Abstract
We report the investigation of structure, morphology, photocatalytic degradation of methyl blue using ZnO nanorods (NRs). The ZnO NRs samples were fabricated using electrochemical deposition from aqueous solution of zinc nitrate and hexamethylenetetramine on ITO substrates. The effect of the growth time on the structure, morphology and photocatalytic properties of ZnO NRs was investigated in details.XRD analysis showed that the fabricated NRs have hexagonal wurtzite structure and growth direction along the (002) plane. The morphology of the prepared samples investigated by SEM showed that growth rate of the NRs depends on the time of the growth. The surface area and cross-sectional area of the fabricated ZnO NRs increased with increasing the growth time and led to improvements in the NRs aspect ratios. It was also found that the photocatalytic activity of the grown nanorods enhances by increasing the deposition time. The present results are discussed in terms of the observed structural morphology of the investigated ZnO NRs and correlated with the photocatalytic performance of the grown NRs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
ZnO is one of the widely studied materials because of its interesting physical properties and numerous technological applications [1,2,3,4]. In addition, it is characterized as a semiconductor material with wide band gap of about 3.3 eV and possess large excitonic binding energy = 60 meV which makes it a very promising material for UV lasing applications [5]. Furthermore, ZnO can be a candidate material for different applications, including, gas-sensing devices and solar cells [6,7,8,9]. The ZnO nanorods are among the most interesting ZnO structures and have been utilized in various promising applications [10]. They have been fabricated with different expensive methods such as chemical vapor deposition and metal organic chemical vapor deposition [11,12,13].
Recently, the electrochemical method is commonly used for the fabrication of ZnO nanorods due to its green, inexpensive, low temperature, easy control of growth and morphology [14, 15]. Furthermore, the structure and morphology of the fabricated materials using electrochemical deposition method can be controlled by various parameters including the electrolyte type, concentration, temperature, deposition time, applied potential or current and substrate type [15, 16]. For example, the applied potential has been utilized by Tezcan et al. to control the morphology and the thickness of the electrochemically grown ZnO NRs and to optimize their photo-electrochemical response for water splitting reactions [17]. In another study by Yang et al., the effect of the deposition time, the applied voltage and the deposition temperature on the grown ZnO nanorods along with the growth mechanism has been investigated [18]. They have reported that by changing the aforementioned parameters ZnO structure with different morphologies ranging from nanorods to spindle, flakes and lamellar ZnO structures were produced. These examples in addition to many other reports on the electrochemical growth and properties of ZnO structures highlight their potential for many applications, especially when considering the advantages of the low-cost electrochemical deposition technique. However, efforts are still needed to explore the growth-related parameters and their utilizations to optimize the performance of the electrochemically deposited ZnO NRs for various applications. ZnO nanostructures behavior is highly sensitive to their size and shape due to the predominant surface contribution resulting from the large surface to bulk ratio. The surface effects in nanostructures are very pronounced by the high surface-to-volume ratio, for NRs equal 2/r, and an enhancement of their properties is expected at the surface due to surface related effects. The bending of the surface band at the NR surface is expected to play a crucial role as well. Therefore, the objective of the present work is to investigate the effect of the fabrication conditions on the structure, morphology and photocatalytic activity of ZnO nanorods prepared by electrochemical method.
2 Experimental procedures
ZnO NRs were prepared by electrodeposition method. In the preparation experiments, three-electrode cell, containing a working electrode (WE), a reference electrode (RE) and a counter electrode (CE), is connected to Autolab PGSTAT101 using NOVA electrochemical software. The potential and current were controlled and reordered via the Nova software. The reference electrode is Ag/AgCl in saturated KCl solution, while working electrode is inert platinum wire. The ZnO nanorods were grown on clean ITO glass substrate (working electrode) which was ultrasonically cleaned using isopropanol and deionized water. The aqueous solution was prepared by mixing of 0.03 M Zn(NO3)26H2O and 0.03 M hexamethylenetetramine (HMT) in a clean beaker containing a triple-distilled water. In the next step, the solution was stirred for 10 min before the deposition process. The three electrodes (WE, RE and CE) were lowered into the cell containing the solution with the WE electrode (cathode) facing the CE electrode, and the solution was stirred constantly during the deposition process. In the deposition experiments, the potentiostatic mode was used with potential = − 1.0 V with respect to the reference electrode. The ZnO samples were electrochemically deposited at a temperature of 80 °C and different periods (30, 60 and 120 min). The ZnO nanorods were grown on the cathode electrode in terms of following chemical reactions:
More \({\text{OH}}^{ - }\) ions are produced by the chemical reaction of HMT (\({\text{C}}_{6} {\text{H}}_{12} {\text{N}}_{4}\)) with the water at a temperature of 80° as follows:
At the end of the deposition experiments, the prepared ZnO nanorods samples were carefully removed from deposition cell and immersed several times in deionized water for washing and then were dried in air. Three samples, denoted as samples 1, 2 and 3 corresponding to the nanorods grown at 30, 60 and 120 min, respectively, were used for the characterizations.
The structure of ZnO nanorods samples was investigated using a Philips X-ray diffractometer (Model-1710), while the morphology was examined by using a FEI Quanta 250 SEM.
On the other hand, the photocatalytic experiments were performed using the fabricated ZnO nanorods on ITO substrate. Three ZnO samples prepared at different deposition times (30, 60 and 120 min) were immersed in methylene blue (MB) which has a concentration C0 = 10–5 M. Then Pyrex cell containing the methylene solution was exposed to UV radiation from an artificial sunlight simulator of 450 W/m2 (Model Oriel SO12A) associated with an ultraviolet filter. The organic dye degradation was investigated by measuring the optical absorbance spectra as a function of the irradiation time. The absorption spectra were reordered after different irradiation exposure durations using spectrophotometer (Lambda 750). The mechanism of photocatalytic degradation of methylene blue using ZnO (as a catalyst) could take place according to the following reactions [19]:
Based on the above reactions, the exposure of ZnO samples to the radiation of UV causes a generation of electron–hole pairs. The reaction of these free carriers with the water produces hydroxyl and superoxide radicals which react with the dye (methylene blue) and form oxidation products. The residual concentration of methylene blue \(C\) after irradiation for a period time can be calculated using the optical absorption (A):
where e is the molar absorptivity with the unit of L/mol cm and b is the path length of the sample in centimeters. According to this given information, Eq. (14) is written in the following form:
3 Results and discussion
3.1 Structural and morphology
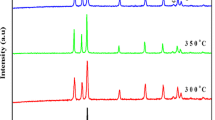
The structural properties of the ZnO nanorods, grown on ITO substrates, were determined by XRD technique as shown in Fig. 1. The crystal structure of the nanorods is hexagonal with lattice parameters a = 3.2568, b = 3.2568 and c = 5.2125 Å. Several XRD peaks are observed which match to the reported data of the bulk ZnO crystal (PDF number: 01-079-0207). The peaks labeled (*) correspond to the ITO substrate. One can see that XRD intensity of some peaks increases with increasing the time of the deposition. The highest intensity corresponds to the sharp (002) peak at 34.18°. In addition, it is clearly seen that the intensity of this peak increases with increasing the deposition time. On the other hand, the number of peaks is increased with the growth time, indicating improvement of the crystallinity and different orientation of deposited nanorods.
The texture coefficient \(R_{hkl}\), which is defined as the ratio of intensity of a particular XRD peak to the sum of the intensities of major peaks, can be used to determine the growth direction of the nanorods. The factor \(R_{hkl}\) of a particular orientation (hkl) is calculated by using the following relation [20]:
where \(I_{hkl}\) is the measured intensity of a particular plane \(hkl\), \(I_{{o\left( {hkl} \right)}}\) is its relative intensity from the reported PDF data, and N is the number of XRD peaks. The factor \(R_{hkl}\) of the 002 plane increases with increasing the growth time as shown in Fig. 2a. This reveals that the growth direction of the NRs is along the (002) plane.
The crystallite size (S) of the investigated ZnO NRs was calculated using the Scherrer equation [21]:
where \(\lambda\) is the X-ray radiation wavelength, B half maximum full-width of diffraction intensity, and \(\theta\) is the angle of X-ray diffraction. The values of the crystallite size (S) of ZnO samples are shown in Table 1 and plotted against the growth time in Fig. 2b. One can see that the average crystallite size of the studied nanorods decreases from 19.40 to 18.48 and then increases to 21.69 nm with increasing the growth time from 30 up to 120 min. This indicates that the sample 2 possesses lower crystallite size.
The morphological images of the grown nanorods at different times are shown in Fig. 3. The images show clearly that the deposition time has considerable influence on the growth of the nanorods as well as their morphologies. The sample grown at 30 min shows formation of highly dense regular as well as irregular hexagonal grains with an average size of about 0.4 μm2. After increasing the deposition time to 60 min, an array of thinner hexagonal ZnO nanorods is formed in the sample as seen from the Fig. 3b. Further increase in the deposition time resulted in an increase in the length and diameter of the grown nanorods as seen from part (c) in the figure for the sample grown for 120 min.
The effect of growth time on the grown nanorods is shown in Fig. 4a. It can be seen from the figure that the average growth rate at 30 min is about 0.04 μm/min and decreases to about 0.032 μm/min and 0.026 μm/min as the growth time increases from 30 to 120 min, respectively. The decrease in the growth rate with increasing the growth time is, in fact, expected as the amount of the precursors used in the growth solution is reduced over the time and leads to reduced growth rate. On the other hand, the average surface area of the grown nanorods increases with increasing growth time as shown in the figure. After 30 min of growth, the nanorods exhibit an average surface area of about 2.7 μm2 which increased by about 42.3% to 3.9 μm2 when the nanorods were left to grow for 60 min. However, a substantial increase in the surface area by about 147.8% of the grown nanorods was achieved by increasing the growth time to 120 min. Increasing the growth time allows the nanorods to grow more in both length and diameter and, as a result, to have an increased surface area.
The effect of growth time on the average cross-sectional area of the grown NRs and their density was also investigated and is shown in Fig. 4b. The variation in the cross section of the NRs is mainly due to the change in the diameter during the growth process and is also related to the density of the grown nanorods. As seen from the figure, the cross-sectional area of the nanorods is on average about 0.4 μm2 for the nanorods grown for 30 min and decreased to less than 0.3 μm2when the growth time was increased to 60 min and then increased to about 0.73 μm2forming thick nanorods for the samples grown for 120 min. When linked to the obtained SEM images of the samples, the variation of the cross-sectional area of the grown nanorods can be explained based on their growth stages times. At the first stages of the growth process, nucleation stages, a development of aggregates of hexagonal and also non-hexagonal grains takes place as seen from the morphological images in Fig. 3a of the sample grown for 30 min. These grains form the nucleation centers that grow later to from the nanorods. After that, the growth of the nanorods starts to gradually progress. The nanorods at the beginning grow mainly in the longitudinal direction and then start to grow in both the longitudinal and lateral directions as in the case of sample grown for 60 min and 120 min, respectively. The growth rate in the longitudinal and lateral directions depends on the ratio of Zn ions diffusion rate to the hydroxide ions generation rate [22]. High OH− concentration, especially with the presence of HMT, hinders the lateral growth of the nanorods, and the growth becomes more dominant along the vertical direction. However, at prolonged deposition times, the diffusion rate of Zn++ and the generation rate of OH− become comparable which permits the growth in both the vertical and lateral directions. This leads to the thickening of the NRs as seen from the SEM images of the sample grown at 120 min as well as the observed increase in the NRs cross-sectional area. As a result, a variation in the nanorods density occurs with increasing the deposition time as seen from Fig. 4a. The highest density was obtained at a deposition time of 60 min and with increasing the deposition time the density decreases due to the increased nanorods lateral growth. The increase in the diameter of the grown NRs as the deposition time is increased can also be related to the calculated crystallite size values (Table 1) and used to explain the observed improvement in the crystallinity of the grown ZnO NRs.
3.2 Photocatalytic properties
The photocatalytic degradation of methylene blue, using the fabricated ZnO NRs which act as catalyst, is investigated by measuring the changes of the absorption spectra as a result of the effect of the UV irradiation exposure. Figure 5 shows the absorption spectra of the methylene blue in the presence of sample 3 (nanorods fabricated at 120 min) at different irradiation times from 30 up to 175 min and all investigated samples as a function of the growth time. The UV–Vis absorption spectra showed two MB peaks which have maximum absorption value at \(\lambda_{\max }\) = 294 and 664 nm. Furthermore, the absorption values of MB decrease with increasing the time of irradiation and that of NRs growth. This is an indication that both irradiation and growth time have an effect on the photodegradation of MB. In addition, the difference between the absorbance values of the sample grown at 30 and 60 min is little bit small.
The photocatalytic efficiency \(\beta\) of MB is calculated using the relation [23]:
where \(C\) and \(C_{0}\) are residual (after irradiation of time) and initial concentrations (without irradiation) of MB, respectively. \(A_{0}\) and \(A\) are the optical absorption at \(\lambda_{\max }\) =664 nm, corresponding to the initial C0 and residual concentration C of MB, respectively.
The effect of time of irradiation on the the relative concentration (C/C0) of MB as well as efficiency (\(\beta\)) of the nanorods grown at 120 min is shown in Fig. 6. As observed in the figure, the photodegradation enhances by increasing the time of irradiation. In addition, the degradation efficiency increases from 7.78 to 31.36% with increasing the irradiation time from 30 to 175 min. This reveals that the photocatalytic activity of ZnO NRs is enhanced by increasing the irradiation time. This could be due to increasing the free radicals which react with MB, thereby increasing the photodegradation of the organic dye.
As shown in Fig. 7, the fabricated ZnO NRs at deposition time of 120 min exhibit better photocatalytic activity (degradation efficiency of 7.78% in 30 min) as compared to the NRs samples prepared at 30 and 60 min (degradation efficiency of 4.45% in 30 min for both samples). In addition, it is clear that the samples 1 and 2 show the same degradation efficiency (4.45%).
There are many factors which could affect the photocatalytic degradation of organic dye. Among them are the type and morphology of catalyst, crystal structure, nanostructure surface area, mean grain size, crystallite size, irradiation time, organic dye, etc. [15]. In our case, as observed in the SEM images the average surface of the grown ZnO NRs increases with increasing the growth time. This could allow for producing more active sites which are responsible for the generation of hydroxyl radicals and thus improving the photocatalytic activity of the investigated ZnO NRs. In addition, sample 3 prepared at deposition time of 120 min shows higher surface area. This could cause higher adsorption of MB upon the irradiation of UV and result in higher photocatalytic activity of the fabricated NRs. On the other hand, the samples (1 and 2) grown at 30 and 60 min, respectively, exhibit almost the same photocatalytic efficiency value (4.45%) and this could be attributed to the slight difference in the surface areas of the two samples in comparison with that of sample 3 grown for 120 min.
Concerning the effect of the crystallite size on the photocatalytic performance, some published studies showed that the crystallite size has a positive influence on the enhancement of the photocatalytic process [27], while others confirmed its negative impact [28. 29]. Furthermore, other researchers have reported that the effect of the crystallite size on the photocatalytic of the nanostructures cannot be measured precisely. This could be due to the involvement of other parameters having a great impact on the improvement of the photocatalysis properties. In our present study, sample 3 shows the highest crystallite size value, while sample 2 possesses the lowest one (18.48 nm) with slight difference compared with sample 1 (19.40 nm). Since the sample 3 grown at 120 min has the highest photocatalytic activity and crystallite size as well. Therefore, the crystallite size may have a role in improving the photocatalytic activity of investigated ZnO NRs grown at 120 min.
On the other hand, the formation of the defects on the surface of the NRs is an important factor which could result in an enhancement of the photocatalysis activity [23, 30]. These defects can trap the photoinduced electrons and thus reduce the electrons–holes recombination rate [23, 31]. Structural defects are observed on the surface of ZnO nanorods as shown in SEM images (Fig. 3). Furthermore, it is expected that with increasing the time of growth more defects could be created on the surface of the fabricated nanorods. These defects could participate in improving the photocatalytic activity of the fabricated ZnO NRs. However, further investigations by other techniques are needed in order to address the correlation between the photocatalytic activity and the defects which could be formed on the surface of ZnO nanorods.
4 Conclusion
ZnO NRs were successfully fabricated by electrochemical deposition on ITO substrates. The fabricated NRs showed the hexagonal crystal structure. The crystallinity was improved by increasing the deposition time. The growth time played an important role in changing the dimensions of the ZnO NRs. Both the radius and length of the NRs were affected by the variation of the growth time from 30 to 120 min. The SEM images analysis revealed that the surface morphology of ZnO NRs is controlled by the variation of the growth time. Consequently, these changes in the surface morphology have a considerable effect on photodegradation process of the methylene blue. The present results indicate that the efficiency of the photocatalytic degradation enhances by increasing the growth time of ZnO NRs up to 120 min which could be attributed to either increasing the surface area or crystallite size. Thus the electrochemical deposition parameters play an important role in controlling the structural and surface morphology of the fabricated NRs making them a promising candidates for photocatalytic applications.
References
A. Sedky, S.A. Amin, M. Mohamed, Appl. Phys. A 125, 308 (2019)
A. Sedky, A.M. Ali, M. Mohamed, Opt. Quantum Electron. 52, 42 (2020)
J. Jiang, J. Pi, J. Cai, Bioinorg. Chem. Appl. 2018, 1 (2018).
D. Pourjafari, G. Oska. Nanomater. Sol. Cell Appl. 1 (2019).
J. Tian, G. Weng, Y. Wang, X. Hu, S. Chen, J. Chu, ACS Appl. Nano Mater. 4, 2 (2019).
E. Oh, H.Y. Choi, S.H. Jung, S. Cho, J.C. Kim, K.H. Lee, S.W. Kang, J. Kim, J.Y. Yun, S.H. Jeong, Sens. Actuators B 141, 239 (2009)
M.R. Alenezi, T.H. Alzanki, A.M. Almeshal, A.S. Alshammari, M.J. Beliatis, S.J. Henley, S.R.P. Silva, RSC Adv. 4, 49521 (2014)
F.A. Al-Agel, M.S. Akhtar, H. Alshammari, A. Alshammari, S.A. Khan, Mater. Lett. 147, 119 (2015)
Y. Hames, Z. Alpaslan, A. Kösemen, S.E. San, Y. Yerli, Sol. Energy 84, 426 (2010)
G.C. Yi, C. Wang, W.I. Park, Semicond. Sci. Technol. 0, 22 (2005)
Z. Ye, T. Wang, S. Wu, X. Ji, Q. Zhang, J. Alloys Compd. 690, 189 (2017)
S.K. Mohanta, D.C. Kim, H.K. Cho, S.J. Chua, S. Tripathy, J. Cryst. Growth 310, 3208 (2008)
D.N. Montenegro, V. Hortelano, O. Martínez, M.C. Martínez-Tomas, V. Sallet, V. Muñoz-Sanjosé, J. Jiménez, J. Appl. Phys. 113, 143513 (2013)
S. Sun, S. Jiao, K. Zhang, D. Wang, H. Li, S. Gao, J. Wang, Q. Yu, F. Guo, L. Zhao, S. Su, J. Mater. Sci. Mater. Electron. 24, 85 (2013)
S.A. Taha, A.M. Abd-Elnaiem, M. Mohamed, S. Mostafa, M.S. Mostafa, Desalin. WATER Treat. 100, 160 (2017)
M. Mohamed, S. Moustafa, S.A. Taha, A.M. Abd-Elnaiem, Mater. Res. Express 6, 035026 (2019)
F. Tezcan, A. Mahmood, G. Kardaş, J. Mater. Sci. Mater. Electron. 29, 9547 (2018)
J. Yang, X. Wang, T. Jiang, Y. Li, Q. Ma, J. Han, J. Chen, J. Wang, Y. Wang, Superlatt. Microstruct. 72, 91 (2014)
J. Lv, W. Gong, K. Huang, J. Zhu, F. Meng, X. Song, Z. Sun, Superlatt. Microstruct. 50, 98 (2011)
J. Sakaliuniene, J. Čyviene, B. Abakevičiene, J. Dudonis, Acta Phys. Pol. A 120, 63 (2011)
U. Holzwarth, N. Gibson, Nat. Nanotechnol. 6, 534 (2011)
M. Skompska, K. Zarȩbska, Electrochim. Acta 127, 467 (2014)
S. Baruah, M.A. Mahmood, M.T.Z. Myint, T. Bora, J. Dutta, Beilstein J. Nanotechnol. 1, 14 (2010)
M. Rashad, N.M. Shaalan, M.M. Hafiz, Dig. J. Nanomater. Biostruct. 10, 823 (2015)
H. Ogawa, T. Higuchi, A. Nakamura, S. Tokita, D. Miyazaki, T. Hattori, T. Tsukamoto, J. Alloys Compd. 449, 375 (2008)
A.B.D. Nandiyanto, R. Zaen, R. Oktiani, Arab. J. Chem. 13, 1283 (2020)
K. Tanaka, M.F.V. Capule, T. Hisanaga, Chem. Phys. Lett. 187, 73 (1991)
J. Sharma, M. Vashishtha, D.O. Shah, Glob. J. Sci. Front. Res. B Chem. 14, 19 (2014)
T. Peng, D. Zhao, K. Dai, W. Shi, K. Hirao, J. Phys. Chem. B 109, 4947 (2005)
S. Baruah, M. Jaisai, R. Imani, M.M. Nazhad, J. Dutta, Sci. Technol. Adv. Mater. 11, 055002 (2010)
L.R. Toporovska, A.M. Hryzak, B.I. Turko, V.P. Rudyk, V.S. Tsybulskyi, R.Y. Serkiz, Opt. Quant. Electron. 49, 1 (2017)
Acknowledgements
This research has been funded by Scientific Research Deanship at University of Ha’il, Saudi Arabia, through project number RG-191247. The authors thank the Deanship of Scientific Research at University of Ha’il for funding this research project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohamed, M., Alshammari, A.S. & Almokhtar, M. Structure, morphology and photocatalytic activity of ZnO nanorods fabricated by electrochemical deposition. Appl. Phys. A 126, 596 (2020). https://doi.org/10.1007/s00339-020-03786-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-020-03786-x