized via sol-gel route. The weighed chemical powders were dissolved in de-ionized water to have different solutions, which were mixed to make a final solution. The solution was continuously stirred and added with ethylene glycol (a stabilizing agent) until converted into gel form. The solidification of the obtained gel was achieved by continued heating at 383 K. For getting ultimate samples, the solid compounds were ground and subsequently sintered at 1273 K for 08 h. The pellets of the sample powder were prepared with a hydraulic press, followed by silver coating and heating at 423 K for 10 h to make them suitable for electrical measurements. The phase formation of developed samples was confirmed by an X-ray diffractometer (Rigaku Ultima IV) with Cu-Kα radiation. Impedance analyzer (IM3570-Hioki) was used for dielectric measurements at temperature values ranging from 303 K to 423 K. The EC-Lab 10.4 program was utilized for equivalent circuit analysis.
3 Results and discussion
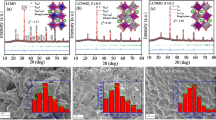
3.1 XRD
X-ray diffraction patterns were recorded at room temperature between 20˚−80˚ (2θ) at 2˚/min scan rate to confirm the phase formation of all developed compositions, which are depicted in Fig. 1. XRD findings confirmed the synthesis of samples with successful Fe substitution in LNMO double perovskite. XRD peak profile is in agreement with monoclinic structure (space group P21/n) [20, 21]. However, the structure of LNMO can be partially disordered due to antisite disorders at the Ni/Mn site and localized inhomogeneity in crystal structures [12, 15]. Rietveld refinement of the prepared compounds revealed a mixture of ordered monoclinic (P21/n) and disordered rhombohedral (R3c) & orthorhombic (Pbnm) phases with different percentages [22]. Debye Scherrer equation is used to determine the crystalline size which can be expressed as [23]:
Where D represents crystallite size, β is FWHM (Full Width at Half Maximum) of Bragg’s peak, K is Scherrer constant typically taken as 0.9, and λ is the wavelength of X-ray beam. The determined values of the crystallite size for all the samples are given in Table 1.
3.2 Williamson-Hall (W-H) plot
Debye Scherrer equation associates peak width with crystallite size only. However, the peak width is related to both size and lattice strain. To overcome the drawback of Scherrer method, W-H analysis is used to obtain the crystallite size and strain broadening by considering peak width as a function of 2 θ [24, 25]. The strain broadening (βε) is induced through structural distortions, point defects, stacking faults, etc. present in the system. W-H analysis covers all these constraints and provides a more accurate estimate of crystallite size [26]. W-H plot deconvolutes broadening into crystallite size (D) and strain (ε) as expressed below [26]:
The strain-induced broadening can be obtained from the following expression [27]:
Where ε is compressive/tensile strain and C is a constant, whose value is generally assumed as 4. Therefore, the W-H equation takes the form as [27]:
Where K is Scherrer constant, λ is the wavelength of the X-ray beam, and θ is Bragg’s angle. On rearranging, Eq. (4) takes the form:
Equation 5 accounts for the isotropic behavior of crystal structure i.e. physical characteristics of materials are independent of direction. W-H plots between 𝛽cos𝜃 and sin𝜃 for all the samples are drawn and depicted in Fig. 2. The crystallite size and strain are estimated from the intercept and slope of the best-fit line of the plot respectively. The positive slopes represent the tensile strains. Table 1 contains the obtained crystallite size and strain values for all compositions. The findings showed that the strain of the synthesized samples decreased with an increasing Fe substitution.
3.3 HRTEM
High-resolution transmission electron microscopy is employed to visualize atomic level details and lattice fringes in high resolution depicted in Fig. 3(a) which authenticates the crystalline nature of the synthesized samples of LNMO double perovskites [28]. The interplanar spacing (d) is found 0.249 nm for the plane (-101) for the pure LNMO sample which is also harmonious with XRD analysis. Figure 3(b) depicts selected area electron diffraction patterns (SAED) for the LNMO double perovskite sample. The well-defined sharp diffraction spots observed in the pattern confirm the high degree of purity of the prepared sample.
3.4 X-ray photoelectron spectroscopy (XPS)
XPS is an extensively utilized technique based on the photoelectric effect commonly employed for surface analysis [29]. The energy of electrons gets completely transferred to core-level electrons when the surface of the sample specimen is bombarded with high-energy X-rays and the kinetic energy of these electrons is measured. Distinct chemical states of the constituent elements that are present in the sample can be detected with the help of this technique [30]. Also, it is suitable for scrutinizing the surface sensitivity of the synthesized samples. The full scan spectrum of the synthesized LNMO double perovskite sample is depicted in Fig. 4(a). It confirms the presence of the constituent elements. Figure 4(b) shows spectra related to La 3d and confirms the presence of La3+ along with the multiplet splits of 3d5/2 and 3d3/2. Figure 4(c) depicts spectra corresponding to Ni 2p, where the peaks detected after deconvolution confirm the presence of both Ni2+ and Ni3+ species along with a satellite peak observed around binding energy value 871.6 eV. The peaks spotted at binding energy values 850.8 eV and 854.2 eV correlate with Ni 2p1/2 whereas peaks around 861.1 eV and 864.7 eV correspond to Ni 2p3/2. Figure 4(d) shows the spectra of Mn 2p, where after deconvolution of the spectra four peaks are detected with no observed satellite peak. The peaks spotted at binding energy 638.5 eV and 650.2 eV are correlated with Mn3+ while at 640.5 eV and 652.5 eV corresponds to Mn4+. Figure 4(e) displays spectra for O 1s and deconvolution suggested the presence of two peaks around binding energy values, 526.4 eV attributing to surface lattice oxygen and 528.5 eV which implies towards absorbed oxygen.
3.5 Dielectric constant and tangent loss
Figure 5(a) shows the frequency dependence of dielectric constant for all the synthesized samples at 303 K and Fig. 5(b) represents the LNFMO2 sample at different temperatures ranging from 323 K to 423 K. Dielectric constant shows a decreasing trend for all samples as frequency increases [31]. The behavior of both parameters was constant towards high frequency whereas a dispersive behavior was noticed at low frequency. Koop’s theory [32] and Maxwell-Wagner effect [33] well explained the frequency variation of dielectric constant. The different polarization mechanisms such as electronic, ionic, dipolar, and interfacial or space polarization are the main contributors to the dielectric response of a material at different frequencies. At low frequencies, almost all mechanisms promote dielectric response [34] whereas, at high frequencies, electronic polarization was the only main contributor to the dielectric constant [35]. Koop’s theory is based on the assumption that the dielectrics are comprised of grains with a high conducting nature and grain boundaries with a relatively lower conducting nature. The grain boundaries contribute more to the dielectric constant in the low-frequency region, while grains have more contribution in the high-frequency region [32]. The region along the grain boundary gets polarized when an electric field is applied that can be attributed to available charged carriers. In the low-frequency range, the grain boundaries offer more resistance to charge carriers, resulting in charge accumulation at interfaces of grains and grain boundaries and developing space charges [36, 37]. These space charges alter the field distribution and, consecutively the dielectric constant and loss. A high space charge polarization along grain boundaries increases the dielectric constant at low frequencies [38]. The crystalline structure especially cations ordering can also play a great role in explaining the dielectric behavior of prepared double perovskites. The polar regions having an intense localized polarizing effect can be formed in the samples due to Ni2+ and Mn4+ ordering [2]. The charge carriers hop among Ni and Mn ions in an externally applied electric field due to which polar regions get reversed and enhanced dielectric response was observed in prepared samples. The charge carriers become delocalized as the temperature rises which leads to an increase in the hopping rate between ions and subsequently boosts the polar regions reversal which further enhances the dielectric response as well as corresponding AC conductivity and tangent loss peaks. In the higher frequency region, the polar regions unfollow the applied field due to which the dielectric constant declines. In addition to this, the dropping point temperature of the dielectric constant also shifts to lower temperatures as frequency decreases and the dielectric constant for the prepared samples has a relaxor-type dispersive behavior at lower frequencies. LNFMO1 sample was observed with a higher dielectric constant than LNMO which can be attributed to the existence of a relatively higher cationic disorder which intensifies the dielectric performance of a material [39]. However, the dielectric constant for the LNFMO2 sample decreases dramatically despite an increase in disorder.
(a) Variation of dielectric constant with frequency for all synthesized samples at 303 K (b) Variation of dielectric constant with frequency for LNFMO2 at different temperatures (c) Variation of tangent loss with frequency for all synthesized samples at 303 K (d) Variation of tangent loss with frequency for LNFMO2 at different temperatures
The dissipation of intrinsic energy of the synthesized samples is represented by tangent loss (tanδ) [40]. Figure 5(c) & (d) shows the frequency variation of tangent loss for the developed DPs at 303 K and for LNFMO2 in the temperature range from 323 K to 423 K. Tangent loss exhibits a similar trend as shown by the dielectric constant. Koop’s theory can also be applied to understand the variation of tangent loss, where at low frequencies the charged carrier needs a large amount of energy to overpower the opposition because of highly resistive grain boundaries which results in larger values of tangent loss. Whereas at higher frequencies, the charged carrier needs a lesser amount of energy and therefore tangent loss decreases [32]. The temperature variation of ε′ as well as tanδ for the LNFMO2 sample at different frequencies is exhibited in Fig. 6(a) & (b). ε′ and tanδ both were increased with temperature rise. The increased ε′ with temperature might be because of an increase in electron-phonon interactions at higher temperatures. A sudden rise in tangent loss at higher temperatures can be attributed to the scattering of charged carriers due to thermal activation. The increase in tangent loss at high temperatures can also be attributed to the domination of conductivity [41].
3.6 Complex impedance
Figure 7(a) depicts the dependence of Z′′ (an imaginary component of impedance) versus logf for LNFMO2 at different values of temperature. The decreasing trend of Z′′ towards higher temperatures indicates to enhancement in conductivity. It can be attributed to an increase in the hopping rate as a result of thermal activation. The observed peak in the plot at a certain frequency (called relaxation frequency, fr), signifies the occurrence of relaxation in the sample. The low-temperature relaxation can be attributed to inactive charge carriers while high-temperature relaxation occurs because of defects and vacancies present in the dielectric materials [41]. The dielectrics get polarized due to induced dipoles when an electric field is applied and the diploes delay while realigning with a change in the field’s direction. Such type of delay time is recognized as relaxation time (1/fr) and it reduces with temperature increase which highlights the temperature-dependent relaxation process in samples. As temperature increases, the peak shifts towards higher frequency which can be attributed to relaxation-time. At a higher frequency (f > fr) i.e. frequency of the applied field exceeds relaxation frequency, induced dipoles stop following the applied field as a result lower dielectric constant was observed towards high frequency [42].
(a) Frequency dependence of imaginary part of impedance (Z″) for LNFMO2 at different temperatures (b) Frequency dependence of imaginary part of electric modulus (M″) for LNFMO2 at different temperatures (c) Master curve of imaginary part of electric modulus (M″) for LNFMO2 at different temperatures (d) Combined variation of imaginary impedance (Z″) and imaginary electric modulus (M″) for LNFMO2 at 423 K
3.7 Equivalent circuits
The cole-cole plots and equivalent circuits provide a better understanding of grain and grain boundary effects on the conduction process [43]. The cole-cole plots for all the compositions at 303 K are depicted in Fig. 8(a) and the LNFMO2 sample at different temperature values are depicted in Fig. 8(b). The observed cole-cole plots comprise depressed semicircular arcs with the centre lying below Z′-axis (real axis) rather than ideal semicircular arcs with the centre lying on Z′-axis. The depression in semicircular arcs indicates non-Debye relaxation in the developed sample system. An imperfect capacitor i.e. constant phase element (CPE) exists instead of a perfect capacitor for such type of depressed semicircular arcs [44]. The depressed semicircular arcs cut intercepts on the Z′-axis which determine Rb (bulk resistance) for the sample. As the temperature increases, the diameter of semicircles decreases which points towards the increased conductivity and decline in Rb values. It suggests that the samples exhibit NTCR (negative-temperature coefficient of resistance) nature.
EC-Lab 10.4 program was utilized to fit equivalent circuits which can be useful for understanding the nature of prepared samples. The equivalent circuits are the combination of resistors (R) and constant phase elements (Q). Here, R is associated with bulk resistance and Q is the replacement for perfect capacitors due to the non-Debye like nature of materials which was related to the dielectric effect at electrodes as a result of space-charge polarization [45]. The cole-cole plots have two semicircles; the large semicircle belongs to grains while the small one belongs to grain boundaries [46]. The equivalent circuit for all synthesized samples consists of two series-connected subcircuits where each subcircuit contains a parallelly connected resistor (R) and CPE (Q) where one subcircuit signifies the grains effect and another one signifies the grain boundary effect. The following equation was utilized for the determination of complex impedance (Z) for such equivalent circuit:
Here, α1 (relaxation time distribution), R1 & Q1 parameters are associated with grains, and α2, R2 & Q2 parameters are associated with grain boundaries. Both α1 & α2 have values amid ~ 0.51 and ~ 1.0 for all the compositions which signify non-Debye like relaxations in samples [47]. Table 2 contains the values of all these parameters determined through fitment of impedance data for all the synthesized samples at 303 K and Table 3 contains the corresponding values for LNFMO2 at different values of temperature. The fitment data and experimental data show a good agreement. The resistances associated with grain boundaries have larger values as compared to corresponding values for grains, however, the capacitance values associated with the grains are larger than that of grain boundaries. The high values for dielectric constant can be attributed to these large values of resistances for grain boundary due to interfacial hindrance for charge carriers [48].
Among the prepared double perovskite samples, LNFMO1 sample was observed with the highest dielectric constant making the sample suitable for energy storage devices such as supercapacitors [49, 50].
3.8 Electric modulus
The modulus analysis is typically used to understand various effects such as polarization effect at electrodes, conduction effect of grain boundary, conductive behavior of a material, relaxation time, etc. Figure 7(b) depicts the plot of M′′ (an imaginary component of the modulus) versus logf for the LNFMO2 sample at distinct temperatures. The variable width of peaks (M′′max) directs towards dispersed relaxation and suggests non-Debye like relaxation [41]. The shifting of peak to the high-frequency region as the temperature rises points towards increases in the hopping rate of ions due to thermal activation. The charge carriers at lower frequencies (f < fr) undergo successfully hopping to neighboring sites because of their long-range mobilities, whereas in the high-frequency zone (f > fr), the charge carriers have short-range mobilities because of their confined movements in potential wells. The peak occurring at the frequency (fr) signifies the relaxation phenomenon and also the point where long-range mobilities switched to short-range mobilities [51]. The plot of M′′/M′′max (a normalized imaginary component of the modulus) versus logf for LNFMO2 at different temperatures is depicted in Fig. 7(c). All the curves overlap to give a sole relaxation curve which highlights towards occurrence of a sole relaxation process for different temperatures in developed materials. The collective plot of both M′′ and Z′′ for the LNFMO2 sample at 423 K is depicted in Fig. 7(d). M′′ represents the relaxation phenomena owing to the smallest capacitance of the sample (i.e. highest conductivity) whereas Z′′ corresponds to the largest resistances of the sample [52]. The occurrence of M′′ and Z′′ peaks at the same frequency suggested that charge carriers with long-range mobilities are involved in relaxation phenomena whereas the occurrence of both peaks at different values of frequency signifies the involvement of short-range mobilities. The combined plot reveals that the M′′ and Z′′ peaks occur at different frequencies for a given temperature which can be confirmed by the short-range order of mobilities for charge carriers and the non-Debye like nature of the sample [41]. Any overlapping temperature was not observed over the full range of measured temperatures which highlights the contributions from localized charge carriers as well as long-range conductivities [35].
3.9 AC conductivity
Figure 9(a) depicts AC conductivity versus frequency plot for the developed DP system at 303 K. The following equation is used to study the variation of conductivity and conduction mechanism involved for all the compositions [53].
Here σ0 is the frequency-independent DC conductivity term and s denotes frequency-exponent which has different values for different materials and represents the intensity of interactions between lattice and mobile ions. A is a constant term that ascertains the polarizability strength.
The AC plot with frequency can be understood by dividing the plot into low and high-frequency regions. The portion of the plot towards lower frequencies signifies the frequency-independent DC term, whereas the portion towards higher frequencies signifies the frequency-dependent conductivity term which has an increasing tendency and shows dispersion [54]. An intensified hopping of charge carriers between localized sites may be responsible for this increasing trend of conductivity which can be explained using the Jump-relaxation model and the Maxwell-Wagner effect [55]. The increase in AC conductivity can also be attributed to enhanced possibility of tunneling of electrons due to thermally oscillating sites [56]. Jonscher’s double power law is used to fit AC conductivity which can be expressed as [57]:
A1ωs1 term, where (0 < s1 < 1) signifies the translational hops which are dominating in the low-frequency region while A2ωs2 term, where (1 < s2 < 2) signifies the localized or re-orientation hopping [57]. Figure 9(b) depicts the AC conductivity variation with frequency along with JPL fitting (using Eq. 8) for the LNFMO2 sample at different temperature values. AC conductivity increases as temperature increases due to an increase in the mobility of electrons with temperature. A similar kind of behavior was also seen for other developed DPs.
Conductivity decreased overall with the introduction of Fe; however, the conductivity may be typically affected by the degree of cations disorder existing in the samples. LNFMO1 was observed to have higher conductivity in comparison with LNMO due to the presence of a relatively higher degree of disordered phase. However, a decrease in conductivity was observed for LNFMO2 despite an increase in the degree of disorderness. This may be attributed to the suppression of double exchange interactions among Ni/Fe-O-Mn [58].
4 Conclusion
La2Ni1 − xFexMnO6 double perovskites samples were synthesized using Sol-gel method. The crystallite size (D) observed an irregular trend, however, strain (ε) decreased with increasing Fe amount in the synthesized samples. HRTEM) with SAED confirmed the crystalline nature and the purity of the synthesized sample. The presence of different oxidation states was confirmed through XPS analysis. The variations in dielectric constant and ac conductivity were significant and showed a decreasing trend with Fe-substitution except for the LNFMO1 sample. LNFMO1 sample (x = 0.2 composition) attained the highest value of dielectric constant (~ 2400 at 1 kHz) and AC conductivity (~ 8 × 10− 2 S/m at 1 kHz) at room temperature among all the synthesized samples, making it suitable for possible applications in energy storage devices, transducers, supercapacitors, electrical tunable devices, etc.
Data availability
The authors state that the data supporting the results of this work are available within this research article. Further, the raw data collected during the present work are available with the corresponding author and may be obtained, if required, upon reasonable request.
References
D.D. Sarma, A new class of magnetic materials: Sr2FeMoO6 and related compounds. Curr. Opin. Solid State Mater. Sci. 5, 261–268 (2001)
Y.Q. Lin, X.M. Chen, X.Q. Liu, Relaxor-like dielectric behavior in La2NiMnO6 double perovskite ceramics. Solid State Commun. 149, 784–787 (2009). https://doi.org/10.1016/j.ssc.2009.02.028
T. Saha-Dasgupta, Double perovskites with 3d and 4d/5d transition metals: compounds with promises. Mater. Res. Express. 7 (2019). https://doi.org/10.1088/2053-1591/ab6293
M.T. Anderson, K.B. Greenwood, G.A. Taylor, K.R. Poeppelmeiert, B-cation arrangements in double perovskites. Prog. Solid State Chem. 22, 197–233 (1993)
M. Dhilip, N.A. Devi, J.S. Punitha, V. Anbarasu, K.S. Kumar, Conventional synthesis and characterization of cubically ordered La2FeMnO6 double perovskite compound. Vacuum. 167, 16–20 (2019). https://doi.org/10.1016/j.vacuum.2019.05.028
Q. Li, L. Xing, M. Xu, Magnetic properties, resistivity and magnetoresistance effects of double perovskite La2Co1 – xFexMnO6. J. Alloys Compd. 710, 771–777 (2017). https://doi.org/10.1016/J.JALLCOM.2017.03.331
E. Burzo, I. Balasz, M. Valeanu, I.G. Pop, The effects of thermal treatment on the physical properties of Sr2FeMo1 – xMxO6 perovskite with M = W, Ta and x ≤ 0.3. J. Alloys Compd. 509, 105–113 (2011). https://doi.org/10.1016/J.JALLCOM.2010.08.126
T. Sarkar, K. Manna, S. Elizabeth, Investigation of multiferroicity, spin-phonon coupling, and unusual magnetic ordering close to room temperature in LuMn ARTICLES YOU MAY BE INTERESTED IN. J. Appl. Phys. 121, 84102 (2017). https://doi.org/10.1063/1.4977103
N.S. Rogado, J. Li, A.W. Sleight, M.A. Subramanian, Magnetocapacitance and magnetoresistance near room temperature in a ferromagnetic semiconductor: La2NiMnO6. Adv. Mater. 17, 2225–2227 (2005). https://doi.org/10.1002/adma.200500737
C.L. Bull, D. Gleeson, K.S. Knight, Determination of B-site ordering and structural transformations in the mixed transition metal perovskites La2CoMnO6 and La2NiMnO6, Journal of Physics: Condensed Matter 15 (2003) 4927–4936. http://iopscience.iop.org/0953-8984/15/29/304
F.N. Sayed, S.N. Achary, O.D. Jayakumar, S.K. Deshpande, P.S.R. Krishna, S. Chatterjee, P. Ayyub, A.K. Tyagi, Role of annealing conditions on the ferromagnetic and dielectric properties of La2NiMnO6. J. Mater. Res. 26, 567–577 (2011). https://doi.org/10.1557/jmr.2011.4
I. Dass, J.Q. Yan, B. Goodenough, Oxygen stoichiometry, ferromagnetism, and transport properties of La2-xNiMnO6 + δ. Phys. Rev. B Condens. Matter Mater. Phys. 68 (2003). https://doi.org/10.1103/PhysRevB.68.064415
J. Blasco, M.C. Sa Ânchez, J. Pe, J. Ârez-Cacho, G. Garcõ Âa, J. Subõ Âas, Campo, Synthesis and structural study of LaNi 12x Mn x O 31d perovskites. J. Phys. Chem. Solids. 63, 781–792 (2002). www.elsevier.com/locate/jpcs
K. Garello, E. Benevent, J.P. Michel, D. Cros, B. Viala, Magnetodielectric thin film heterostructure with high permeability and permittivity. IEEE Trans. Magn. 45, 4325–4328 (2009). https://doi.org/10.1109/TMAG.2009.2025182
D. Choudhury, P. Mandal, R. Mathieu, A. Hazarika, S. Rajan, A. Sundaresan, U.V. Waghmare, R. Knut, O. Karis, P. Nordblad, D.D. Sarma, Near-room-temperature colossal magnetodielectricity and multiglass properties in partially disordered La 2NiMnO 6. Phys. Rev. Lett. 108 (2012). https://doi.org/10.1103/PhysRevLett.108.127201
P. Padhan, H.Z. Guo, P. Leclair, A. Gupta, Dielectric relaxation and magnetodielectric response in epitaxial thin films of La2NiMnO6. Appl. Phys. Lett. 92 (2008). https://doi.org/10.1063/1.2832642
M.H. Tang, J.W. Hou, J. Zhang, G.J. Dong, W. Shu, The giant dielectric tunability effect in bulk La2NiMnO 6 around room temperature. Solid State Commun. 150, 1453–1456 (2010). https://doi.org/10.1016/j.ssc.2010.05.029
Y.Q. Lin, X.M. Chen, Dielectric relaxation and polaronic conduction in double perovskite La 2 MgMnO6. Appl. Phys. Lett. 96 (2010). https://doi.org/10.1063/1.3377906
G. Jagadish Kumar, E. Senthil Kumar, M. Navaneethan, K. Kamala, Bharathi, Exchange bias, magnetic, and dielectric properties of La2FeMnO6 nanoparticles. J. Mater. Sci.: Mater. Electron. 33, 8467–8472 (2022). https://doi.org/10.1007/s10854-021-06343-1
M. ’Nasir, A.K. ’ ’Pathak, J. ’Kubik, D. ’Malaviya, V. ’Krupa, A. ’Dasgupta, S. ’Sen, Enhanced B-site cation ordering and improved magnetic properties: role of A-site Bi substitution in La2NiMnO6 double perovskites. J. Alloys Compd. 896, 162713 (2022)
Y. Guo, L. Shi, S. Zhou, J. Zhao, W. Liu, Near room-temperature magnetoresistance effect in double perovskite La2NiMnO6. Appl. Phys. Lett. 102, 222401 (2013). https://doi.org/10.1063/1.4808437
A. Hooda, M.S. Rathore, Role of Fe supplantation in tailoring structural, electrical conductivity, and magnetic properties in La2Ni1-xFexMnO6 double perovskites. Phys. B Condens. Matter. 691, 416319 (2024). https://doi.org/10.1016/j.physb.2024.416319
N. Ahmad, S. Khan, Effect of (Mn-Co) co-doping on the structural, morphological, optical, photoluminescence and electrical properties of SnO2. J. Alloys Compd. 720, 502–509 (2017). https://doi.org/10.1016/j.jallcom.2017.05.293
V.D. Mote, Y. Purushotham, B.N. Dole, Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. J. Theoretical Appl. Phys. 6, 6 (2012). https://doi.org/10.1186/2251-7235-6-6
S. Sharma, N. Ahmad, S. Khan, Effect on structural, optical, electrical, and magnetic properties of ce and Ni co-doped SmFeO3 nanostructures. J. Mater. Sci.: Mater. Electron. 34, 476 (2023). https://doi.org/10.1007/s10854-023-09917-3
A. Somvanshi, S. Husain, S. Manzoor, N. Zarrin, N. Ahmad, B. Want, W. Khan, Tuning of magnetic properties and multiferroic nature: case study of cobalt-doped NdFeO3. Appl. Phys. A 127, 174 (2021). https://doi.org/10.1007/s00339-021-04329-8
S. Aarif, U. Islam, M. Ikram, Structural stability improvement, Williamson Hall analysis and band-gap tailoring through A-site Sr doping in rare earth based double perovskite La 2 NiMnO 6. Rare Met. 38, 805–813 (2019). https://doi.org/10.1007/s12598-019-01207-4
A.M. Fahim, R.E. Abouzeid, S.A. Al Kiey, S. Dacrory, Development of semiconductive foams based on cellulose- benzenesulfonate/CuFe2O4- nanoparticles and theoretical studies with DFT/ B3PW91/LANDZ2 basis set. J. Mol. Struct. 1247, 131390 (2022). https://doi.org/10.1016/j.molstruc.2021.131390
A.U. Rehman, A.M. Afzal, M.W. Iqbal, M. Ali, S.M. Wabaidur, E.A. Al-Ammar, S. Mumtaz, E.H. Choi, Highly efficient and stable layered AgZnS@WS2 nanocomposite electrode as superior charge transfer and active redox sites for energy harvesting device. J. Energy Storage. 71, 108022 (2023). https://doi.org/10.1016/j.est.2023.108022
A. Yasmeen, A.M. Afzal, M.W. Iqbal, A. Zaka, H. ul Hassan, T. Abbas, M. Usman, L. Wang, Y.M. Alanazi, S. Mumtaz, Enhanced the Stability and Storage Capability of Sulfide-based material with the incorporation of Carbon Nanotube for High-Performance Supercapattery device. J. Electrochem. Energy Convers. Storage. 21 (2023). https://doi.org/10.1115/1.4062642
W. Li, Z. Ma, L. Gao, F. Wang, Preparation and electrical properties of La0.9Sr0.1TiO3 + δ. Materials. 8, 1176–1186 (2015). https://doi.org/10.3390/ma8031176
C.G. Koops, On the dispersion of resistivity and dielectric constant of some semiconductors at audiofrequencies. Phys. Rev. 83, 121–124 (1951). https://doi.org/10.1103/PhysRev.83.121
R. Das, R.N.P. Choudhary, Studies of structural, dielectric relaxation and impedance spectroscopy of lead-free double perovskite: Dy2NiMnO6. J. Mater. Sci.: Mater. Electron. 29, 19099–19110 (2018). https://doi.org/10.1007/s10854-018-0036-7
R. Das, R.N.P. Choudhary, Studies of electrical, magnetic and leakage-current characteristics of double perovskite: Dy2CoMnO6. J. Alloys Compd. 853 (2021). https://doi.org/10.1016/j.jallcom.2020.157240
K. Sultan, M. Ikram, K. Asokan, Effect of Mn doping on structural, morphological and dielectric properties of EuFeO3 ceramics. RSC Adv. 5, 93867–93876 (2015). https://doi.org/10.1039/c5ra20514j
S. Halder, S. Bhuyan, R.N.P. Choudhary, Structural, dielectric and electrical characteristics of lead-free electro-ceramic:Bi(Ni2/3Ta1/3)O3, Engineering Science and Technology. Int. J. 22, 376–384 (2019). https://doi.org/10.1016/j.jestch.2018.11.007
M. Zaid, Y. Altowairqi, S.S. Majid, A. Somvanshi, M. Shariq, S.K. Ali, S.S. Ashraf, A. Khan, N. Ahmad, Comparative structural, optical, and dielectric studies of Zn1 – xMnx/2Ax/2O (A = Ni, Co and x = 0.24) nanoparticles. Appl. Phys. A 128, 1002 (2022). https://doi.org/10.1007/s00339-022-06138-z
S.A. Ul Islam, F.A. Andrabi, F. Mohmed, K. Sultan, M. Ikram, K. Asokan, Ba doping induced modifications in the structural, morphological and dielectric properties of double perovskite La2NiMnO6 ceramics. J. Solid State Chem. 290 (2020). https://doi.org/10.1016/j.jssc.2020.121597
A.J. Barón-González, C. Frontera, J.L. García-Mũoz, B. Rivas-Murias, J. Blasco, Effect of cation disorder on structural, magnetic and dielectric properties of La2MnCoO6 double perovskite. J. Phys. Condens. Matter. 23 (2011). https://doi.org/10.1088/0953-8984/23/49/496003
S.A. Al Kiey, S.A.M. Abdel-Hameed, M.A. Marzouk, Influence of Transition metals on the development of Semiconducting and low thermal expansion TiO2-Borosilicate glasses and Glass ceramics. Silicon. 16, 2945–2953 (2024). https://doi.org/10.1007/s12633-024-02896-y
N. Panda, B.N. Parida, R. Padhee, R.N.P. Choudhary, Dielectric and Electrical properties of the double Perovskite PbBaBiNbO6. J. Electron. Mater. 44, 4275–4282 (2015). https://doi.org/10.1007/s11664-015-3939-6
S. Sil, J. Datta, M. Das, R. Jana, S. Halder, A. Biswas, D. Sanyal, P.P. Ray, Bias dependent conduction and relaxation mechanism study of Cu5FeS4 film and its significance in signal transport network. J. Mater. Sci.: Mater. Electron. 29, 5014–5024 (2018). https://doi.org/10.1007/s10854-017-8463-4
N. Hooda, R. Sharma, A. Hooda, S. Khasa, Enhanced dielectric, magnetic and magnetoelectric properties in 0.5BaFe12O19 - xBaTiO3 - (0.5-x)CoFe2O4 composites. Ceram. Int. 50, 12059–12073 (2024). https://doi.org/10.1016/j.ceramint.2024.01.109
R. Das, R.N.P. Choudhary, Structural, electrical, and leakage-current characteristics of double perovskite: Sm2CoMnO6. Appl. Phys. Mater. Sci. Process. 125 (2019). https://doi.org/10.1007/s00339-019-3163-y
A. Darwish, N.S. El-Sayed, S.A. Al Kiey, S. Kamel, G. Turky, Polyanionic electrically conductive superabsorbent hydrogel based on sodium alginate-g-poly (AM-co-ECA-co-AMPS): Broadband dielectric spectroscopy investigations. Int. J. Biol. Macromol. 232, 123443 (2023). https://doi.org/10.1016/j.ijbiomac.2023.123443
P.G.R. Achary, A.A. Nayak, R.K. Bhuyan, R.N.P. Choudhary, S.K. Parida, Effect of cerium dopant on the structural and electrical properties of SrMnO3 single perovskite. J. Mol. Struct. 1226 (2021). https://doi.org/10.1016/j.molstruc.2020.129391
M. Manjusha, K.L. Rawat, Yadav, Structural, dielectric, ferroelectric and magnetic properties of (x) CoFe 2 O 4 -(1-x) BaTiO 3 composite. IEEE Trans. Dielectr. Electr. Insul. 22, 1462–1469 (2015). https://doi.org/10.1109/TDEI.2015.004774
S. Dagar, A. Hooda, S. Khasa, M. Malik, Structural refinement, investigation of dielectric and magnetic properties of NBT doped BaFe12O19 novel composite system. J. Alloys Compd. 826, 154214 (2020). https://doi.org/10.1016/j.jallcom.2020.154214
A.U. Rehman, A.M. Afzal, M.W. Iqbal, S.M. Wabaidur, E.A. Al-Ammar, S. Mumtaz, E.H. Choi, M. Ali, Exploring the potential of hydrothermally synthesized AgZnS@Polyaniline composites as electrode material for high-performance supercapattery device. Phys. Scr. 98, 085009 (2023). https://doi.org/10.1088/1402-4896/ace560
M. Imran, A.M. Afzal, M.W. Iqbal, H.H. Hegazy, M.Z. Iqbal, S. Mumtaz, R. Qureshi, Manganese (Sulfide/Oxide) based electrode materials advancement in supercapattery devices. Mater. Sci. Semicond. Process. 158, 107366 (2023). https://doi.org/10.1016/j.mssp.2023.107366
D.K. Mahato, T.P. Sinha, Electrical conductivity and dielectric relaxation in Pr2CoZrO6 double perovskite. J. Alloys Compd. 634, 246–252 (2015). https://doi.org/10.1016/j.jallcom.2015.01.098
R.S. Yadav, I. Kuřitka, J. Vilcakova, P. Urbánek, M. Machovsky, M. Masař, M. Holek, Structural, magnetic, optical, dielectric, electrical and modulus spectroscopic characteristics of ZnFe2O4 spinel ferrite nanoparticles synthesized via honey-mediated sol-gel combustion method. J. Phys. Chem. Solids. 110, 87–99 (2017). https://doi.org/10.1016/j.jpcs.2017.05.029
A. Jonscher, The ‘universal’ dielectric response. Nature. 267, 673–679 (1977). https://doi.org/10.1038/267673a0
R. Sharma, N. Hooda, A. Hooda, S. Khasa, Enhanced dielectric and magnetic properties of magnetodielectric La2 – xPrxCoMnO6 for spintronic applications. J. Alloys Compd. 965, 171394 (2023). https://doi.org/10.1016/j.jallcom.2023.171394
S. Karmakar, H.S. Mohanty, D. Behera, Exploration of alternating current conduction mechanism and dielectric relaxation with Maxwell–Wagner effect in NiO–CdO–Gd2O3 nanocomposites. Eur. Phys. J. Plus. 136 (2021). https://doi.org/10.1140/epjp/s13360-021-01998-5
A. Jamil, M.F. Afsar, F. Sher, M.A. Rafiq, Temperature and composition dependent density of states extracted using overlapping large polaron tunnelling model in MnxCo1 – xFe2O4 (x = 0.25, 0.5, 0.75) nanoparticles. Phys. B Condens. Matter. 509, 76–83 (2017). https://doi.org/10.1016/j.physb.2017.01.005
C. Léon, A. Rivera, A. Várez, J. Sanz, J. Santamaria, K.L. Ngai, Origin of constant loss in ionic conductors. Phys. Rev. Lett. 86, 1279–1282 (2001). https://doi.org/10.1103/PhysRevLett.86.1279
X. Yang, J. Chen, D. Panthi, B. Niu, L. Lei, Z. Yuan, Y. Du, Y. Li, F. Chen, T. He, Electron Doping of Sr 2 FeMoO 6-: δ as high performance anode materials for solid oxide fuel cells. J. Mater. Chem. Mater. 7, 733–743 (2019). https://doi.org/10.1039/c8ta10061f
Funding
The authors declare that no funds or grants were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Amit Hooda: Conceptualization, methodology, data collection, investigation, formal analysis, Writing-original draft, data curation, software. Mahendra Singh Rathore: Supervision and writing- review & editing.
Corresponding author
Ethics declarations
Ethical approval
This study does not contain any human-related information and no animals were used. This work did not require any ethical approval. The work is completely original based on the research carried out by authors.
Consent for publication
All authors declare their consent for publication of the manuscript.
Conflict of interest
The authors declare that they have no any competing financial interest or personal relationship that affects this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hooda, A., Rathore, M. Dielectric tunability of La2Ni1-xFexMnO6 double perovskites for energy storage applications. Appl. Phys. A 130, 666 (2024). https://doi.org/10.1007/s00339-024-07829-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-024-07829-5