Abstract
(1 − x)K0.5Na0.5NbO3–xLa(Zn0.5Zr0.5)O3 [(1 − x)KNN–xLZZ, x = 0.005, 0.010, 0.015, 0.020] ceramics were synthesized to fulfill the application requirement of high-temperature ceramic capacitors. The influence of LZZ content on the phase structure, microstructure morphology, dielectric and conductivity behaviors of this system was conducted. An orthorhombic phase for 0.005 ≤ x ≤ 0.010 and an orthorhombic-tetragonal coexisting phase for 0.015 ≤ x ≤ 0.020 were determined by the X-ray diffraction, Rietveld refinement, and dielectric spectra. The ceramic with x = 0.015 exhibits an ultrahigh and stable permittivity (ε' ~ 1892, ± 15% variation) and a low dielectric loss (tanδ ≤ 0.05) in a wide temperature region of 82–382 °C owing to the composition heterogeneity-induced shoulder-like peak and diffuse phase transition from tetragonal to cubic. The activation energies from the high-temperature dielectric relaxation and conductivity behaviors initially enhance greatly and then increase slowly, which could be related to the synergy effect of decreased oxygen vacancies and microstructure morphology evolution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ceramic capacitors with coupling, filtering, bypass, and other functions are the core electronic components in many fields such as computer, mobile phone, and motor vehicle. The actual temperature of widely used BaTiO3-based X8R ceramic capacitors is restrained to 150 °C due to their low Curie temperature (Tc) [1, 2]. However, some harsh operational conditions (e.g., engine control, deep oil exploration) express the development demand of high-temperature ceramic capacitors (HTCC) that operate stably above 200 °C or even 300 °C [3, 4]. Moreover, the development direction of HTCC is high reliability, small volume, and eco-friendliness. Therefore, to fulfill the practical working requirement of HTCC, lead-free dielectric ceramics with a high and stable permittivity (ε') and a low dielectric loss (tanδ) across a broad temperature range have received considerable concern [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20].
Perovskite oxides have drawn much attention in diverse electrical devices owing to their excellent dielectric, pyroelectric, piezoelectric, ferroelectric, photoelectric, and catalytic properties [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. In recent years, a great number of lead-free dielectric materials with perovskite structure for HTCC application have been extensively investigated, e.g., BaTiO3-based [5, 6], Bi0.5Na0.5TiO3-based [7,8,9], and K0.5Na0.5NbO3 (KNN)-based dielectric ceramics [10, 11]. Among them, KNN-based ceramics by composition modulation are considered to be potential materials for HTCC application [10,11,12]. Generally, unmodified KNN ceramic presents an intense temperature dependence of permittivity which is caused by two phase transitions from tetragonal to cubic (TC ~ 410 °C) and orthorhombic to tetragonal (TO-T ~ 200 °C) [12]. An alternative approach that improves its temperature stability of permittivity is to achieve a ferroelectric relaxor with diffuse phase transition by introducing dopants or forming solid solutions [13,14,15,16,17,18].
The Bi-based ABO3-type perovskites are usually used to obtain good temperature stability of permittivity in KNN-based solid solutions [13,14,15,16,17]. For example, Bi(Zn2/3Nb1/3)O3-doped KNN was reported to show a stable permittivity from 79 to 342 °C [17]. However, the volatilization of bismuth during sintering at high temperature produces intrinsic oxygen vacancies, leading to the increase of high-temperature dielectric loss. Recently, Liu et al. reported that the La(Zn0.5Ti0.5)O3-modified KNN showed a stable permittivity with a low tanδ in a wide temperature region of 95–350 °C [18]. Furthermore, the relaxor feature with diffuse phase transition has been found in the KNN–AZrO3 [A = Ba2+, Sr2+] solid solutions [19, 20], which can effectively enhance the temperature stability of permittivity and broaden the operational temperature range.
Combined with the above studies, it is anticipated that the introduction of La(Zn0.5Zr0.5)O3 into KNN can produces excellent dielectric properties for HTCC application. Therefore, La(Zn0.5Zr0.5)O3-doped KNN ceramics were prepared. To get deep insight into the dielectric properties of this system, the phase evolution was identified by X-ray diffraction (XRD), Rietveld refinement, and dielectric spectra, and the dielectric relaxation and conductivity behaviors at high temperature were characterized by the Arrhenius law and the universal dielectric response (UDR) law.
2 Experimental procedure
Lead-free (1 − x)K0.5Na0.5NbO3–xLa(Zn0.5Zr0.5)O3 [(1 − x)KNN–xLZZ, x = 0.005, 0.010, 0.015, 0.020] ceramics were synthesized using the raw powders Na2CO3 (99.8%), K2CO3 (99%), Nb2O5 (99.9%), La2O3 (99.99%), ZnO (99.99%), and ZrO2 (99.99%) (Shanghai Aladdin Bio-Chem Technology Co., Ltd., China) by a solid state sintering route. First, these powders were fired in an oven of 120 °C for 24 h and weighed according to the nominal (1 − x)KNN–xLZZ ratios, then ball-milled for 12 h and calcined at 900 °C for 4 h. Second, the calcined powders were re-milled for 12 h, then fired and ground with a 5 wt% polyvinyl alcohol (PVA) binder. Finally, the resultant powders were pressed into pellets 10 mm in diameter at ~ 350 MPa, and the pellets covered with the same composition powders were sintered at 1150–1160 °C for 4 h in an alumina crucible.
The phase structure of (1 − x)KNN–xLZZ samples at room temperature was measured using an automatic X-ray diffractometer (PANalytical, X’Pert PRO, Netherlands) with Cu Kα radiation. The structural parameters were obtained using the Rietveld refinement of GSAS program. The microstructure morphology of thermally etched samples with painted gold was observed using a scanning electron microscopy (SEM, JSM 6380, Japan). The density and porosity percentage were obtained by the Archimedes method. For the dielectric test, the sintered pellets were polished, and two surfaces were brushed with silver paste and then heated up to 650 °C for 0.5 h to generate the electrodes. The permittivity (ε') and dielectric loss (tanδ) from room temperature (RT) to 550 °C and the impedance data at 20 Hz–1 MHz in a temperature range of 480–560 °C were recorded using a high-precision LCR meter (KEYSIGHT E4980AL, USA).
3 Results and discussion
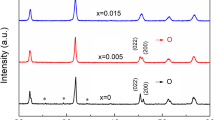
The XRD patterns of (1 − x)KNN–xLZZ ceramics at RT in a reflection angle (2θ) range of 20–60° are displayed in Fig. 1a. The magnified XRD patterns from 44° to 47° are shown in Fig. 1b. It can be seen from Fig. 1a that all the samples have a single perovskite structure except that an impurity of K6Nb10.8O30 indexed by X'Pert HighScore Plus software appears in the sample with x = 0.005. It indicates that LZZ has diffused into the KNN matrix to obtain the (1 − x)KNN–xLZZ solid solution. Moreover, according to the peak shape in Fig. 1b, the small addition of LZZ dramatically changes the phase structure of KNN. The samples with x = 0.005 and 0.010 have an orthorhombic phase (Amm2) similar to pristine KNN, which is characterized by the intensity of (022) peak higher than that of the (200) peak near 45° [28, 29]. With further increase in LZZ, the sample with x = 0.015 evolved into an orthorhombic and tetragonal coexisting phase based on the intensity of (022)/(002) peak close to that of the (200) peak, which is in accordance with the Bi(Zn2/3Nb1/3)O3-modified KNN system [17]. Although the sample with x = 0.020 shows a typical tetragonal phase (P4mm) characteristic of the higher intensity of the (200) peak than that of the (002) peak in the vicinity of 45° [29], the following Rietveld refinement and dielectric spectra confirm that it still maintains the orthorhombic and tetragonal coexisting phase.
To better understand the phase structure evolution of (1 − x)KNN–xLZZ ceramics, the Rietveld refinement of the measured XRD data was carried out using the GSAS software. The space groups were specified according to the XRD results and the incipient crystal parameters were adopted from Ref. [30]. During the structural refinement, the relevant parameters (e.g., scale factor, background, cell parameters) were continuously adjusted to decrease the difference between the measured XRD data and fitted values. The refinement results (e.g., observation, calculation, and difference values) are shown in Fig. 2. The small difference values suggest that the measured XRD data are well matched to the refined model. The crystal parameters, weighted profile residual factor (Rwp), and profile residual factor (Rp) are listed in Table 1. The Rwp and Rp factors of 4–8% prove the rationality of the phase structure.
As shown in the inset of Fig. 2, the Amm2 and P4mm unit cells contain two octahedral units and one octahedral unit, respectively. From the cell volume of Table 2, the average volume of the octahedral unit can be calculated: 63.2080 Å3 for x = 0.005, 63.1327 Å3 for x = 0.010, 63.0343 Å3 for x = 0.015, and 62.9976 Å3 for x = 0.020. The average volume of the octahedral unit has a slight decrease with increasing LZZ, which is associated with ionic substitution. For the (1 − x)KNN–xLZZ solid solution, according to the radius match rule of ABO3-type perovskite structure, the K+ [1.64 Å, coordination number (CN) = 12], Na+ (1.39 Å, CN = 12) and La3+ (1.34 Å, CN = 12) with larger size occupy the A site, whereas the Nb5+ (0.64 Å, CN = 12), Zn2+ (0.74 Å, CN = 12) and Zr4+ (0.72 Å, CN = 12) with smaller size occupy the B site [31]. Consequently, the incorporation of LZZ into KNN slightly shrinks the average volume of the octahedral unit.
The SEM morphology of (1 − x)KNN–xLZZ ceramics is shown in Fig. 3a–d. The samples with 0.005 ≤ x ≤ 0.015 present dense microstructure with few pores in Fig. 3a–c. With further increase in LZZ, a great number of pores are generated in the sample with x = 0.020 as in Fig. 3d. Figure 4a–d presents the distribution of grain sizes of different samples measured by the ImageJ software. The calculated average grain sizes are 7.84 µm, 5.04 µm, 11.22 µm, and 12.94 µm for the samples with x = 0.005, 0.010, 0.015, and 0.020, respectively. The average grain size initially declines for 0.005 ≤ x ≤ 0.010 and then increases with further increase of LZZ content. The decrease of average grain size could be because a low concentration of La3+ aggregates at the grain boundary to restrict the grain growth and a similar phenomenon was also observed in the La-modified KNN ceramics [32]. The increase of average grain size could be beacuse ZnO promotes grain growth at the sintering aid due to its low melting point and liquid-phase effect similar to the ZnO- and MnO2-added KNN system [33]. In addition, the densities of the samples with x = 0.005, 0.010, 0.015, and 0.020 are 4.36 g/cm3, 4.38 g/cm3, 4.33 g/cm3, and 4.23 g/cm3, respectively. The porosity percentages of the samples with x = 0.005, 0.010, 0.015, and 0.020 are 4.73%, 3.02%, 4.93%, and 8.64%, respectively. It can be found that the density and porosity percentage of 0.005 ≤ x ≤ 0.015 are both much higher than those of x = 0.020, which is in agreement with the result of microstructure morphology.
The temperature dependent permittivity (ε') of (1 − x)KNN–xLZZ ceramics at 100 kHz is shown in Fig. 5. Similar to pristine KNN, the samples with x = 0.005 and 0.010 present the permittivity peaks of Tm and TO-T higher than RT, indicating an orthorhombic phase at RT. With the increase of LZZ, Tm rapidly shifts to a lower temperature. It is notable that the samples with x = 0.010, 0.015 and 0.020 exhibit a shoulder-like peak (Ts) around 380 °C. Such a shoulder-like peak could be attributed to the composition heterogeneity (core–shell structure) and a similar phenomenon has been also reported in the KNN–AZrO3 (A = Sr2+, (Bi0.5K0.5)2+) solid solutions [20, 34]. As a result, the samples with x = 0.015 and 0.020 possess an orthorhombic and tetragonal coexisting phase, which is consistent with the analysis of XRD and Rietveld refinement. However, the composition heterogeneity distribution cannot be found by energy-dispersive spectroscopy (EDS) owing to the slight element difference. In addition, the samples with 0.015 ≤ x ≤ 0.020 exhibit a low-temperature diffuse phase transition from tetragonal to cubic, which has been largely reported in the ferroelectric relaxors [34,35,36,37]. Such a diffuse phase transition in this study is assigned to the thermal evolution of polar nano-regions (PNRs) size/volume produced by the heterovalent ion substitutions (e.g., La3+ for (K0.5Na0.5)+ at the A site, (Zn0.5Zr0.5)3+ for Nb5+ at B site). Consequently, the temperature stability of permittivity for the samples with x = 0.015 is significantly improved owing to the composition heterogeneity-induced shoulder-like peak and the diffuse phase transition from tetragonal to cubic.
The temperature stability of permittivity is a key indicator for practical application of HTCC materials. In Fig. 6a, the temperature variation of permittivity (Δε'/ε'270 °C, Δε' = ε' − ε'270 °C) for (1 − x)KNN–xLZZ ceramics at 100 kHz shows that the addition of LZZ has a great effect on the temperature stability of permittivity. Especially, the sample with x = 0.015 exhibits a wide operational temperature range of 82–382 °C based on the ± 15% tolerance of Electronic Industries Alliance (EIA) X8R capacitor standards [17, 18], as shown by the gray box in Fig. 6a. The temperature-dependent dielectric loss (tanδ) for (1 − x)KNN–xLZZ ceramics at 100 kHz is shown in Fig. 6b. The tanδ of the sample with x = 0.015 is maintained low (≤ 0.05), from 63 to 443 °C. The dielectric properties of the 0.985KNN–0.015LZZ ceramic and some reported KNN-based HTCC ceramics are listed in Table 2 [10, 16,17,18, 36]. By a comparison of the dielectric properties, the 0.985KNN–0.015LZZ ceramic with an ultrahigh and stable ε' (~ 1892, ± 15% variation) and a low tanδ (≤ 5%) from 82 to 382 °C is a potential candidate for HTCC application. It is notable that tanδ of the samples increases dramatically above 450 °C, which is related to a thermally induced conductivity behavior [8, 38].
To provide a better understanding of the conductivity behavior of (1 − x)KNN–xLZZ ceramics at high temperature, the normalized imaginary parts of impedance (\(Z^{\prime \prime } /Z_{{{\text{max}}}}^{\prime \prime }\)) for this system as a function of frequency at measured temperatures are shown in Fig. 7a–d. For each temperature of different samples, the measured frequency region shows an asymmetric peak. With the increase in temperature, the peak/relaxation frequency (fmax) gradually shifts toward a higher frequency, which suggests a thermally induced relaxation behavior [39, 40]. This relaxation behavior is because increase in temperature promotes the hopping/migration of charge carriers (e.g., holes, electrons, ions). Generally, the connection between fmax and the relevant temperature can be expressed using the following Arrhenius equation:
where f0 is the pre-constant, Erel denotes the activation energy from relaxation behavior, and kB and T denote the Boltzmann constant (8.617 × 10–5 eV/K) and kelvin temperature (K), respectively. The Arrhenius plots of relaxation frequency (fmax) were fitted based on Eq. (1), as shown by the straight lines in Fig. 8. The Erel values can be solved according to the slope of fitted straight lines: Erel = 1.264 eV for x = 0.005, Erel = 1.352 eV for x = 0.010, Erel = 1.440 eV for x = 0.015, and Erel = 1.470 eV for x = 0.020. The Erel monotonously increases with the increase in LZZ.
The frequency-dependent AC conductivity (σAC) of (1 − x)KNN–xLZZ ceramics at measured temperatures is shown in Fig. 9a–d. With increasing frequency, σAC initially presents a constant platform close to the DC conductivity (σDC) and then significantly increases, due to the contribution of localized charge carriers’ hopping/migration to conductivity. This conductivity behavior originates from the many-body interplay between the dipoles and charge carriers, which can be expressed using the following universal dielectric response (UDR) equation [39, 40]:
where σ0, f and s are the pre-constant, probing frequency, and exponent factor (0 < s ≤ 1), respectively. As shown by the red lines in Fig. 9, the nonlinear fitting between σAC and probing frequency was carried out and the σDC was solved based on Eq. (2). The connection between the obtained σDC and relevant temperature can be expressed using the following Arrhenius equation:
where σ1 is the pre-constant and Econ denotes the activation energy from DC conductivity. The Arrhenius plots of DC conductivity (σDC) were fitted based on Eq. (3), as shown by the straight lines in Fig. 10. The Econ values can be solved according to the slope of fitted straight lines: Econ = 0.856 eV for x = 0.005, Econ = 0.968 eV for x = 0.010, Econ = 1.171 eV for x = 0.015, and Econ = 1.222 eV for x = 0.020. The variation trend of Econ is consistent with that of Erel. Furthermore, the Erel values are higher than those of Econ for all the samples. Generally, Erel includes the free energy of localized charge carriers’ hopping/migration between the neighboring lattice sites, whereas Econ contains both the long-distance hopping/migration and creation of free energies of charge carriers [41, 42]. Thus, the difference between Erel and Econ is mainly connected with the creation of charge carriers, suggesting that charge carriers are released from the traps.
Frequency-dependent AC conductivity (σAC) of (1 − x)KNN–xLZZ ceramics at the measured temperatures: a x = 0.005, b x = 0.010, c x = 0.015, and d x = 0.020. The red lines are nonlinear fitting based on Eq. (2)
As one of the most common charged carriers in perovskite oxides, oxygen vacancies play a crucial part in high-temperature conductivity behavior. Previous studies have reported that the activation energy is in the range of 0.3–0.5 eV and 0.6–1.2 eV for the single-ionized and doubly ionized oxygen vacancies, respectively [43]. According to the Econ and Erel values of all the samples, the doubly ionized oxygen vacancies are regarded as the dominant charged carriers of (1 − x)KNN–xLZZ ceramics at high temperature. In the sintering process of KNN ceramic, oxygen vacancies are easily produced due to the volatility of K/Na from the lattice sites. Nevertheless, the substitution of LZZ into KNN does not generate the oxygen vacancies compensation, because all charged carriers produced from the La3+ are balanced by Zn2+ and Zr4+ [44]. The relevant Kroger–Vink equations can be described as:
where \({\text{V}}_{{\text{A}}}^{\prime }\), \({\text{V}}_{{\text{O}}}^{ \cdot \cdot }\), \({\text{La}}_{{\text{A}}}^{ \cdot \cdot }\), \({\text{Zn}}_{{{\text{Nb}}}}^{\prime \prime \prime }\), and \({\text{Zr}}_{{{\text{Nb}}}}^{\prime }\) are the K/Na vacancy, doubly ionized oxygen vacancies, La3+ at the K+/Na+ site, Zn2+ at the Nb5+ site, and Zr4+ at the Nb5+ site, respectively. According to Eqs. (4) and (5), the introduction of LZZ decreases the concentration of oxygen vacancies, leading to the increase of activation energy in agreement with the variation trend of Econ and Erel. It is notable that with the addition of LZZ, both Erel and Econ initially enhance greatly for 0.005 ≤ x ≤ 0.015 and then increase slowly for 0.015 ≤ x ≤ 0.020, as shown in Fig. 11. Generally, the variation trend of activation energy is associated with the oxygen vacancy concentration, microstructure morphology, and lattice deformation [15,16,17,18]. Since the LZZ content has little effect on the octahedral unit volume of (1 − x)KNN–xLZZ solid solution from the Rietveld refinement results, such a variation trend of activation energy is considered to be associated with the oxygen vacancy concentration and microstructure morphology. With the increase of LZZ, the samples with 0.005 ≤ x ≤ 0.015 are all dense and the decreased oxygen vacancy concentration plays a major part in the great enhancement of activation energy. With further increase in LZZ content up to x = 0.020, the generation of a great number of pores gives rise to the large surface on the grains [42], and the slight increase of activation energy could be attributed to the synergy effect of decreased oxygen vacancy concentration and increased pore numbers.
4 Conclusions
(1 − x)KNN–xLZZ ceramics were synthesized by the solid state sintering method. The combined analysis of XRD, Rietveld refinement, and dielectric spectra indicates that the introduction of LZZ into KNN induces a change of crystal structure from an orthorhombic phase for 0.005 ≤ x ≤ 0.010 to an orthorhombic–tetragonal coexisting phase for 0.015 ≤ x ≤ 0.020 and a decrease of octahedral unit volume. Owing to the composition heterogeneity-induced shoulder-like dielectric peak and diffuse phase transition from tetragonal to cubic, the 0.985KNN–0.015LZZ ceramic exhibits a ultrahigh and stable ε' (~ 1892, ± 15% variation) and a low tanδ (≤ 0.05) in a wide temperature region of 82–382 °C, which is a potential candidate for HTCC application. The activation energies from the high-temperature dielectric relaxation and conductivity behaviors initially enhance greatly, attributed to the decline of oxygen vacancy concentration, and then increase slowly due to the synergy effect of decreased oxygen vacancy concentration and increased pore numbers.
References
K. Kobayashi, M. Ryu, Y. Doshida et al., J. Am. Ceram. Soc. 96, 531 (2013)
J. Watson, G. Castro, J. Mater. Sci. Mater. Electron. 26, 9226 (2015)
N. Raengthon, H. Brown-Shaklee, G. Brennecka et al., J. Mater. Sci. 48, 2245 (2013)
P. Ren, J. He, F. Yan et al., J. Alloy. Compd. 807, 151676 (2019)
Z. Chen, G. Li, X. Sun et al., Ceram. Int. 41, 11057 (2015)
R. Muhammad, Y. Iqbal, I. Reaney, J. Am. Ceram. Soc. 99, 2089 (2016)
M. Acosta, J. Zang, W. Jo et al., J. Eur. Ceram. Soc. 32, 4327 (2012)
Q. Xu, Z. Song, W. Tang et al., J. Am. Ceram. Soc. 98, 3119 (2015)
D. Li, Y. Lin, M. Zhang et al., Chem. Eng. J. 392, 123729 (2020)
H. Du, W. Zhou, F. Luo, J. Appl. Phys. 105, 124104 (2009)
H. Cheng, W. Zhou, H. Du et al., J. Alloy. Compd. 579, 192 (2013)
H. Zhang, X. Li, X. Chen et al., J. Electron. Mater. 48, 4017 (2019)
H. Du, W. Zhou, F. Luo et al., J. Appl. Phys. 104, 044104 (2008)
H. Cheng, H. Du, W. Zhou et al., J. Am. Ceram. Soc. 96, 833 (2013)
Z. Liu, H. Fan, M. Li, J. Mater. Chem. C 3, 5851 (2015)
T. Yan, F. Han, S. Ren et al., Appl. Phys. A Mater. 124, 338 (2018)
T. Yan, K. Chen, C. Li et al., J. Adv. Ceram. 10, 809 (2021)
Z. Liu, A. Zhang, X. Geng et al., Ceram. Int. 45, 16842 (2019)
R. Wang, H. Bando, T. Katsumata et al., Phys. Status Solidi RRL 3, 142 (2009)
Z. Liu, F. Fan, S. Lei et al., J. Eur. Ceram. Soc. 37, 115 (2017)
Y. Slimani, A. Selmi, E. Hannachi et al., J. Mater. Sci: Mater. Electron. 30, 13509 (2019)
Y. Slimani, A. Selmi, E. Hannachi et al., J. Mater. Sci. Mater. Electron. 30, 9520 (2019)
Y. Slimani, A. Selmi, E. Hannachi et al., J. Phy. Chem. Solids 156, 110183 (2021)
E. Hannachi, M. Sayyed, K. Mahmoud et al., Appl. Phys. A 127, 970 (2021)
E. Hannachi, M. Sayyed, B. Albarzan et al., Ceram. Int. 47, 28528 (2021)
E. Hannachi, K. Mahmoud, M. Sayyed et al., Mat. Sci. Semicon. Proc. 145, 106629 (2022)
V. Jha, S. Alam et al., J. Supercond. Nov. Magn. 33, 455 (2020)
R. Zuo, X. Fang, C. Ye, Appl. Phys. Lett. 90, 092904 (2007)
C. Long, T. Li, H. Fan et al., J. Alloy. Compd. 658, 839 (2016)
A. Hewat, J. Phys. C: Solid State Phys. 6, 2559 (1973)
R. Shannon, Acta Crystallogr. 32, 751 (1976)
D. Gao, K. Kwok, D. Lin et al., J. Phys. D Appl. Phys. 42, 035411 (2009)
I. Kang, I. Seo, Y. Cha et al., J. Eur. Ceram. Soc. 32, 2381 (2012)
T. Yan, S. Ren, X. Ma et al., J. Electron. Mater. 47, 7106 (2018)
H. Du, W. Zhou, F. Luo, J. Am. Ceram. Soc. 91, 2903 (2008)
X. Chen, J. Chen, D. Ma et al., Mater. Lett. 145, 247 (2015)
L. Liu, M. Knapp, H. Ehrenberg et al., J. Eur. Ceram. Soc. 37, 1387 (2017)
S. Zhou, T. Yan, K. Chen et al., J. Electroceram. 46, 72 (2021)
M. Boukriba, F. Sediri, N. Gharbi, Mater. Res. Bull. 48, 574 (2013)
X. Chen, X. Yan, X. Li et al., J. Alloy. Compd. 762, 697 (2018)
Z. Abdelkafi, N. Abdelmoula, H. Khemakhem et al., J. Appl. Phys. 100, 114111 (2006)
T. Li, H. Fan, C. Long et al., J. Alloys. Compd. 609, 60 (2014)
L. Liu, H. Fan, L. Fang et al., Mater. Chem. Phys. 117, 138 (2009)
S. Dwivedi, T. Pareek, M. Badole et al., J. Appl. Phys. 127, 094104 (2020)
Acknowledgements
This work was financially supported by the Natural Science Foundation of China (Grant No. 12264012), the Natural Science Foundation of Guangxi (Nos. 2019GXNSFBA245069, GA245006, AA21238001, ZY22096019, BA297029, and AD18281042); Opening Project of Key Laboratory of Inorganic Functional Materials and Devices, Chinese Academy of Sciences (No. KLIFMD202207); Guilin University of Technology (No. GUTQDJJ20176612037); the High Level Innovation Team and Outstanding Scholar Program of Guangxi Institutes.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cui, Y., Yan, T., Wang, Z. et al. Phase evolution, dielectric and conductivity behaviors of (K0.5Na0.5)NbO3–La(Zn0.5Zr0.5)O3 ceramics. Appl. Phys. A 128, 954 (2022). https://doi.org/10.1007/s00339-022-06105-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-06105-8