Abstract
The optical properties of silver nanoparticles originating from the collective oscillations of their conduction electrons (LSPR) play a major role in the development of nanotechnology-based optical devices, from optical sensors to bactericide materials via non-linear optical devices. In this study, nanocomposites polymer films embedding silver nanoparticles were prepared by thermal annealing of poly-(vinyl alcohol) films doped by silver nitrate using a “one-pot synthesis” method. In this upscalable solution-based process, the nanoparticles are directly grown in the polymer matrix, yielding a plasmonic nanocomposite with a high density of nano-objects. Low (2.5% w:w) and high (25% w:w) doping concentration of silver were considered as well as their effect on the optical properties of thin (30 nm) and thick (300 nm and more) films. The topography and the optical properties (refractive index n and extinction coefficient k) of such films were studied by atomic force microscopy and spectroscopic ellipsometry. Unexpectedly, for a given doping level, the parameters of the surface plasmon resonance (amplitude, position and width) were shown to be thickness-dependent. A multivariate statistical analysis technique was used to identify the differences in the optical behavior of thick and thin films.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Plasmonic nanocomposites (PNCs) belong to an emerging class of materials whose optical properties take advantage of the surface plasmon resonance of the metal nanoparticles (NPs) embedded in a dielectric matrix. They are highly customizable by tuning the nanostructures shape and size as well as the mechanical behavior (stiffness, flexibility, Young’s modulus) and physical properties (glass transition) of the matrix [1]. PNCs have numerous applications such as optical sensors, non-linear optical devices [2,3,4,5], biosensors or bactericide materials [6,7,8,9,10]. The study of their optical properties is an active research field and is driven by the interest for the Localized Surface Plasmon Resonance (LSPR). Well-known in its effect since e.g. the optical aspect of the Lycurcus cup (4th CE) or of the mosaics of Saint Sabina (5th CE) [11], the LSPR appears when free electrons of the metal are excited by the electromagnetic field associated to the propagation of light [12, 13]. The most popular way to prepare these PNCs is the “bottom-up” approach: metal NPs are synthesized in colloidal solutions and stabilized with or without further specific coating to prevent their aggregation. In this synthesis, several experimental factors can provide a considerable control of the formation of NPs. However, to produce PNCs, the NPs in the colloidal solution have to be dispersed and immobilized in a dielectric matrix such as glass or polymer (see e.g. [14,15,16,17] and the references therein). This approach has therefore two separate steps: the synthesis of the NPs and their dispersion in the matrix.
Another upscalable solution-based process is to grow the NPs directly in the polymer matrix. This is the process retained for our study. According to this “one-pot” synthesis method, a noble metal salt (\(\hbox {AgNO}_{3}\), in our case) is directly mixed with a polymer. This approach leads to a high concentration of NPs in the material [18]. The basic principle of the in situ methods is the use of one of the matrix components as a reducing agent of a metal salt and to speed-up the reduction of the metal cations in situ by irradiating or thermally annealing the solid phase. Polymers such poly-(vinyl) alcohol (PVA) are excellent host materials for metal or semiconductor NPs [19] because they play the role of reducing agent but also contribute to the stabilization of the NPs. In the polyols method, the reduction step of the silver cations uses electrons originating from the -OH groups of the PVA molecules (Fig. 1a). Depending on the temperature, cross-linking of the polymer matrix can also be induced, reducing the solubility of the composite (Fig. 1b) [20]. However, in the case of polymer-based PNCs, the reproductibility of the NPs distributions in size and shape remains difficult to achieve and slight variations in the experimental conditions leads to different results evidenced by modifications of the plasmon resonance band parameters. Moreover, the detailed mechanism linking the optical properties of the nanocomposite to their structural parameters is today not fully understood although still being the subject of an increasing number of publications.
a Reduction scheme for the silver cations and b Cross-linking of the PVA chains at high temperature (redrawn from [20])
A large number of publications has been devoted to the study of the optical properties of NPs in solutions, considering the effects of solvent, temperature synthesis, surfactants, ... but much less authors have considered the growth of the NPs in a polymer film. Concerning the experimental technique, the optical properties of PNCs are usually probed using reflection or transmission spectrophotometry in the UV-visible spectral range. In our previous publications, we used spectroscopic ellipsometry (SE) [21, 22] or imaging ellipsometry (IE) [18, 23] to study the optical properties of Ag- or Au-doped PVA films produced by thermal annealing. As explained hereinafter, this optical analysis has the advantages of being non-destructive and, moreover, of allowing the simultaneous determination of the film thickness and of its complex refractive index values. To date, only a reduced number of studies have been carried out using SE as an analysis technique [21, 22, 24,25,26]. Extensive reviews have been recently published by Oates, Wormeester and Arwin on spectroscopic ellipsometry studies of plasmon resonances at metal-dielectric interfaces of thin films and metallic nanostructures [27, 28].
As previously mentioned, interest for the Ag/PVA PNCs lays in the possible applications of their optical enhancing properties [29,30,31]. Some articles have been devoted to the study of the role of the polymer matrix [32] or of the chemical nature of the metal salt [33, 34]. The effects of annealing time and temperature have also been intensively studied (see e.g. [35,36,37,38]). On the contrary, the influence of the thickness of the film has only been considered in very few references [39] and most of the time a systematic study of its influence was not carried out. Moreover, the intrinsic optical properties of the nanocomposite is usually only considered to be a function of the optical properties of the metal and of the polymer and of their volume fraction.
In this article, we report in details on the influence the film thickness and on the silver doping of the PNCs on the plasmon resonance parameters. More precisely, we compare the resonance parameters in thin (less than 50 nm) and thick films (more than 300 nm) at a given Ag-doping level. We will show how, starting from the same silver-to-polymer mass ratio, nanocomposites with different refractive index can be obtained by simply tuning the film thickness. More precisely, the statistical distribution of the plasmon resonance parameters will be analyzed by multivariate statistics leading to the conclusion that at a given \(\hbox {Ag}^{+}\) doping level, thin and thick films behave differently. This effect is probably related to the growth process of the NPs in films with different thickness.
2 Materials and methods
2.1 Nanocomposite film preparation
Polymer films were prepared according to the experimental procedure described in our previous publications [21, 22] and based on the original protocol of Porel and coworkers [40, 41]. A 10% (w:w) poly(vinyl alcohol) solution in MilliQ water was prepared as a stock solution (PVA, MW = 13,000–23,000, 98% hydrolyzed, Sigma-Aldrich). Dissolution of the polymer was achieved by heating the solution at 85\(^{\circ }\hbox {C}\). \(\hbox {AgNO}_{3}\) (99.99%, Sigma-Aldrich) was added to the polymer solution after cooling to room temperature to obtain a silver concentration of 2.5% or 25%. Percentage corresponds to the w:w silver-to-polymer ratio. Required amount of water was added to the polymer stock solution to obtain 2% and 8% polymer solutions.
A small volume (\(\simeq\) 400 \(\mu\)L) of the polymer solution containing the \(\hbox {Ag}^{+}\) cations was spin-coated on piranha-cleaned silicon wafers fragments (\(<100>\), ACM, France). Silicon substrates were chosen because of their high optical contrast with polymer films and their low intrinsic roughness. Depending on the coating conditions, the final thickness of the dried film ranged from 18–25 nm (2% PVA, 60 s at 6000 rpm) to 300 nm and above (8% PVA, 60 s at 1600 rpm). After being dried in open-air atmosphere, the coatings were annealed 60 min at 110\(^{\circ }\hbox {C}\). Contrary to the case of glass substrates in which, by increasing the temperature, the coatings became yellowish-brown in color, in the case of silicon substrates, the color of the samples is mainly dominated by the interferometric color and the contribution of the plasmon resonance to the color change can hardly be seen by naked eyes. For comparison, reference PVA films were prepared according to the same method but omitting the \(\hbox {AgNO}_{3}\) in the polymer solution.
Four types of samples were prepared by varying the film thickness (‘T’: thick ; ‘t’: thin) and the doping level in silver (‘H’: high (25%) ; ‘l’: low (2.5%)). In the following, these categorical variables will be referred to as the class of the sample (‘TH’, ‘Tl’, ‘tH’or ‘tl’) (Table 1).
2.2 Surface topography characterization
The topography of the films was studied by AFM (Park XE70, Park Systems Corp., Korea). The AFM operated in the air in intermittent contact mode with commercial ACTA tips (resonance frequency: 309 kHz). Areas of 5 \(\mu\)m \(\times\) 5 \(\mu\)m, 2 \(\mu\)m \(\times\) 2 \(\mu\)m and 1 \(\mu\)m \(\times\) 1 \(\mu\)m were imaged for every sample. The resolution of the images was 256 pixels \(\times\) 256 pixels. The data were processed with the Gwyddion 2.53 software (http://gwyddion.net/) [42].
2.3 Ellipsometry
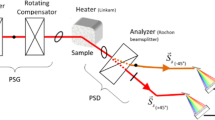
The ellipticity of the samples was measured using a SOPRA GES5E rotating polariser spectroscopic ellipsometer with a 1024 channels CCD spectrograph allowing fast and accurate measurements in the range 1.37 to 4.96 eV. The angle of incidence (AOI) was set to 70 deg. and the ellipsometer operated in parallel beam configuration (beam small axis : 4 mm). SE results were processed using the SOPRA Winelli II software.
2.4 Multivariate statistical analysis
The multivariate analysis of the data was achieved using the R statistical software [43]. The e1071 package [44] was used to carry out the classification tasks of the resonance peaks parameters.
3 Results and discussion
3.1 Surface topography of the nanocomposite
The topography of the surface was measured by AFM in intermittent contact mode after the annealing of the film at 110\(^{\circ }\hbox {C}\) during 60 min. Figure 2a, b represent the topography and phase images (1 \(\mu\)m \(\times\) 1 \(\mu\)m) of a typical 30 nm-thick film. The mass ratio of Ag/PVA was 25% (w:w). The nanoparticles of silver were clearly observed by a variation of the phase value, which provides a local information of the nature of the nanocomposite, and a local variation of the height. In Fig. 2c, d, we represented the topography and the phase images corresponding to a 300 nm-thick film at the same Ag/PVA ratio. Figure 3 presents the AFM images (topography and phase) of the control films. They don’t show a structuration at the nanoscale. The height and the phase values are normally distributed. The surfaces are smooth.
A first observation of these images of Fig. 2 shows that the size of the particles near the surface is influenced by the thickness of the film even if the silver-to-polymer weight ratio is kept constant. Figure 4 represents a boxplot of the radii r of the particles as determined from the AFM images. The radii were corrected for the embedding of the spherical particles in the matrix following \(r = \left( r_{eq}^2+h^2\right) /(2h)\) where \(r_{eq}\) is the apparent radius of the particles and h the height emerging from the polymer matrix. The mean values are 8.2 ± 2.4 nm and 4.0 ± 2.5 nm for the 25 nm (tH) and 300 nm-thick (TH) films respectively. The whiskers corresponds to the interquartile range (IQR). According to this nanoscale analysis, it is clear that the particles embedded at the surface of the thick samples are smaller than the particles in the thinner ones.
The roughness of doped and non-doped representative samples was characterized by the average surface roughness parameter (\(S_a\)) and by the root-mean-square surface roughness parameter (\(S_q\)) [45]. The measurements show a significant difference between doped and non-doped film in both cases (thick or thin films) (Table 2). As seen from both the \(S_a\) and the \(S_q\) surface roughness values, the growth of the nanoparticles induced by the annealing of the film controls the roughness of the surface, although it remains quite low (\(\le\) 1 nm).
3.2 Optical properties
The purpose of this study was to compare the optical properties of thick and thin films embedding AgNPs grown in situ from the same polymer solution. To that purpose, we prepared a series of nanocomposite films on a glass substrate and investigated the onset of the LSPR peak as a function the annealing time (Sect. 3.2.1).
To compare the optical responses of the PNCs, optical models are required to calculate the complex refractive index of the layers from SE measurements, i.e. from the \(\varPsi\) and \(\varDelta\) spectra. This second part of the study was achieved by preparing nanocomposite films on silicon substrates which are known to be more convenient for an ellipsometric study due to the high optical contrast between the substrate and the doped polymer film (Sect. 3.2.2).
3.2.1 Absorbance in the UV-VIS range
In the first series of experiments, the optimum annealing time range of the samples was determined by measuring the evolution with the annealing time of the height of the LSPR peak. These measurements were carried out using a Genesys 20 UV-Vis spectrometer (Thermo, USA). To that purpose, cleaned glass substrates were coated with the PVA 8% solution at a 25% Ag/PVA mass ratio and annealed at 110\(^{\circ }\hbox {C}\). The annealing time was varied from 10 to 60 min and the spectra were recorded from 300 to 800 nm. The reference spectrum was measured on an uncoated glass substrate. As expected the plasmon resonance was observed slightly above 415 nm and the position of the peak did not significantly vary with the annealing time (Fig. 5).
Absorbance spectra of a characteristic series of AgPVA films as a function of their annealing time (T = 110\(^{\circ }\hbox {C}\), Ag/PVA mass ratio: 25%, film thickness \(\simeq\) 400 nm). No significant change in the LSPR peak position is observed (\(\varLambda _0 = 416.6 \pm 0.4\) nm, open circles)
As shown in Fig. 6, the absorbance stabilizes after 40 to 50 min, suggesting the complete reduction of the \(\hbox {Ag}^{+}\) ions. The fluctuations are due to the changes in thickness from one sample to the other. The absence of a significant shift in the LSPR peak position shows that, in a first approximation, the average size of the AgNPs does not significantly vary.
3.2.2 Spectroscopic ellipsometry
Spectroscopic ellipsometry (SE) is a widely used optical analysis technique based on the change of polarization of light after reflection of an incident light beam on a substrate or on a stratified sample [46, 47]. Due to its non-destructive nature, SE is a powerful technique to analyze at once the optical properties of materials and the thickness of the different layers of the sample. More specifically, one measures at a given wavelength the ellipticity \(\rho\) which is defined by
where \(r_p\) and \(r_s\) are the complex reflectance coefficients of the \(p-\) and \(s-\)components of the incident light, respectively. \(\varPsi\) and \(\varDelta\) are the ellipsometric angles. They are explicitly defined by
where \(\delta _p\) and \(\delta _s\) are the phase-shifts undergone by the \(p-\) and the \(s-\)components of the incident wave during the reflection. The angle \(\varDelta\) therefore measures the relative phase-shift between the perpendicular components of the propagating light wave. Except in a very limited number of cases, \(\varPsi\) and \(\varDelta\) cannot be directly converted into the refractive index and/or film thickness. Indeed, the ellipticity is a complex quantity which is a function of the angle of incidence, of the optical properties of the materials and of the thickness of the layers. Taking into account the dispersion of the dielectric function, Eq. 1 has to be inverted in most cases using numerical methods, assuming specular reflection of light at ideal planar interfaces. Contrarily to the UV-VIS spectrophotometric measurements [48, 49], reflection, absorption and transmission of light are considered at once in a SE measurement.
3.2.3 Modeling of the optical properties
The models used to interpret the SE data are described hereinafter and illustrated in Fig. 7.
PVA layer: A one-layer Cauchy model was chosen to represent the optical properties of the PVA films in the transparent range (\(k_{PVA}(\lambda ) = 0\))
Schematic representation of the optical models used to interpret SE data: a PVA layer on silicon, b Cauchy model used to describe the optical properties of the PVA layer, c AgNPs in PVA layer on Si and d a Cauchy model and Lorentzian oscillator used to describe the optical properties of AgNPS in PVA matrix. Shaded area between the polymer layer and the substrate in a) and c) schemes: native \(\hbox {SiO}_{2}\) layer. (2 nm, not to scale)
Ag-PVA layer: To the Cauchy model described above, a Lorentzian oscillator was added to account for the localized absorption of the plasmon resonance in visible range [47]. The specific contribution of the plasmon resonance to the wavelength-dependent complex dielectric function \(\varepsilon (\lambda )\) is given by
where \(\lambda\) denotes the wavelength. \(\varLambda _0\) is the resonance wavelength of the oscillator, A its oscillator strength (i.e. amplitude of the resonance) and \(\varGamma _0\) its full-width at half maximum (FWHM) and is hereinafter referenced to as the ‘width’ of the resonance. \(\varepsilon _\infty\) represents the contributions to \(\varepsilon _r\) from the resonances at wavelengths much greater than the measurable frequency range.
Ellipsometric spectra of Ag nanoparticles-doped PVA films (Ag/PVA ratio: 25 % w:w): a ‘tH’ thin films (thickness: 25.4 nm); b ‘TH’ thick film (thickness: 293.9 nm). Experimental data: \(\alpha = \cos (2 \varPsi )\) (filled circles) and \(\beta = \sin (2\varPsi ) \cos (\varDelta )\) (open circles). Dashed lines: optimized results from the optical model
One of the main advantages of SE over conventional spectrophotometric methods is that it allows in most cases a simultaneous determination of the optical properties and of the thickness of the layers and not only the measurement of absorption or of transmission spectra. Figure 8 represents the SE data corresponding to a 25.4 nm-thick film (Fig. 8a) and to a 293.9 nm-thick film (Fig. 8b). Instead of representing the \(\varPsi\) and \(\varDelta\) ellipsometric angles, we have chosen to present the \(\alpha = \cos (2\varPsi )\) and \(\beta = \sin (2\varPsi ) \cos (\varDelta )\) Fourier coefficients, which are closer to the experimental quantities measured by the ellipsometer. With respect to reference films of equivalent thickness, the ellipsometric data present spectra distortion in the 3.0–3.5 eV range. This is attributed to the localized plasmon resonance associated with the silver NPs. This effect is more noticeable for the thick films (Fig. 8b): one can see three peaks in the \(\alpha\)-spectrum (filled symbols) but the second is considerably damped due to the energy absorption at the resonance. The data generated by the optimized optical model are also presented in Fig. 8 (dashed lines). They are in close agreement with the experimental data: the residual \(\chi ^2\) values are respectively equal to 2.7 10\(^{-4}\) and 2.9 10\(^{-3}\) for the thin and thick films.
As previously described [21], to model the optical behavior of our samples, a one-layer Cauchy model was chosen to represent the optical properties of the polymer matrix and a Lorentzian oscillator was added to account for the localized absorption of the plasmon resonance in the visible range. The use of the Cauchy model for the polymer matrix is justified by the monotonous increase of the refractive index of the polymer when going from the infrared to the UV, as expected for dielectric materials far from the fundamental absorption edge. The effect of the presence of the Lorentzian oscillator on the model is equivalent to using the Maxwell-Garnett effective medium approximation (MG-EMA) in a view of obtaining the dielectric function of the nanocomposite films [28, 50, 51]. One may expect our approach to be less dependent on the shape/size of the embedded NPs than the MG-EMA for which the depolarization factors linked to the particles shape have to be specified. Therefore, with our approach, two problems are implicitly solved : the dependence of the dielectric function of the silver NPs and the generalization of the MG-EMA to take into account the size and shape statistical distributions of the NPs. These two problems can easily be solved for spherical NPs [12, 26, 52] but require more complex approaches for non-spherical particles (see e.g. [13, 53, 54]). Moreover, these advanced models have to be supported by complementary experiments based e.g. on the quantitative analysis of transmission electron microscopy images to obtain the size distribution of the NPs. Also is avoided the influence of the doping level of the films: at high doping levels, it is expected that the nanocomposite films cannot be considered anymore as diluted solutions of NPs in a polymer matrix. The analysis of the optical response of nanocomposite films containing a high concentration of metal nanoparticles is more relevant of the metal island model [55, 56] which has been recently generalized by Wormeester and co-workers [57, 58]. Adding roughness or void inclusions did not significantly improved the quality of the fits, even for the thin samples.
The optical properties of the NPs-doped films are represented in Fig. 9a. For each type of film, the refractive index n and the extinction coefficient k of two different samples are represented to show the reproducibility of the analysis and indirectly, of the sample preparation. The presence of the silver NPs considerably modifies the optical properties in the doped polymer films: the presence of the absorption peak in the 2.5–3.5 eV range of the extinction coefficient spectrum (Fig. 9b) induces a large oscillation in the refractive index value (Fig. 9a) because of the Kramers-Krönig consistency of the optical properties and of the Lorentzian model of the peak. The perturbation of the refractive index occurs over the 1.5–4.5 eV range although the variation of the absorption peak is limited and the refractive index values are higher than the ones of a pure polymer film (see e.g. Fig. 5 in reference [22]). The optical properties of thin and thick films are clearly different as experimentally demonstrated here: starting from the same silver-to-polymer mass ratio, nanocomposites with different refractive index can be prepared by simply tuning the film thickness. The optimized parameters of the plasmon resonance peak are given in Table 3. In thin films, the amplitude of the resonance is higher, the position of the resonance smaller and the peaks wider than in thick ones.
3.3 Multivariate analysis
Once the optical properties of the nanocomposites are determined and remembering that they are normally decorrelated from the thickness of the film, we considered the dispersion of the data cloud, the optical response of each nanocomposite being characterized by 3 parameters (A, \(\varLambda _0\) and \(\varGamma _0\)) where A represents the oscillator strength or the amplitude of the resonance, \(\varLambda _0\) correspond to the central resonance wavelength and depends on the size of the NPs, and finally where \(\varGamma _0\) describes the width of the resonance and can be used to characterize the polydispersity of the NPs in the film. Statistical classes were defined on the basis of the thickness and doping of the samples (Table 4).
3.3.1 Data clouds of the resonance parameters
The samples considered for this study are distributed among 4 classes of relatively equivalent sizes. Each of the four classes are represented by a different color. Let us first consider the link between the thickness of the film and the strength of the plasmon band A (Fig. 10). One can easily see that the data cloud is subdivided into four areas, each of them being assigned to one type of nanocomposite. Samples with high doping level are on the right side of the plot with a higher value of the absorbance A in both case (thin and thick films, green and red symbols, respectively).
Scatterplot of the thickness of the film (in \(\mu\)m) versus the strength of the oscillator. Color coding of the categorical variables are given in the legend and refer to Table 1
After this first analysis between the amplitude of the resonance and the thickness, it is interesting to plot the dispersion cloud for each pair of parameters, yielding a symmetric scatter plot matrix (Fig. 11). In the \(\varLambda _0 - A\) and \(\varGamma - A\) plots, different behaviors can be identified between the PNCs prepared from solutions with a high \(\hbox {AgNO}_{3}\) concentration (red and green circles) and those prepared from diluted \(\hbox {AgNO}_{3}\) solutions (blue and yellow circles). However, it seems more complicated to separate samples from different categories in the same subcloud: for example, red and green symbols are overlapping in the right subcloud for both graphs.
On the contrary, in the \(\varGamma _0\) - \(\varLambda _0\) plot, all subclouds are strongly overlapping and the interpretation of the plot is rather more complicated. This complicated link between the width of the resonance and its position has already been considered by Berg and coworkers for mono- and polydispersed silver NPs in a glass matrix [59]. They observed a non monotonous variation. Starting from small particles (ca. 2 nm), an increase of the size of particles lead to a decrease of the FWHM and a blue shift of the position of the resonance peak until the radius of the particle has reached 10 nm. For larger particles, both FWMH and position of the resonance peak increase.
This global picture of the data cloud is reinforced by the statistical dispersion of the resonance parameters in terms of box-and-whiskers plots (Fig. 12).
As expected, the strength of the oscillator A is strongly dependent on the silver content of the film (Fig. 12a). Two trends emerge from the peak position dispersion: an increase of the amount of \(\hbox {Ag}^{+}\) in the films (\(l \rightarrow H\)) blue-shifts the resonance peak. A decrease of the thickness of the films (\(T \rightarrow t\)) slightly red-shifts the resonance wavelength (Fig. 12b). This supports the fact that thickness plays an important role in the resonance parameters and therefore on the in situ synthesis of the AgNPs.
The evolution of the resonance width is more complicated (Fig. 12c). For example, increasing the film thickness (\(t \rightarrow T\)) leads to an increase of the FWMH for high doped samples and a decrease of FWMH for low doped samples. Looking at the decrease of the amount of \(\hbox {Ag}^{+}\) (\(l \rightarrow H\)) leads to an increase of the FWMH for thick samples and a decrease of the FWMH for thin samples. By considering only the FWMH, it is complicated to draw a trend. For that reason, the behaviors of the films with low and high silver contents have to be separately considered in the remaining parts of this analysis.
3.3.2 Classification in the \(\varGamma _0 - \varLambda _0\) plane
As we have seen from the scatter plot matrix and the box-and-whiskers plots, thick and thin films differently behave when we consider the resonance width (\(\varGamma _0\)) and the position of the plasmonic band (\(\varLambda _0\)). We, therefore, attempted to classify the films as a function of the silver content of the coated solution (2.5% or 25%). The classification task was performed for thin and thick films separately (Fig. 13) using support vector machines (SVM). They are statistical classification algorithms used to find the best classifier (i.e. separator) between two sets of (eventually overlapping) data points [60].
When the data are presented in the \(\varGamma _0 - \varLambda _0\) plane, one can see that the set of data points corresponding to the highly doped films (circles) has only a slight overlap with the cloud of the films prepared from the 2.5% solution (triangles). To perform the classification task in a quantitative way, we used a SVM algorithm [44] derived from the original classification algorithm proposed by Vapnik [60]. In the case of overlapping data clouds, the classifier has to be tuned with respect to the penalty applied when an event is misclassified. Optimum results for the classifiers are represented in Fig. 13. The figure shows that, when applied to our data, SVMs allowed us to discriminate between the high- and low-doping level films in a very efficient way: only 3 films over 33 were misclassified in the thin films case and 3 over 54 in the thick films one. The most striking feature of the classification is the behaviour of the classifiers: when comparing panels (A) and (B) in Fig. 13, one can easily see that their slope have opposite signs. The classifiers slopes are respectively \(-0.36\) (thick films) and \(+0.30\) (thin films). The opposite sign in the slope of the separator confirm the difference in the optical behavior between thin and thick films in terms of position and width of the plasmon band.
3.4 Growth of nanoparticles
These different optical behaviours are probably strongly related to the growth process of the NPs in films of different thickness. We will discuss this point in the following paragraphs. Since the pioneer work of LaMer and Dinegar [61], the kinetics of growth of nanoparticles in solution has been the subject of an increasing number of publications and reported mechanism has been partially transposed to explain the growth of NPs in films. For both types of systems (films and solutions), one may expect the precursor solute to reach a critical level for nucleation. This prenucleation step is fed by the spatial distribution of the precursors (in our case, the \(\hbox {Ag}^{+}\) ions) which we can consider to be isotropic within the film volume. As pointed out by Sugimoto [62], in films or sol-gel system, nucleation and growth of the generated nuclei are separated due to the reduction of the supersaturation with the stabilization of the matrix, in contrast to the Lamer mechanism. In our case, it seems that, after the nucleation, the growth process is limited by the diffusion of \(\hbox {Ag}^{+}\) ions or of the \(\hbox {Ag}^{0}\) atoms in the film. The shape of AgNPs are spherical in both case (i.e. thin and thick films) but their sizes are different as clearly demonstrated in Fig. 4. A possible explanation of this different growth process can be associated with the diffusion process. In fact, in films whose thickness corresponds to a couple of NPs equilibrium diameter, NPs grow in a 2D-like matrix and the diffusion of silver ions is constrained in 2 dimensions by the substrate and the film/air interface. On the contrary, in thick films, the interfacial effect is expected to be less marked and the NPs rapidly form a 3D structure. The possible interpretation of the spatial distribution of the AgNPs is schematized in Fig. 14. A similar effect has been recently reported in \(\hbox {Au}-\hbox {PVA}\) films for which imaging ellipsometry shows local inhomogeneity of the optical response [18].
4 Conclusions
In summary, we have studied the surface topography and the optical properties of nanocomposite containing in situ grown silver nanoparticles. In particular, the study has been focused on the influence of the thickness of the film and of the silver concentration in the polymer matrix on the optical properties of the nanocomposite. We experimentally observed a different behaviour of thick and thin films as a function of the silver concentration. Topography and phase images of thin and thick nanocomposites were measured by AFM and demonstrated that the size of the AgNPs seems to be larger in thin films than in thick films. The statistical distribution of the plasmon resonance parameters (A, \(\varLambda _0\) and \(\varGamma _0\)), obtained by spectroscopic ellipsometry, has been analyzed using large margin classifiers. This multivariate analysis method leads to the conclusion that at a given \(\hbox {Ag}^{+}\) doping level, thin and thick films behave differently, probably due to a different spatial distribution of the NPs in the polymer matrix limited by the diffusion process of ions or of the silver atoms in the film. This variation in the spatial distribution in thin and thick films is a possible explanation for the different behavior in the optical properties despite the fact that the diffusion process of ions in thin solid films still remains an open question.
References
S.W. Hsu, A.L. Rodarte, M. Som, G. Arya, A.R. Tao, Chem. Rev. 118, 3100 (2018)
R. Kuladeep, L. Jyothi, S. Chakradhar, D. Rao, Opt. Eng. 53, 071823 (2014)
Y. Deng, Y. Sun, P. Wang, D. Zhang, H. Ming, Q. Zhang, Physica E 40, 911 (2008)
S. Anthony, S. Porel, D. Rao, T. Radhakrishnan, Pramana J. Phys. 65, 871 (2005)
X. Chen, J. Tao, G. Zou, Q. Zhang, P. Wang, Appl. Phys. A Mater. Sci. Process. 100, 223 (2010)
J.R. Morones, J.L. Elechiguerra, A. Camacho, K. Holt, J.B. Kouri, J.T. Ramírez, M.J. Yacaman, Nanotechnology 16, 2346 (2005)
N. Bhat, N. Karmakar, D. Kothari, Int. J. Nanosci. 12, 1350029 (2013)
A. Fuchs, S. Ritz, S. Pütz, V. Mailänder, K. Landfester, U. Ziener, Biomater. Sci. 1, 470 (2013)
T. Galya, V. Sedlarik, I. Kuritka, R. Novotny, J. Sedlarikova, P. Saha, J. Appl. Polym. Sci. 110, 3178 (2008)
N. Durán, M. Durán, M.B. de Jesus, A.B. Seabra, W.J. Fávaro, G. Nakazato, Nanomed. Nanotechnol. Biol. Med. 12, 789 (2016)
Y. Wei, H. Ebendorff-Heidepriem, J.T. Zhao, Adv. Opt. Mater. p. 1900702 (2019)
U. Kreibig, M. Vollmer, Optical Properties of Metal Clusters (Springer, New York, 1995)
D.D. Evanoff, G. Chumanov, ChemPhysChem 6, 1221 (2005)
K.L. Kelly, E. Coronado, L.L. Zhao, G.C. Schatz, J. Phys. Chem. B 107, 668 (2003)
C.J. Murphy, T.K. Sau, A.M. Gole, C.J. Orendorff, J. Gao, L. Gou, S.E. Hunyadi, T. Li, J. Phys. Chem. B 109, 13857 (2005)
X. Huang, S. Neretina, M.A. El-Sayed, Adv. Mater. 21, 4880 (2009)
C.J. Murphy, L.B. Thompson, D.J. Chernak, J.A. Yang, S.T. Sivapalan, S.P. Boulos, J. Huang, A.M. Alkilany, P.N. Sisco, Curr. Opin. Colloid Interface Sci. 16, 128 (2011)
C. Guyot, P. Vandestrick, I. Marenne, O. Deparis, M. Voué, Beilstein J. Nanotechnol. 10, 1768 (2019)
Z.H. Mbhele, M.G. Salemane, C.G.C.E. van Sittert, J.M. Nedeljković, V. Djoković, A.S. Luyt, Chem. Mater. 15, 5019 (2003)
L. Nicolais, G. Carotenuto, Nanocomposites: In Situ Synthesis of Polymer-Embedded Nanostructures (Wiley, Amsterdam, 2014)
N. Dahmouchène, S. Coppée, M. Voué, J. De Coninck, Physica Status Solid. C 5, 1210 (2008)
M. Voué, N. Dahmouchène, J. De Coninck, Thin Solid Films 519, 2963 (2011)
C. Guyot, P. Leclère, M. Voué, J. Vac. Sci. Technol. B 38, 013602 (2020)
S. Kurbitz, J. Porstendorfer, K.J. Berg, G. Berg, Appl. Phys. B Lasers. Opt. 73, 333 (2001)
T. Oates, Appl. Phys. Lett. 88, 213115 (2006)
T. Oates, E. Christalle, J. Phys. Chem. C 111, 182 (2007)
T. Oates, H. Wormeester, H. Arwin, Prog. Surf. Sci. 86, 328 (2011)
H. Wormeester, T. Oates, in Ellipsometry at the Nanoscale, ed. by M. Losurdo, K. Hingerl (Springer, Berlin, 2013), chap. 6, pp. 225–256
T. Baker, O. Monti, D. Nesbitt, J. Phys. Chem. C 115, 9861 (2011)
T. Jiang, J. Li, L. Zhang, B. Wang, J. Zhou, J. Alloy. Compd. 602, 94 (2014)
D. Whitcomb, B. Stwertka, S. Chen, P. Cowdery-Corvan, J. Raman Spectrosc. 39, 421 (2008)
R. Abargues, K. Abderrafi, E. Pedrueza, R. Gradess, J. Marqués-Hueso, J.L. Valdés, J. Martínez-Pastor, New J. Chem. 33, 1720 (2009)
O. Yeshchenko, I. Dmitruk, A. Dmytruk, A. Alexeenko, Mater. Sci. Eng. B 137, 247 (2007)
C. Sun, R. Qu, C. Ji, Y. Meng, C. Wang, Y. Sun, L. Qi, J. Nanopart. Res. 11, 1005 (2009)
G.V. Ramesh, S. Porel, T.P. Radhakrishnan, Chem. Soc. Rev. 38, 2646 (2009)
G. Ramesh, B. Sreedhar, T. Radhakrishnan, Phys. Chem. Chem. Phys. 11, 10059 (2009)
S. Clémenson, L. David, E. Espuche, J. Polym. Sci. Part A: Polym. Chem. 45, 2657 (2007)
S. Clémenson, D. Léonard, D. Sage, L. David, E. Espuche, J. Polym. Sci. Part A: Polym. Chem. 46, 2062 (2008)
S. Clémenson, P. Alcouffe, L. David, E. Espuche, Desalination 200, 437 (2006)
S. Porel, S. Singh, S.S. Harsha, D.N. Rao, T.P. Radhakrishnan, Chem. Mater. 17, 9 (2005)
S. Porel, N. Venkatram, D.N. Rao, T.P. Radhakrishnan, J. Nanosci. Nanotechnol. 7, 1887 (2007)
D. Necas, P. Klapetek, Cent. Eur. J. Phys. 10, 181 (2012)
R Core Team, R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria (2012). http://www.R-project.org/. ISBN 3-900051-07-0
D. Meyer, E. Dimitriadou, K. Hornik, A. Weingessel, F. Leisch, e1071: Misc Functions of the Department of Statistics (e1071), TU Wien (2014). http://CRAN.R-project.org/package=e1071. R package version 1.6-2
P. Klapetek, Quantitative Data Processing in Scanning Probe Microscopy - SPM Applications for Nanometrology (Elsevier, Amsterdam, 2012)
R.M.A. Azzam, N.M. Bashara, Ellipsometry and Polarized Light (North-Holland, Amsterdam, 1977)
H. Tompkins, E. Irene (eds.), Handbook of Ellipsometry (Springer, New York, 2005)
E.M. El-Menyawy, G.M. Mahmoud, R.S. Ibrahim, F.S. Terra, H. El-Zahed, I.K. El Zawawi, J. Mater. Sci. Mater. Electron. 27, 10070 (2016)
S. Aziz, M. Rasheed, H. Ahmed, Polymers 9, 194 (2017)
D.E. Aspnes, J.B. Theeten, F. Hottier, Phys. Rev. B 20, 3292 (1979)
D.E. Aspnes, in Ellipsometry at the Nanoscale, ed. by M. Losurdo, K. Hingerl (Springer-Verlag, 2013), chap. 5, pp. 225–256
A.S. Keita, A.E. Naciri, Phys. Rev. B 84, 125436 (2011)
J. Toudert, L. Simonot, S. Camelio, D. Babonneau, Phys. Rev. B Condens. Mater. Mater. Phys. 86, 045415 (2012)
Y. Battie, A.E. Naciri, W. Chamorro, D. Horwat, J. Phys. Chem. C 118, 4899 (2014)
J. Lekner, Theory of Reflection of Electromagnetic and Particle Waves (Martinus Nijhoff Publishers, Leiden, 1987)
D. Bedeaux, J. Vlieger, Optical Properties of Surfaces (Imperial College Press, London, 2002)
E.S. Kooij, H. Wormeester, E.A.M. Brouwer, E. van Vroonhoven, A. van Silfhout, B. Poelsema, Langmuir 18, 4401 (2002)
H. Wormeester, E.S. Kooij, B. Poelsema, Phys. Rev. B 68, 085406 (2003)
K.J. Berg, A. Berger, H. Hofmeister, Zeitschrift für Physik D Atoms Mole. Clust. 20, 309 (1991)
C. Cortes, V. Vapnik, Mach. Learn. 20, 273 (1995)
V.K. LaMer, R.H. Dinegar, J. Am. Chem. Soc. 72, 4847 (1950)
T. Sugimoto, J. Colloid Interface Sci. 309, 106 (2007)
Acknowledgements
This work was supported by the Fonds de la Recherche Scientifique - FNRS under Grant nr 1926111.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guyot, C., Voué, M. Intrinsic optical properties of Ag-doped poly-(vinyl alcohol) nanocomposites: an analysis of the film thickness effect on the plasmonic resonance parameters. Appl. Phys. A 126, 870 (2020). https://doi.org/10.1007/s00339-020-04055-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-020-04055-7