Abstract
In this study, an indium oxide–zinc oxide (In2O3–ZnO) nanocomposite is successfully synthesized via a facile hydrothermal process. The samples are characterized using X-ray diffraction, scanning electron microscopy, high-resolution transmission electron microscopy, ultraviolet–visible diffuse reflectance spectroscopy, Fourier–transform infrared spectroscopy, and so on. The photocatalytic test results show that the photocatalytic degradation of methyl orange efficiency of 15% In2O3–ZnO nanocomposite can be more than 96.62% after 120 min, which is much higher than that of In2O3 and ZnO particles. The enhanced photocatalytic activity is attributed to the reduction in electrons (e−) and holes (h+) recombination in the photocatalytic process; it allows the rapid separation of photo-generated charge carriers, thus enhancing their photocatalytic activity. Finally, a possible photocatalytic reaction mechanism is proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, the environmental pollution problem has become increasingly severe due to the rapid development of industries [1, 2]. A significant amount of industrial wastewater is discharged into the natural environment, and the sewage purification and treatment issues have attracted people’s attention [2, 3]. Dyes have various applications and generates the most severe water pollution among diverse pollutants and are difficult to decompose [3]. Photocatalytic technology is considered one of the most promising approaches among various water pollution remediation methods [4, 5]. Relevant research results show that sunlight is a significant and sustainable strategy for degrading pollutants and producing highly efficient clean energy [6,7,8].
Oxide semiconductor photocatalysts, such as ZnO, In2O3, and TiO2, have attracted much attention due to their potential applications in environmental protection and energy production [9,10,11]. ZnO, with a wide bandgap of 3.37 eV and electron binding energy (60 meV), is a promising solution in the field of environmental pollution, among other oxide semiconductor materials, and has certain applications in photocatalytic, conductive materials, chemical sensors, and gas sensors, particularly showing potential advantages in the photocatalytic degradation of dyes [12]. However, the recombination process of ZnO photogenerated carriers in the photocatalytic process is much faster than the transporting process, resulting in lower photocatalytic efficiency of ZnO [13, 14]. Researchers have investigated different strategies in the past few decades to improve the photocatalytic efficiency of ZnO, including doping, metal loading, and heterostructure construction [15,16,17]. Among them, the combination of two semiconductor types to form semiconductor composites is considered an effective and economical technology for degrading organic pollutants [18]. ZnO is combined with other semiconductor materials to form nanocomposites, and various interfacial interactions will produce new properties [19]. The formation of semiconductors with an appropriate band gap, band edges, and high lattice matching degrees in a nanocomposite can facilitate the separation of photogenerated electrons and holes [20]. Zhao et al. [21] successfully synthesized ZnO nanocomposite doped with In2O3 using a microwave hydrothermal method, which beneficially reduced the electrons and holes recombination during the photocatalytic process and promoted the active substances (•OH and •O2−). Martha et al. [22] successfully synthesized In2O3 − modified ZnO nanocomposite using a coprecipitation method, which demonstrated high photocatalytic activity. Liu et al. [23] successfully synthesized In2O3–ZnO nanocomposite with heterostructure using a one-step hydrothermal method, which promoted the separation of photogenerated carriers. The construction of heterostructures in nanocomposites can significantly improve the performance of nanocomposites, however, the choice of semiconductor materials when constructing heterostructures is crucial.
In2O3 is an indirect band semiconductor with a 2.56 eV bandgap and is a highly effective sensitizer to extend the absorption spectra of the transition metal oxide photocatalyst from ultraviolet (UV) to the visible region [24, 25]. Its valence and conduction band arrangement is irregular compared with ZnO. Several studies have been reported on In2O3–ZnO nanocomposites, which relate to organic compound degradation and photocatalysis hydrogen production [26, 27]. These results suggest that doping In2O3 into ZnO nanostructures can significantly inhibit the recombination of photogenerated electron–hole pairs to enhance photocatalysis. In addition, p–n junctions can also be formed at the interface between the ZnO and In2O3 phases in the In2O3–ZnO nanocomposites, which help separate the electron and hole pairs [28]. Consequently, the fabrication of In2O3–ZnO nanocomposites has attracted significant attention.
This study successfully synthesized In2O3–ZnO nanocomposites with enhanced photocatalytic properties via a hydrothermal process. The microstructure, phase, and optical properties of the samples were investigated. The photocatalytic properties of the as-synthesized samples were investigated by the degradation of methyl orange (MO) in an aqueous solution under UV light irradiation. As anticipated, the obtained In2O3–ZnO nanocomposite exhibits excellent charge separation efficiency and higher photocatalytic properties than In2O3 and ZnO particles. Concurrently, we proposed and discussed the charge transfer and probable photocatalytic mechanism.
2 Experimental details
2.1 Materials
All chemicals applied in this experiment including zinc nitrate hexahydrate (Zn(NO3)2·6H2O, Shandong West Asia Chemical Industry Co., Ltd.), sodium hydroxide (NaOH), polyethylene glycol (PEG), indium nitrate, and ethanol were purchased and used without any further purification. All the chemical reagents are analytical grade. Highpurity deionized water was used throughout all experiments.
2.2 Preparation of ZnO nanoparticles
ZnO nanoparticles are synthesized using the hydrothermal process. First, Zn(NO3)2·6H2O (5.950 g), NaOH (5.000 g), and PEG (2.000 g) are mixed and stirred for 1.5 h at room temperature to yield a white precipitate. Further, the precipitate is diverted into the Teflon autoclave and maintained for 6 h at 150 °C. The precipitate is then naturally cooled from 150 °C to room temperature, and the precipitate is washed three times using deionized water and ethanol anhydrous. Finally, the precipitate is vacuum–dried at 60 °C for 12 h, ground, and the dried samples are calcined at 500 °C for 2 h.
2.3 Preparation of In2O3–ZnO nanocomposite
The experiment adopted the hydrothermal process to synthesize the In2O3–ZnO nanocomposites. First, Zn(NO3)2·6H2O (5.950 g), NaOH (5.000 g), and PEG (2.000 g) were mixed and stirred for 1.5 h at room temperature to yield a white precipitate. Further, Zn(NO3)2·6H2O (5.950 g), NaOH (5.000 g), PEG (2.000 g), and indium nitrate (corresponding molar ratio: 0%, 1%, 2%, 5%, 10%, 15%, and 20%) are mixed and stirred for 1.5 h at room temperature, and the product is added to the above precipitate and stirred for 0.5 h at room temperature to yield a white precipitate. Afterward, the above precipitate is diverted into the Teflon autoclave and maintained for 6 h at 150 °C. In addition, the precipitate is naturally cooled from 150 °C to room temperature, and the precipitate is washed three times using deionized water and ethanol anhydrous. Finally, the precipitate is vacuum–dried at 60 °C for 12 h, ground, and the dried samples are calcined at 500 °C for 2 h.
2.4 Characterization
The crystal structures are studied using powder X–ray diffraction (XRD). The morphologies of the obtained samples are measured using scanning electron microscopy (SEM). The obtained samples are measured using high-resolution transmission electron microscopy (HRTEM) at an accelerating voltage of 200 kV. The ultraviolet–visible diffused reflectance spectra (UV–Vis DRS) of the samples are obtained using a spectrophotometer.
2.5 Photocatalytic properties test
The photocatalytic activities of the as-synthesized samples are evaluated by the photocatalytic degradation of MO. First, 50 mg of the photocatalysts is ultrasonically dispersed in 50 mL of 10 mg L−1 MO aqueous solution. It is magnetically stirred in the dark for 30 min. Further, UV light irradiation is conducted using a 420–W Mercury lamp as the light source. At specified intervals, 4 mL aliquots are analyzed by obtaining variations in the absorption band (464 nm) in the UV–Vis spectra of MO.
3 Results and discussion
3.1 Structural and morphological characterization
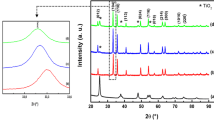
Figure 1 shows the XRD patterns of pure ZnO and In2O3–ZnO nanocomposites. The diffraction peaks of pure ZnO at 31.79°, 34.44°, 36.27°, 47.56°, 56.63°, 62.87°, 66.40°, 67.92°, and 68.99° in 2θ correspond to the ZnO hexagonal wurtzite structure (100), (002), (101), (102), (110), (103), (200), (112), and (201) crystal planes, respectively, which are consistent with PDF (JCPDS NO. 36–1451) [29]. All diffraction peaks in the pure ZnO pattern can be expressed as a hexagonal phase structure [29]. The diffraction profile of In2O3–ZnO nanocomposites shows that the nanocomposites are composed of the hexagonal phase of ZnO (JPCDS, 36–1451) and the body–centered cubic structure of In2O3 (JCPDS, 71–2194). The 15% In2O3–ZnO spectrum line in the figure shows that the diffraction peak at 2θ of 30.68°, 32.71°, 35.51°, 45.77°, 51.13°, and 60.84° is consistent with that of PDF (JCPDS NO. 06–0415), except for the characteristic peak of ZnO, which may be In2O3 diffraction peaks, indicating that the In2O3–ZnO nanocomposite is an In2O3 cubic ferromanganese structure (C-In2O3) [30]. The diffraction peak intensity and crystallinity of C-In2O3 gradually increase with the C-In2O3 content, whereas the diffraction peak intensities of ZnO gradually decrease, which may imply that the characteristic peaks of ZnO are inhibited by the In2O3 composite ZnO. The sharp and intense diffraction peaks show that the samples are highly crystalline [31].
Figure 2 shows the SEM images (a and b) and particle size (c and d) of pure ZnO (a, c) and 15% In2O3–ZnO nanocomposite (b, d). Figure 2a shows that the pure ZnO are spindle particles, with a 200-nm-length and 100-nm-diameter. Figure 2b shows that the spherical small particles in the 15% In2O3–ZnO nanocomposite adhere to the spindle–like ZnO crystal. These small particles are In2O3 nanoparticles with a particle size of about 30 nm. The morphology and size of the spindle–like ZnO particles remain unchanged. This may be because the ZnO crystal size is not affected by the In2O3 composite, and the In2O3 nanoparticles adhere to the ZnO surface and form a composite with it [32]. Figure 2c and d shows that the particle size distributions of pure ZnO and 15% In2O3–ZnO nanocomposite are obviously narrowed, and the particles are more uniform.
Figure 3 shows the EDS diagram of the 15% In2O3–ZnO nanocomposite. The mass of Zn, In, and O elements present in the In2O3–ZnO nanocomposite are about 67.91%, 17.05%, and 15.04%, respectively, which is consistent with the theoretical mass percentage of 15% In2O3–ZnO nanocomposite, indicating that In2O3 and ZnO are aggregated as composite [33].
Figure 4 shows the TEM (a) and (c) and HRTEM (b) and (d) images of pure ZnO (a, b) and 15% In2O3–ZnO nanocomposite (c, d). Figure 4a and c show that ZnO and 15% In2O3-ZnO nanocomposite are composed of nanoparticles with a particle diameter of 25∼30 nm. The HRTEM image of ZnO and 15% In2O3–ZnO is shown in Fig. 4b and d, and the clear lattice stripes can be observed, which indicates the high crystallinity of hexagonal ZnO and the high crystallinity of cubic In2O3 and hexagonal ZnO.
3.2 UV–Vis DRS analysis
Figure 5 shows the UV–Vis DRS of ZnO and 15% In2O3–ZnO nanocomposite. The 15% In2O3–ZnO nanocomposite absorption in the UV region is considerably more than ZnO and may improve the ZnO’s absorption capacity in the UV region. Further, a redshift occurs along the 15% In2O3–ZnO nanocomposite absorption band, probably due to the increase in the visible light absorption range of the In2O3 composite ZnO [34].
3.3 FTIR analysis
Figure 6 shows the FTIR results of ZnO and 15% In2O3–ZnO nanocomposite. The characteristic peaks of ZnO and 15% In2O3–ZnO nanocomposite at 506 correspond to Zn–O peak [35]. The characteristic peaks of the 15% In2O3–ZnO nanocomposite at 600 and 1158 cm−1 correspond to the In2O3 characteristic and δ (OH) vibration peaks [36], respectively. The characteristic peaks of ZnO and 15% In2O3–ZnO nanocomposite at 866 correspond to r (OH) vibration peak [35]. The ZnO characteristic peaks at 840 and 902 cm−1 correspond to the r (OH) and δ (OH) vibration peaks, respectively [36]. The characteristic peaks of ZnO and 15% In2O3–ZnO nanocomposite at 1385 cm−1 correspond to Zn(OH)2 vibration peak [35]. The characteristic peaks of ZnO and 15% In2O3–ZnO nanocomposite at 1454 and 1439 cm−1, respectively, correspond to the O–H bond bending vibration peaks, resulting in the absorption of atmospheric moisture from the sample [35]. The wide characteristic peaks of ZnO and 15% In2O3–ZnO nanocomposite at 3503 and 3515 cm−1, respectively, may correspond to vibration peaks of product crystalline water or hydroxy hydroxide bending [36].
3.4 Photocatalytic activity and stability
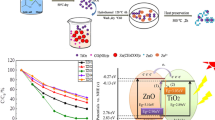
Photocatalytic activity of In2O3–ZnO nanocomposites is evaluated by MO degradation under UV light. Figure 7 shows the experimental results of the MO photocatalytic degradation in ZnO and In2O3–ZnO nanocomposites. The degradation of MO in In2O3, ZnO, and In2O3–ZnO nanocomposites is very slow in the first 20 min. This shows that the adsorption capacity of In2O3, ZnO, and In2O3–ZnO nanocomposites to MO is relatively weak. The MO photocatalyst degradation rate in ZnO and In2O3 after 120 min are 83.31% and 81.56%, respectively, which are attributed to its high bandgap energy, indicating that ZnO and In2O3 have certain photocatalytic activities in the UV region. Alternatively, the MO degradation rate in In2O3–ZnO nanocomposites first increases after 120 min before decreasing with the percentage content; In2O3 composite ZnO may promote the transfer of photoelectron, inhibit the recombination of photoelectron–hole pairs, and improve the photocatalytic activity, indicating that an optimal percentage content is essential for enhancing the photocatalytic activity of In2O3–ZnO nanocomposites [37]. In2O3 and ZnO promote the recombination of photoraw electron–hole pairs when the percentage content reaches a certain level, thereby reducing their photocatalytic activity. The photocatalytic activity of 15% In2O3–ZnO nanocomposite is the highest among others, and the MO degradation rate under UV light after 120 min was up to 96.62%. The results show that the appropriate content of nanocomposites allows for rapid separation of photogenerated charge carriers, thus enhancing their photocatalytic activity.
ZnO has poor stability in degrading organic pollutants. Therefore, investigating the photostability and repeatability of ZnO–based photocatalyst is critical. The recycling experiment is conducted using 15% In2O3–ZnO nanocomposite degradation of MO solution under UV irradiation [38]. Figure 8 shows the degradation efficiency of MO decreased from 96.62 to 92.02% after three cycles. Therefore, 15% In2O3–ZnO nanocomposite is stable in the photodegradation of organic pollutants.
3.5 Proposed photocatalytic mechanism
A mechanism is proposed to explain the enhanced photocatalytic performance of In2O3–ZnO nanocomposite based on the aforementioned results [39]. The photocatalytic performance of In2O3–ZnO nanocomposite is stronger than that of pure In2O3 and pure ZnO. This is primarily because under ultraviolet radiation, the In2O3 nanoparticles can absorb ultraviolet light and excite their own electrons, while ZnO conduction band is located between In2O3 conduction and valence bands. Thereupon, the photoexcited electrons on the surface of In2O3 can be easily transferred to the conduction band of ZnO, and the photoexcited holes remain on In2O3. In this way, photogenerated electrons and holes can be effectively separated, further improving their photocatalytic performance. In addition, due to the excellent crystallization performance of In2O3, electron migration has limited resistance, which is conducive to the effective separation of photogenerated electron–hole pairs, so as to increase the lifetime of loaded streamer. The bottom and top of the conduction band (CB) and valence band (VB) of ZnO are at − 0.4 and 2.8 eV, respectively, whereas the bottom and top of the CB and VB of In2O3 are at − 0.63 and 2.17 eV, respectively. Figure 9 shows that the bottom and top of the CB and VB of ZnO are located below those of In2O3. Further, In2O3 can be excited by UV light when combined with ZnO, and electrons in In2O3 migrate to the ZnO CB. In addition, the electron transport resistance is extremely low, and electron–hole recombination is reduced due to the high crystallinity of In2O3. Therefore, effective charge separation increases the lifetime of charge carriers and improves the efficiency of interfacial charge transfer to the adsorption matrix, resulting in higher photocatalytic activity of In2O3–ZnO nanocomposite. The suitable substrate can be oxidized and the original In2O3 recycled since the In2O3 (h+) radical redox potential is about 2.17 eV. In our experiment, the MO photocatalytic degradation mechanism is proposed as follows:
Under UV light, the photoraw electrons in In2O3 move freely to the ZnO, whereas the photoraw holes remain in the In2O3 VB. Dissolved oxygen molecules react with ZnO surface electrons to generate superoxygen radical anion ·O2−, which produces hydrogen peroxide radical HO2· at protonation, thus producing hydroxy OH·, which is a strong oxidizer that decomposes organic dyes [40].
4 Conclusions
The In2O3–ZnO nanocomposite is successfully synthesized using the hydrothermal method. XRD analysis reveals that the In2O3 composite ZnO suppressed the ZnO characteristic peaks. SEM analysis shows that spindle–like ZnO crystal size in In2O3–ZnO nanocomposite did not change due to In2O3 composite. EDS analysis reveals that the Zn, In, and O in the In2O3–ZnO nanocomposite has a percent mass fraction of 15%. TEM analysis shows that 15% In2O3-ZnO nanocomposite is composed of nanoparticles with a particle diameter of 25∼30 nm. UV–Vis DRS analysis shows that In2O3 composite ZnO can broaden the range of ZnO visible light absorption. FTIR analysis reveals that the ZnO and In2O3 phases in In2O3–ZnO nanocomposites exist individually. The experimental results of photocatalytic degradation show that the photocatalytic activity of 15% In2O3–ZnO nanocomposite is the best, and the MO degradation rate after 120 min can be up to 96.62%, thereby improving ZnO’s photocatalytic performance.
Data availability
All data used to support this study are included within the paper.
References
M. Lan, G. Fan, L. Yang, F. Li, Enhanced visible-light-induced photocatalytic performance of a novel ternary semiconductor coupling system based on hybrid Zn-In mixed metal oxide/g-C3N4 composites. RSC Adv. 5, 5725 (2015)
C.B. Ong, L.Y. Ng, A.W. Mohammad, A review of ZnO nanoparticles as solar photocatalysts: synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 81, 536–551 (2018)
W. K. Zhang, Q. Y. Zhong, X. M. Liu, Q. Lu, Inorganic functionalization of CdSexS1-x/ZnS core-shell quantum dots and their photoelectric properties. Physica Status Solidi (A). 217, 2000010 (2020)
H. Cheng, W.K. Zhang, X.M. Liu, T.F. Tang, J.H. Xiong, Fabrication of titanium dioxide/carbon fiber (TiO2/CF) composites for removal of methylene blue (MB) from aqueous solution with enhanced photocatalytic activity. J. Chem. 9, 1–11 (2021)
H. Cheng, X.N. Li, T.H. Li, D.F. Qin, T.F. Tang, Y.Q. Li, G.X. Wang, Electrospun nanofibers with high specific surface area to prepare modified electrodes for electrochemiluminescence detection of azithromycin. J. Nanomater. 93, 1–10 (2021)
X.M. Liu, F.I. Chowdhury, L. Meng, Q. Xu, X. Wang, Luminescent films employing quantum dot-cellulose nanocrystal hybrid nanomaterials. Mater. Lett. 294, 129737 (2021)
X.M. Liu, Q.Y. Zhong, W.M. Guo, W.K. Zhang, Y.Y. Ya, Y.Q. Xia, Novel platycladus orientalis-shaped Fe-doped ZnO hierarchical nanoflower decorated with Ag nanoparticles for photocatalytic application. J. Alloys Compd. 880, 160501 (2021)
X.M. Liu, Y. Liu, W.K. Zhang, Q.Y. Zhong, X.Y. Ma, In situ self-assembly of 3D hierarchical 2D/2D CdS/g-C3N4 hereojunction with excellent photocatalytic performance. Mater. Sci. Semicond. Process. 105, 104734 (2020)
X. M. Liu, S. B. Yao, Y. Liu, W. K. Zhang, F. M. Fu, H. Y. He, Metal ion-induced synthesis of hierarchical ZnO architectures with various morphologies and their photocatalytic performances. Springer Singapore. (2017).
H.R. Liu, C.J. Hu, H.F. Zhai, J. Yang, X.G. Liu, H.S. Jia, Fabrication of In2O3/ZnO@Ag nanowire ternary composites with enhanced visible light photocatalytic activity. RSC Adv. 7, 37220–37229 (2017)
N. Talebian, M.R. Nilforoushan, Z. Salehi, Effect of heterojunction on photocatalytic properties of multilayered ZnO-based thin films. Ceram. Int. 38, 4623–4630 (2012)
S.G. Kumar, K.S.R.K. Rao, Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3 and ZnO). Appl. Surf. Sci. 391, 124–148 (2017)
X. Xiang, L.S. Xie, Z.W. Li, F. Li, Ternary MgO/ZnO/In2O3 heterostructured photocatalysts derived from a layered precursor and visible-light-induced photocatalytic activity. Chem. Eng. J. 221, 222–229 (2013)
N. Talebian, M.R. Nilforoushan, R.R. Ghasem, Enhanced photocatalytic activities of ZnO thin films: a comparative study of hybrid semiconductor nanomaterials. J. Sol-Gel. Sci. Technol. 64, 36–46 (2012)
N. Talebian, M.R. Nilforoushan, Comparative study of the structural, optical and photocatalytic properties of semiconductor metal oxides toward degradation of methylene blue. Thin Solid Films 518, 2210–2215 (2010)
X.N. Liu, Q.F. Lu, J.H. Liu, Electrospinning preparation of one-dimensional ZnO/Bi2WO6 heterostructured sub-microbelts with excellent photocatalytic performance. J. Alloys Compd. 662, 598 (2016)
C. Han, M.Q. Yang, B. Weng, Y.J. Xu, Improving the photocatalytic activity and anti-photocorrosion of semiconductor ZnO by coupling with versatile carbon. Phys. Chem. Chem. Phys. 16, 16891 (2014)
Y.L. Xing, W.X. Que, X.T. Yin, Z.L. He, X.B. Liu, Y.W. Yang, J.Y. Shao, L.B. Kong, In2O3/Bi2Sn2O7 heterostructured nanoparticles with enhanced photocatalytic activity. Appl. Surf. Sci. 387, 36–44 (2016)
F. Zhao, Q. F. Lu, Z. L. Xiu, C. F. Zhu, Preparation and photocatalytic performance of one-dimensional In2O3 nanofibers, CuO microfibers and CuO/In2O3 heterostructured nanofibers by electrospinning process. Nano: brief reports and reviews, 11, 1650048 (2016)
M.M. Sun, Z.Y. Chen, Enhanced photoelectrochemical cathodic protection performance of the In2O3/TiO2 composite. J. Electrochem. Soc. 162, C96–C104 (2015)
J.K. Zhao, S.S. Ge, D. Pan, Y.L. Pan, V. Murugadoss, R. Li, W. Xie, Y. Lu, T.T. Wu, E.K. Wujcik, Q. Shao, X.M. Mai, Z.H. Guo, Microwave hydrothermal synthesis of In2O3-ZnO nanocomposites and their enhanced photoelectrochemical properties. J. Electrochem. Soc. 166, H3074–H3083 (2019)
S. Martha, K.H. Reddy, K.M. Parida, Fabrication of In2O3 modified ZnO for enhancing stability, optical behaviour, electronic properties and photocatalytic activity for hydrogen production under visible light. J. Mater. Chem. A 2, 3621–3631 (2014)
H.R. Liu, X. He, Y.C. Hu, X.G. Liu, H.S. Jia, B.S. Xu, One-step hydrothermal synthesis of In2O3-ZnO heterostructural composites and their enhanced visible-light photocatalytic activity. Mater. Lett. 131, 104–107 (2014)
N. Lu, C.L. Shao, X.H. Li, F.J. Miao, K.X. Wang, Y.C. Liu, A facile fabrication of nitrogen-doped electrospun In2O3 nanofibers with improved visible-light photocatalytic activity. Appl. Surf. Sci. 391, 668–676 (2017)
F. Zhao, Q.F. Lu, S.W. Liu, Preparation and characterization of In2O3/ZnO heterostructured microbelts by sol-gel combined with electrospinning method. J. Sol-Gel. Sci. Technol. 69, 357–363 (2014)
F.L. Zhou, X.J. Li, J. Shu, J. Wang, Synthesis and visible light photo-electrochemical behaviors of In2O3-sensitized ZnO nanowire array film. J. Photochem. Photobiol., A 219, 132–138 (2011)
Z.Y. Wang, B.B. Huang, Y. Dai, X.Y. Qin, X.Y. Zhang, P. Wang, H.X. Liu, J.X. Yu, Highly photocatalytic ZnO/In2O3 heteronanostructures synthesized by a coprecipitation method. The Journal of Physical Chemistry C 113, 4612–4617 (2009)
F. Zhao, Q.F. Lu, S.W. Liu, C.Q. Wang, In2O3/ZnO heterostructured nanotubes: electrospinning fabrication, characterization, and highly enhanced photocatalytic properties. J. Sol-Gel. Sci. Technol. 72, 137–143 (2014)
B.X. Li, T.X. Liu, Y.F. Wang, Z.F. Wang, ZnO/graphene-oxide nanocomposite with remarkably enhanced visible-light-driven photocatalytic performance. J. Colloid Interface Sci. 377, 114–121 (2012)
Y.X. Du, P. Ding, Synthesis and cathodoluminescence of In2O3-SnO2 nanowires heterostructures. J. Alloy. Compd. 507, 456–459 (2010)
Q.P. Luo, X.Y. Yu, B.X. Lei, H.Y. Chen, D.B. Kuang, C.Y. Su, Reduced graphene oxide-hierarchical ZnO hollow sphere composites with enhanced photocurrent and photocatalytic activity. J. Phys. Chem. C 116, 8111–8117 (2012)
J.B. Mu, C.L. Shao, Z.C. Guo, Z.Y. Zhang, M.Y. Zhang, P. Zhang, B. Chen, Y.C. Liu, High Photocatalytic activity of ZnO-carbon nanofiber heteroarchitectures. ACS Appl. Mater. Interfaces 3, 590–596 (2011)
S. Rehman, R. Ullah, A.M. Butt, N.D. Gohar, Strategies of making TiO2 and ZnO visible light active. ChemInform 170, 560–569 (2010)
M. Samadi, M. Zirak, A. Naseri, E. Khorashadizade, A.Z. Moshfegh, Recent progress on doped ZnO nanostructures for visible-light photocatalysis. Thin Solid Films 605, 2–19 (2016)
R. Saravanan, S. Karthikeyan, V.K. Gupta, G. Sekaran, V. Narayanan, A. Stephen, Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination. Mater. Sci. Eng., C 33, 91–98 (2013)
J.X. Sun, Y.P. Yuan, L.G. Qiu, X. Jiang, A.J. Xie, Y.H. Shen, J.F. Zhu, Fabrication of composite photocatalyst g-C3N4-ZnO and enhancement of photocatalytic activity under visible light. Dalton Trans. 41, 6756–6763 (2012)
R. Saravanan, E. Sacari, F. Gracia, M.M. Khan, E. Mosquera, V.K. Gupta, Conducting PANI stimulated ZnO system for visible light photocatalytic degradation of coloured dyes. J. Mol. Liq. 221, 1029–1033 (2016)
C.G. Tian, Q. Zhang, A.P. Wu, M.J. Jiang, Z.L. Liang, B.J. Jiang, H.G. Fu, Cost-effective large-scale synthesis of ZnO photocatalyst with excellent performance for dye photodegradation. Chem. Commun. 48, 2858–2860 (2012)
X. Zhang, L.Z. Zhang, T.F. Xie, D.J. Wang, Low-temperature synthesis and high visible-light-induced photocatalytic activity of BiOI/TiO2 heterostructures. J. Phys. Chem. C 113, 7371–7378 (2009)
L.P. Xu, Y.L. Hu, C. Pelligra, C.H. Chen, L. Jin, H. Huang, S. Sithambaram, M. Aindow, R. Joesten, S.L. Suib, ZnO with different morphologies synthesized by solvothermal methods for enhanced photocatalytic activity. Chem. Mater. 21, 2875–2885 (2009)
Acknowledgements
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (No. 21968005), the Opening Project of Guangxi Key Laboratory of Green Processing of Sugar Resources (No. GXTZY201701), and the High Levels of Innovation Team and Excellence Scholars Program in Colleges of Guangxi (Gui Jiaoren [2014] No. 7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, W., Huang, W., Wu, Q. et al. Hydrothermal synthesis of In2O3–ZnO nanocomposite and their enhanced photocatalytic properties. Appl. Phys. A 128, 159 (2022). https://doi.org/10.1007/s00339-021-05237-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-021-05237-7