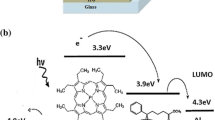
Abstract
In this research, we examined the influence of platinum octaethylporphyrin (PtOEP) concentration on the photovoltaic and impedance spectroscopy of P3HT:PCBM solar cells. Through optimizing the concentration of PtOEP in the blend, the average power conversion efficiency of P3HT:PCBM solar cells was raised from 3.06 to 3.61%, when milligram of PtOEP was added to the blend. According to the impedance spectroscopy, the low concentration of PtOEP significantly increases the recombination process, simultaneously increasing the diffusion coefficient (D) and charge-carrier mobility (μ). These findings suggest that the incorporation of low doses of PtOEP into P3HT:PCBM blends will be a practical method for achieving the high efficiency of polymer solar cells.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Due to their lightweight, low cost, simplicity of large-scale production, and compatibility with flexible substrates, polymer solar cells (PSCs) are the most potent renewable energy source [1]. Numerous attempts have been made recently to improve the performance and reliability of PSCs, including the use of novel materials for greater photon collecting, interface modification for improved charge-carrier selection, and the deployment of novel designs [1,2,3,4]. In addition, a range of procedures, including heat annealing, solvent vapor annealing, and doping processes, have been successfully employed to improve the carrier’s electrical conductivity, shape, and solar cell capacity [4,5,6,7,8]. Following light absorption, photocurrent production occurs in multiple phases, including the creation of excitons, their diffusion to the donor (D)/acceptor (A) interface, their free dissociation, and their transport to the electrodes. Numerous variables contribute to inefficiency. One is recombination at the D/A contact, which prevents excitons from diffusing. As a result, we need to find a strategy to improve the diffusion length in order to optimize the performance of solar cells. It is widely established that phosphorescent dyes or triplet-exciton materials have a greater diffusion length and lifespan. Increase the photocurrent effectively by converting singlet excitons to triplet excitons [9,10,11,12,13]. If singlet excitons can be changed by intersystem crossing into triplet excitons with a longer lifespan, this would benefit exciton dissociation. Using strong atomic effects to boost the performance of polymer solar cells by doping phosphorescent elements such as PtOEP into organic solar cells is a feasible technique. The purpose of this study is to explore the influence of various concentrations of PtOEP, a phosphorescent dye, on the efficiency and impedance spectroscopy (IS) of P3HT:PCBM PSCs.
2 Experimental
2.1 Film and solar cells’ fabrication
PtOEP (Sigma-Aldrich) was added amounts 0.5, 1, 2, 3, and 6 mg per 20 mg mL−1 of P3HT[Mw 50,000–100,000]:PCBM[Mw 910.88] (1:1) (Sigma-Aldrich) and then combined with 1, 2-dichlorobenzene as a solvent. The blends were dynamically stirred at160 rpm under 60 °C for 24 h. To fabricate the solar cell, a layer of poly(3,4-ethylenedioxythiophene)-blend-poly(styrene-sulfonate) (PEDOT:PSS) (Sigma-Aldrich) was spin-coated (at 4000 rpm for 30 s) on top of clean ITO(̴ 10/square) substrates in the air and was dried by thermal annealing using a digitally controlled hotplate at 150 °C for10 min. The P3HT:PCBM:PtOEP blends solution was spin-coated at 1500 rpm for 30 s on the top of insoluble PEDOT:PSS layer with annealing for eliminating any remaining solvent at 150 °C for 5 min under nitrogen N2 atmosphere in a glove box. The Al electrode (~100 nm) was thermally deposited onto the active layer using a vacuum deposition system at a pressure of about 2 × 10−4 Pa through a shadow mask to obtain 20 identical cells on one device with an active area of 0.09 cm2. The complete organic solar cells [ITO/PEDOT:PSS/P3HT:PCBM:PtOEP/Al], with a configuration, are displayed in Fig. 1.
2.2 Measurements
Scanning electron microscopy (SEM, JEOL model JSM-6510) was used to investigate the morphology of the samples at room temperature, and the Raman spectrum was determined using a Raman Spectrometer (Jasco NRS-2100). A spectrophotometer was used to record the optical absorption spectra (JASCO V-570). The external quantum efficiency (EQE) measurements were performed with a halogen lamp and a monochromatic filter. The J/V parameters of the cells were determined using a computer-controlled voltage-current source meter under standard solar irradiation of 100 mW/cm2 (AM1.5 G) at 25 °C (Keithley 238). The IS in the dark was determined at room temperature in the air using an impedance analyzer (Agilent 4284A) at a bias voltage of.
3 Results and discussion
Figure 2a illustrates the effect of PtOEP doping on P3HT:PCBM absorption. In the absorption spectra of the blends, the bands of PtOEP [14, 15] are observed at 374 and 547 nm, respectively. In addition, it is obvious that the peaks for P3HT:PCBM:PtOEP (1 mg) contribute significantly to the formation of a charge-transfer complex (CTC) in the blended films [9]. At concentration (1 mg mL−1) of PtOEP, the polymer degree increase is sensitive to Raman shift C=C [1453 cm−1] stretching to lower wavenumbers [1445 cm−1] (see Fig. 2b) [9, 16]. However, this interaction was diminished at higher PtOEP doping concentrations (6 mg) owing to PtOEP aggregation. However, this interaction is diminished at higher PtOEP doping concentrations (6 mg mL−1) owing to PtOEP aggregation, and SEM images Fig. 3a–c demonstrate the existence of these aggregates. The surface of the active layer’s surface seems to be smooth and homogenous at concentrations (0, 0.5, and 1 mg mL−1) of PtOEP. As the doping percentage increases, PtOEP particles agglomerate on specimen surfaces with varying shapes and sizes. As a result of these aggregates defining isolated zones, charging carriers in such areas would be unable to engage in electrical transport activities effectively.
Figure 4a illustrates the external quantum efficiency (EQE) spectra of the ITO/PEDOT:PSS/P3HT:PCBM:PtOEP (0, 1 and 6 mg mL−1)/Al solar cells are shown in the absorption region for P3HT:PCBM:PtOEP (360–800) nm. Furthermore, at PtOEP (1 mg mL−1), the maximum EQE at 550 nm increases from 49.6 to 56.8%. The effect of PtOEP doping on the efficiency of P3HT:PCBM (1:1) PSCs is seen in Fig. 4b. Table 1 displays the average photovoltaic characteristics for this system P3HT:PCBM:PtOEP (0, 0, 5, 1, 2, 3, and 6 mg mL−1), including open-circuit voltage (VOC), short-circuit current density (JSC), fill factor (FF), and power conversion efficiency (PCE, η%). The obtained results indicate that the VOC, JSC and FF are remarkably improved after adding low concentration of PtOEP. When the dopant concentration is 1 mg mL−1, the J–V curves record the ideal cell parameters, which is compatible with the EQE spectra at the same concentration. In comparison to the reference cell, which had a PCE of 3.06%, the maximum PCE of 3.61% implies a 16.82% improvement. The comparative statistical parameters (including the mean values and standard deviations of JSC and PCE) are also listed in Table 1. However, when the PtOEP ratios are increased (2, 3, and 6 mg mL−1), the photovoltaic performance of the samples decreases, as demonstrated in Table 1 and Fig. 5a, b. The rise in the JSC may be related to an improvement in charge-carrier transfer, which is facilitated by the increasing conjugation length in the P3HT:PCBM blends owing to the PtOEP concentration in the blends. Furthermore, the lower percentage of PtOEP may change excitons from singlet into triplet excitons, resulting in higher exciton diffusion time and solar cell performance [9, 11,12,13, 17]. The improved performance of P3HT:PCBM:PtOEP devices might be attributed to improved morphology for electron and hole transport along the PCBM and P3HT phases. However, at higher PtOEP doping levels, the large quantities of PtOEP form big aggregates within the P3HT:PCBM, decreasing the film adhesion to the ITO substrate. The triplet excitons will be placed or restricted in these isolated areas in the PtOEP aggregation (isolated areas) even though they do not have any effective function. Moreover, these aggregations have a negative impact on solar cell efficiency, resulting in poorer JSC and PCE. The smaller FF also indicates that electron and hole carrier concentrations in systems with high concentrations are lopsided [9, 18]. Under dark conditions, the J–V characteristics of the ITO/PEDOT:PSS/P3HT:PCBM:PtOEP (0, 0, 5, 1, 2, 3, 6 mg mL−1)/Al solar cells are shown in Fig. 4c. When compared to the other additional devices, ITO/PEDOT, PSS/P3HT:PCBM:PtOEP (1 mg mL−1)/Al had the lowest reverse dark current. This indicates that introducing PtOEP (1 mg mL−1) into the active layer may marginally diminish the device’s microscopic leak circuit and surface recombination centers [19]. Impedance spectroscopy is used to validate the photovoltaic properties of P3HT:PCBM:PtOEP (0, 5, 1, 2, 3, 6 mg mL−1). Each Nyquist plot has one semicircle, which designates that there is only one type of interface process [20]. In addition, the equivalent circuit is seen in the inset of Fig. 6a. The Nyquist plot for solar cells fits into a simple circuit, as shown in the inset of Fig. 6a, with the recombination resistance (Rrec) and series resistance (Rs) attributed to ITO and wire contributions [20]. The fitting parameters are reported in Table 2. Furthermore, the width of the Nyquist plot is substantially influenced by the PtOEP concentration. This observation is in accordance with the literature by Hadi et al. [20], Oklobia et al. [21], and Zhang et al. [22]. The bode phase plot (in Fig. 6b) is very valuable for estimation of the diffusion time (τd) of carriers through the active layer, which in turn can be used to compute the diffusion coefficient (D) and charge-carrier mobility (μ) [9, 10] (see Table 1). The lowest values for (Rs, Rrec, and d) and the highest values for D and are observed when 1 mg mL−1 of PtOEP is introduced (see Fig. 6c). It can be assumed that the interface between P3HT and PCBM is more appropriate for charge transport in the case of using a small amount of PtOEP (0.5 and 1 mg mL−1). Therefore, the electron transfer and diffusion length in the active layer is faster and longer, resulting in higher photovoltaic properties [9, 10, 23, 24].
4 Conclusions
In summary, this study clarified the effect of PtOEP (using the blade coating method) as a phosphorescent dye with various doping levels (0.5, 1, 2, 3, and 6 mg mL−1) on photovoltaic efficiency and spectroscopic impedance. We found that P3HT:PCBM devices improved their PCE by 16.82% at P3HT:PCBM:PtOEP (1 mg mL−1) and had the lowest (Rs, Rrec, and d) and highest (D and) values. The IS results indicated that the maximum mobility value and diffusion coefficient were correlated to the maximum photovoltaic properties. Thus, the use of tiny concentrations of PtOEP in P3HT:PCBM blends will be a new strategy to enhance the performance of PSCs.
Data availability
This manuscript has associated data in a data repository. [Authors’ comment: All data included in this manuscript are available upon request by contacting the corresponding author.]
References
C. Lee, S. Lee, G.-U. Kim, W. Lee, B.J. Kim, Chem. Rev. 119, 8028 (2019)
W. Lee, H.D. Lee, J.H. Bae, J.W. Jung, Org. Elect. 39, 85 (2016)
Y. Choia, G. Kima, H. Kima, S.H. Lee, S. Kwon, J. Kima, K. Lee, Nano Energy 30, 200 (2016)
L. Lu, T. Zheng, Q. Wu, A.M. Schneider, D. Zhao, L. Yu, Chem. Rev. 115, 12666 (2015)
W. Aloui, T. Adhikari, J.-M. Nunzi, A. Bouazizi, K. Khirouni, Mater. Sci. Semicond. Process. 39, 575 (2015)
M. Gao, Z. Wang, Mater. Lett. 164, 325 (2016)
Z. Liu, E.-C. Lee, Org. Electron. 24, 101 (2015)
Y.A.M. Ismail, N. Kishi, T. Soga, Thin Solid Films 616, 73 (2016)
A.A. Abuelwafa, M. Dongol, M.M. El-Nahass, T. Soga, J. Mol. Struct. 1202, 127303 (2020)
A.A. Abuelwafa, M. Dongol, M.M. El-Nahass, T. Soga, Appl. Phys. A 124, 266 (2018)
A.A. Abuelwafa, M.S.H. Choudhury, M. Dongol, M.M. El-Nahass, T. Soga, J. Mater Sci: Mater Electron. 29, 4232 (2018)
C. Lee, I. Hwang, C.C. Byeon, B.H. Kim, N.C. Greenham, Adv. Funct. Mater. 20, 2945 (2010)
K. Xiong, L. Hou, P. Wang, Y. Xia, D. Chen, B. Xiao, J. Lumine. 151, 193 (2014)
A.A. Abuelwafa, A. El-Denglawey, M. Dongol, M.M. El-Nahass, T. Soga, J. Alloy. Compd 655, 415 (2016)
A.A. Abuelwafa, A. El-Denglawey, M. Dongol, M.M. El-Nahass, T. Soga, Opt. Mater. 49, 271 (2015)
T.-C. Wang, Y.-H. Su, Y.-K. Hung, C.-S. Yeh, L.-W. Huang, W. Gomulya, L.-H. Lai, M.A. Loi, J.-S. Yanga, J.-J. Wu, Phys. Chem. Chem. Phys. 17, 19854 (2015)
F.A. Angel, C.W. Tang, Org. Electron. 30, 247 (2016)
X. Gong, Y. Jiang, C. Zhang, L. Yang, M. Li, H. Ma, Appl. Phys. A 122, 287 (2016)
C.-E. Cheng, Z. Pei, C.-C. Hsu, C.-S. Chang, F.S.-S. Chien, Sol. Energy Mater. Sol. Cells 121, 80 (2014)
A.A. Abuelwafa, A. El-Denglawey, M. Dongol, M.M. El-Nahass, M.S. Ebied, T. Soga, Appl. Phys. A 124, 33 (2018)
A. Hadi, M.H. Hekmatshoar, F. Abbasi, S. Agbolaghi, Carbon Lett. 31, 107 (2021)
O. Oklobia, S. Komilian, T.S. Shafai, Organ. Electron. 61, 276 (2018)
Y. Zhang, L. Li, S. Yuan, G. Li, W. Zhang, Electrochim. Acta 109, 221 (2013)
W. Aloui, T. Adhikari, J.-M. Nunzi, A. Bouazizi, Mater. Res. Bull. 78, 141 (2016)
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AAA: conceptualization, visualization, formal analysis, methodology, data curation, writing—original draft and writing—review and editing. SE: formal analysis, investigation, visualization, data curation and writing—review and editing. RMDM: formal analysis and data curation. MD: formal analysis and writing—review and editing. MME-N: formal analysis and writing—review and editing. TS: formal analysis and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abuelwafa, A.A., Elnobi, S., Matiur, R.M. et al. Impact of phosphorescent dye on the performance and impedance spectroscopy for P3HT:PCBM solar cells. Appl. Phys. A 129, 65 (2023). https://doi.org/10.1007/s00339-022-06343-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-06343-w