Abstract
A variety of combinations of Y2O3 and Al2O3 were used as sintering aids in the fabrication of Si3N4 ceramics via gas pressure sintering (GPS). Based on the prediction of the residual thermal stress at the interface, the influence of crystal phases on the wear properties of Si3N4 ceramics was analyzed. As a result, with the increase of Y2O3 addition from 1 to 9 wt%, there are four kinds of crystal phases being firmly discovered in the sample, Y2Si2O7, YSiO2N, Y4Si2O7N2, and Y2Si3O3N4. The crystal phases of Y2Si2O7, YSiO2N, and Y4Si2O7N2 inhibit the particle flaking during the friction process, which greatly inhibited the progress of the abrasive wear, and optimize the wear resistance of silicon nitride ceramics. According to Selsing's model, it can be predicted that the interface residual thermal stress caused by the intergranular phase Y2Si3O3N4 is 22–35% higher than that caused by the other three intergranular phases. The crystal phase Y2Si3O3N4 intensifies the shedding of grains during wear, and reduces the wear performance of silicon nitride ceramics. In addition, the silicon nitride ceramics with 5wt% Y2O3 show better wear resistance, and the wear rate is 1.8 × 10−6 mm3 N−1 m−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Si3N4 is an all-purpose ceramic with desirable mechanical properties, including excellent strength and low creep at high temperatures, thermal shock resistance, wear resistance, corrosion resistance, and appreciable theoretical thermal conductivities, which is ideal material for the preparation of wear-resistant basic parts [1,2,3]. At the same time, wear becomes the main reason for the failure of these components. Substantial efforts have been made to improve the wear resistance of silicon nitride ceramics [4,5,6,7]. Previous research has indicated that the wear properties of silicon nitride ceramic are greatly affected by the hardness ratio between the abrasive particles and the material matrix, and the specific wear rate is maximum when the abrasive particles are harder than the matrix [8]. Since the β-Si3N4 particles (20 Gpa) that exfoliated during the wear process are much harder than the Si3N4 (16–17 Gpa) ceramic matrix, the effective ways to improve the wear properties of silicon nitride ceramics are controlling the interfacial debonding. Francisco found that the existence of residual thermal stress at the interface promoted interface debonding [9]. Xue et al. [10] found that the residual thermal stress originating from the thermal expansion coefficient mismatches between the β-Si3N4 and the crystal phase.
Y2O3 is the most frequently used sintering aid in investigating and exploring the mechanical properties of silicon nitride. Quaternary Y–Si–O–N compounds are the frequently detected crystal phase in investigating Si3N4 ceramic when Y2O3 is used as sintering additive. It has been reported that there are six quaternary crystal phase being firmly discovered in the Y–Si–O–N family: Y2SiO5, Y2Si2O7, Y5Si3O12N, YSiO2N, Y4Si4O7N2, and Y2Si3O3N4 [11, 12]. Moreover, the thermal expansion coefficients of these crystalline phases are obviously different [13,14,15].
However, to the authors’ knowledge, little information is available on the relationship between the wear properties of silicon nitride ceramics and Y2O3. Therefore, the present study explores the wear performance of silicon nitride doped with Y2O3, focusing on the influence of crystal phase on wear performance of silicon nitride ceramics. This study provides technical guidance for optimizing the preparation process of Si3N4 ceramic.
2 Estimation model of interface residual thermal stress
2.1 Generation of interface residual thermal stress
Figure 1 shows the transformation process of the α phase to the β phase in Si3N4. SiO2 is an inevitable phase in Si3N4 powders. The sintering aid reacted with the SiO2 film on the surface of the Si3N4 powder in an oxidation–reduction manner, thereby removing SiO2 and generating a liquid phase. α-Si3N4 dissolves in the liquid phase generated by the sintering aid, and when the solution reaches saturation, it precipitated out again as β-Si3N4 and existed in the form of rod-like β-Si3N4 after cooling. In this process, the residual thermal stress caused by thermal expansion mismatch between crystal phase and β-Si3N4 may promote interface debonding of Si3N4 ceramics.
2.2 Thermal expansion coefficient of crystal phase
The thermal expansion coefficient of each crystal phase in the SiO2–Y2O3–Si3N4 system was calculated using the bond valence model. For a multicomponent compound system with n types of binary systems, its thermal expansion coefficient can be obtained on the basis of the definition by the following equation [16]:
\(v_{l}^{\mu }\) and \(\alpha_{l}^{\mu }\) can be calculated as
where ɑ is the linear thermal expansion coefficient of the intergranular phase, γ = 23nNÅ2 (electrons)−2, Rij is the length of the bond between the two atoms i and j, b is a universal constant equal to 0.37 Å, R0 is the bond valence parameter, k is the Boltzmann constant, μ represents the bond type in the unit cell, and n represents the number of binary systems in the unit cell. The binary structural units, bond length, and bond valence parameters of the six crystal phases are shown in Table 1.
Figure 2 shows the calculated and experimental values of the thermal expansion coefficients of the six crystal phases of the SiO2–Y2O3–Si3N4 system. The experimental values were obtained from literatures [13,14,15]. Here, all the experimental thermal expansion coefficients are those at or near room temperature in the available literatures. Wherein, the calculated thermal expansion coefficients of crystal phases Y2SiO5, Y2Si2O7, Y5Si3O12N, YSiO2N, Y4Si2O7N2, and Y2Si3O3N4 are 4.1 × 10–6 K−1, 8.1 × 10–6 K−1, 8.07 × 10–6 K−1, 8.46 × 10–6 K−1, 7.8 × 10–6 K−1, and 9.17 × 10–6 K−1, respectively. The calculated values of thermal expansion coefficient were compared with the experimental data, and the good agreement demonstrates the predictive power of Eq. (1).
2.3 Residual thermal stresses
According to Selsing's model [18, 19], the interfacial thermal residual stress between the crystal phase and silicon nitride could be calculated by
where,\(\sigma_{{\text{r}}}\), \(\Delta \alpha\), \(\Delta T\), \(v,\) and \(E\) are the interfacial thermal residual stress, difference in coefficient of thermal expansion (CTE), temperature range over which stress is not relieved, Poisson's ratio, and elastic modulus, respectively. The subscripts 1 and 2 refer to the crystal phase and silicon nitride, respectively. The physical properties of the β-Si3N4 and the crystal phase are shown in Table 2.
Figure 3 shows the residual thermal stress at the interface between crystal phase and β-Si3N4. The residual thermal stress around crystal phases of Y2SiO5, Y2Si2O7, Y5Si3O12N, YSiO2N, Y4Si2O7N2, and Y2Si3O3N4 are 37.53 MPa, 453.23 MPa, 524.44 MPa, 529.25 MPa, 442.64 MPa, and 678.4 MPa, respectively. The residual thermal stress of the samples with introducing crystal phases of Y2Si2O7, Y4Si2O7N2, Y2SiO5, Y5Si3O12N, and YSiO2N shows a continuous upward trend. At the same time, the generation of the crystal phase Y2Si3O3N4 significantly increases the residual thermal stress at the interface, which may aggravate interface peeling during the wear process and reduce the wear performance of silicon nitride ceramic.
3 Materials and methods
3.1 Raw materials and experimental procedure
Si3N4, (E10 α-Si3N4, UBE, Japan), Y2O3, and Al2O3 powders (Shanghai Naiou Nanotechnology Co., Ltd.) were used as raw materials in this study. The raw materials were mixed according to the ratios given in Table 3.
The α-Si3N4 powder was mixed with Y2O3 and Al2O3 with agate balls in a nylon jar for 12 h in a planetary mill, using ethanol as a mixing medium. The slurries were dried at 80 °C. The mixed powder was milled and sieved through 80-screen sieve. After being uniaxially dry pressed at 50 MPa and cold isostatically pressed at 230 MPa, the green samples were sintered at 1780 °C under 100 bar nitrogen pressure, followed by furnace cooling down to room temperature (22 °C).
3.2 Characterization
An X-ray diffractometer was used to characterize the phase structure of sintered samples. The step size was set at 0.03° in a diffraction angle range of 20°–50°, and the scanning rate of 1°/min.
The wear properties of the samples were tested using the Rtec multifunctional friction and wear tester (MFT-5000, USA). Polished commercial Si3N4 balls (Sinoma High-tech Nitride Ceramics Co., Ltd.) with a diameter of 5.56 mm were used as friction pairs. At room temperature (22 ℃), the friction pairs moves is a reciprocating relative motion. The load, stroke, and frequency of reciprocation were all set to 40 N, 4 mm, and 3 Hz, respectively.
After the wear test, the worn surface morphology of the sample was observed using scanning electron microscopy (SEM).
Meanwhile, the depth of the worn surface was measured using laser confocal microscopy.
Finally, a VHX6000 ultradepth-of-field 3D contour graph (Keyence Corporation, Japan) was used to measure the topography profile curves of worn after the test. Then, using the formula k = A/2(P × f × t) to calculate wear rates. A is the area of topography profile of worn surface which determined by ultradepth-of-field 3D contour graph, P is the applied load, f is sliding frequency, and t is total sliding time.
4 Results and discussion
4.1 Phase composition
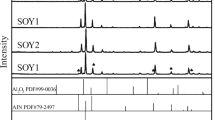
Figure 4 shows the XRD patterns obtained for the as-sintered samples with different Y2O3 content. It can be seen that all main peaks are characteristic β-Si3N4 peaks, which indicates that the α-phase has been completely transformed into the β phase during the sintering. For Y2O3 content of 1 wt%, no other peaks were observed except for the characteristic β-Si3N4 peaks. For the sample with 3 wt% Y2O3, in addition to the β-phase peaks, low-intensity peaks characteristic for Y2Si2O7 were detected. In the samples with 5 wt% Y2O3, the Y2Si2O7 and YSiO2N phase was found. For Y2O3 content of 7 and 9 wt%, the Y4Si2O7N2 phase was found.
The chemical reactions for the formation of the Y2Si2O7, YSiO2N, Y4Si2O7N2, and Y2Si3O3N4 phases are as follows [23,24,25,26]:
It can also be seen from the above that when the ratio of Y2O3/SiO2 is 1/2, 2/1, 4/1, and 1/0, the corresponding crystal phases are Y2Si2O7, YSiO2N, Y4Si2O7N2, and Y2Si3O3N4, respectively.
4.2 Wear behavior
Figure 5 shows the wear surface morphologies of the samples. When the Y2O3 content was 1%, recurrent loads induced surface fatigue and crack, as shown in Fig. 5a. As the wear progressed, the cracks propagated and the scale debris accumulation layer were exfoliated, and then, a new surface was exposed. The surface morphology of the Si3N4 ceramics with 3wt% and 5wt% Y2O3 after Wear test is shown in Fig. 5b, c, respectively. The wear debris adhered to each other during the wear process. Most area of the surface remains smooth, and surface fatigue and crack occurred in a few isolated areas. When the Y2O3 content increased to 7%, wear took away the debris accumulation layer to expose a new surface, and Si3N4 particles’ exfoliation resulted in the wear intensified, as shown in Fig. 5d. When the Y2O3 content increased to 9%, accompanied by large debris accumulation layer removed, the wear surface has the appearance of a typical fracture surface of silicon nitride, indicating a possible intergranular fracture, as shown in Fig. 5e. This observation suggests that interface peeling is the predominant mode of wear.
To analyze wear mechanism of Si3N4 ceramics by adding Y2O3, the debris morphology of wear surface was also observed and analyzed. The morphology of the wear debris of the Si3N4 ceramic with 1, 3, and 5 wt% Y2O3 is block-like debris, as shown in Fig. 6a–c. This showed that surface fatigue is the dominant wear mechanism in Si3N4 ceramics. For the Si3N4 ceramic with 7 and 9 wt% Y2O3, the morphology of the wear debris is particle-like debris and β-Si3N4, as shown in Fig. 6d–f. This showed that abrasive wear is the dominant wear mechanism in Si3N4 ceramics. Based on the above analysis, the existence of Y2Si3O3N4 phases caused a large residual thermal stress at the interface, and thus increased the β-Si3N4 fracture of subsurface during the wear process, which greatly promoted the progress of abrasive wear.
4.2.1 Wear surface depth and wear rate
The wear depth and the wear rate of the silicon nitride ceramics are shown in Fig. 7. When the Y2O3 content increased from 1 to 5%, the wear depth decreased from 35.6 μm to 29.8 μm, and the wear rate of the silicon nitride ceramics decreased from 2.5 × 10−6 mm3 to 1.8 × 10−6 mm3 N−1 m−1. The wear depth and wear rate increased by 35.2% and 53.8% after the Y2O3 content increased from 5 wt% to 9 wt%. Obviously, the silicon nitride ceramics with 5wt% Y2O3 shows better wear resistance. Since the less residual thermal stress is helpful to improved the fracture toughness of the silicon nitride ceramics [27], thereby inhibiting particle flaking during the friction process. As discussed in Sects. 2.3 and 4.2, the crystal phases of Y2Si2O7, YSiO2N, and Y4Si2O7N2 inhibit the particle flaking during the friction process, which greatly inhibited the progress of the abrasive wear and optimize the wear resistance of silicon nitride ceramics. However, the residual thermal stress at the interface caused by the crystal phase Y2Si3O3N4 was 22–35% higher than that caused by the above three crystal phases. The interface between crystal phase Y2Si3O3N4 and Si3N4 matrix would be debonded due to the excessive residual thermal stress, intensifies the shedding of grains during wear, and reduces the wear performance of silicon nitride ceramics.
5 Conclusions
A variety of combinations of Y2O3 and Al2O3 were used as sintering aids in the fabrication of Si3N4 ceramics via gas pressure sintering (GPS). Based on the prediction of the residual thermal stress at the interface, the influence of crystal phases on the wear properties of Si3N4 ceramics was analyzed. The following conclusions were drawn:
-
1.
The crystal phases, Y2Si2O7, YSiO2N, Y4Si2O7N2, and Y2Si3O3N4, were formed in silicon nitride ceramics during sintering. The interface residual thermal stress caused by the crystal phase Y2Si3O3N4 is 22–35% higher than that caused by the other three crystal phases.
-
2.
The crystal phases of Y2Si2O7, YSiO2N, and Y4Si2O7N2 inhibit the particle flaking during the friction process, which greatly inhibit the progress of the abrasive wear and optimize the wear resistance of silicon nitride ceramics.
-
3.
The interface between crystal phase Y2Si3O3N4 and Si3N4 matrix would be debonded due to the excessive residual thermal stress, intensifies the shedding of grains during wear, and reduces the wear performance of silicon nitride ceramics.
Data and code availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also form part of an ongoing study.
References
L. Wang, R.W. Snidle, L. Gu, Rolling contact silicon nitride bearing technology: a review of recent research. Wear 246, 159–173 (2000)
F. Caretto, A.M. Laera, F. Di Nuzzo, R. Iovino, F. Di Benedetto, E. Pesce, M. Re, M. Schwarz, L. Tapfer, Molybdenum disilicide-silicon nitride bushing nozzles tailor-made for basalt fifibers production. Ceram. Int. 42, 11844–11850 (2016)
R. Danzer, M. Lengauera, W. Zleppnig, W. Harrer, Silicon nitride tools for hot rolling of high-alloyed steel and superalloy wire-load analysis and first practical tests. Int. J. Mat. Res. 98, 1104–1114 (2007)
F. Brenscheidt, S. Oswald, A. Miicklich, E. Wieser, W. Mijller, Wear mechanisms in titanium implanted silicon nitride ceramics. Nucl. Inst. Methods Phys. Res. B 129, 483–486 (1997)
J. Kang, M. Hadfield, The influence of heterogeneous porosity on silicon nitride/steel wear in lubricated rolling contact. Ceram. Int. 26, 315–324 (2000)
A. Kumar, S. Ghosh, Aravindan, Grinding performance improvement of silicon nitride ceramics by utilizing nanoflfluids. Ceram. Int. 43, 13411–13421 (2017)
Q. Tang, J. Chen, L. Liu, Tribological behaviours of carbon fifibre reinforced PEEK sliding on silicon nitride lubricated with water. Wear 269, 541–546 (2010)
L. Wang, Z. Qiao, Q. Qi, Y. Yu, T. Li, X. Liu, Z. Huang, H. Tang, W. Liu, Improving abrasive wear resistance of Si3N4 ceramics with self-matching through tungsten induced tribochemical wear. Wear 494–495, 204254 (2022)
S. Funfschilling, T. Fett, M.J. Hoffmann, Mechanisms of toughening in silicon nitrides: The roles of crack bridging and microstructure. Acta Mater. 59, 3978–3989 (2011)
W. Xue, J. Yi, Z. Xie, W. Liua, J. Chen, Enhanced fracture toughness of silicon nitride ceramics at cryogenic temperatures. Scripta Mater. 66, 891–894 (2012)
I.K. Naik, L.J. Gauckler, T.Y. Tien, Solid-liquid equilibria in the system Si3N4-AIN-SiO2-Al2O3. J. Am. Ceram. Soc. 61, 332–335 (1978)
Y.-N. Xu, P. Rulis, W.Y. Ching, Electronic structure and bonding in quaternary crystal Y3Si5N9O. Phys. Rev. B. 72, 1–4 (2005)
Z. Sun, Y. Zhou, J. Wang, M. Li, Thermal Properties and Thermal Shock Resistance of c-Y2Si2O7. J. Am. Ceram. Soc. 91, 2623–2629 (2008)
Z. Sun, J. Wang, M. Li, Y. Zhou, Mechanical properties and damage tolerance of Y2SiO5. J. Eur. Ceram. Soc. 28, 2895–2901 (2008)
L. Sun, B. Liu, J. Wang, J. Wang, Y. Zhou, Hu. Zijun, Y4Si2O7N2: a new oxynitride with low thermal conductivity. J. Am. Ceram. Soc. 95, 3278–3284 (2012)
X. Liu, H. Wang, W. Wan, Fu. Zhengyi, A prediction model of thermal expansion coeffificient for cubic inorganic crystals by the bond valence model. J. Solid State Chem. 299, 1–13 (2021)
L. Sun, B. Liu, J. Wang, Z. Li, J. Wang, Theoretical study on the relationship between crystal chemistry and properties of quaternary Y-Si-O-N oxynitrides. J. Am. Ceram. Soc. 99, 2442–2450 (2016)
R. Wang, W. Li, D. Li, D. Fang, New temperature dependent fracture strength model for the ZrB2-SiC composites. J. Eur. Ceram. Soc. 35, 2957–2962 (2015)
J. Selsing, Internal stresses in ceramics. J. Am. Ceram. Soci. 44, 419 (1961)
I.M. Peterson, T.-Y. Tien, Effect of the grain boundary thermal expansion coefficient on the fracture toughness in silicon nitride. J Am. Cwam Sac. 78, 2345–2352 (1995)
R. van Weeren, S.C. Danforth, The effect of grain boundary phase characteristics on the crack deflection biehavior in asilicon nitride material. Scripta Mater. 34, 1567–1573 (1996)
Y. Zhou, H. Hyuga, D. Kusano, C. Matsunaga, K. Hirao, Effects of yttria and magnesia on densification and thermal conductivity of sintered reaction-bonded silicon nitrides. J. Am. Ceram. Soc. 102, 1579–1588 (2019)
M. Kitayama, Thermal Conductivity of β-Si3N4: Effect of Lattice Oxygen. J. Am. Ceram. Soc. 83, 1985–1992 (2000)
Z. Luo, W. Liu, Qu. Gao, Lu. Anxian, G. Han, Sintering behavior, microstructure and mechanical properties of various fluorine-containing Y-SiAlON glass-ceramics. J. Non-Cryst. Solids 388, 62–67 (2014)
W. Liu, W. Tong, R. He, Wu. Haidong, Wu. Shanghua, Effect of the Y2O3 additive concentration on the properties of a silicon nitride ceramic substrate. Ceram. Int. 42, 18641–18647 (2016)
W. Wang, D. Yaoa, H. Liang, Y. Xia, K. Zuo, J. Yin, Y.-P. Zeng, Effffect of in-situ formed Y2O3 by metal hydride reduction reaction on thermal conductivity of β-Si3N4 ceramics. J. Eur. Ceram. Soc. 40, 5316–5323 (2020)
L.J. Wang, Q. Qi, X. Yang, Mechanical properties optimization of Si3N4 ceramics by in-situ introduction of core-shell structural W-Fe5Si3. Compos. B Eng. 196, 1–9 (2020)
Acknowledgements
This work was supported by Science and Technology Major Project of Shanxi Province, China (20181102015), and Science and Technology Project of Taiyuan, Unveiled Project of Taiyuan.
Author information
Authors and Affiliations
Contributions
QW and CZ: wrote the main manuscript text; CY: analyzed the data; RH and YY: experiment.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest with others.
Ethics approval
No human tissue or animal subjects were involved in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Q., Zhou, C., Yang, C. et al. Analysis of friction and wear performance of Y2O3-doped Si3N4 ceramic using the estimation of stress. Appl. Phys. A 129, 302 (2023). https://doi.org/10.1007/s00339-023-06485-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06485-5