Abstract
A series of Mg-doped NiFe2O4 (NMF) and Co-doped NiFe2O4 (NCF) nanoparticles were synthesized via citrate-gel method. The X-ray diffraction patterns of conventionally heated NMF and NCF nanoparticles confirmed the formation of single-phase cubic spinel structures. Further, the variation of structural parameters as a function of compositions was described. The morphology of NMF and NCF materials was investigated using scanning and transmission electron microscopes (SEM and TEM). In addition, the formation of tetrahedral (A-site) and octahedral (B-site) locations of NMF and NCF was obtained from the Fourier transform infrared spectra (FTIR). Furthermore, the room- and low-temperature magnetic properties were studied for NMF and NCF nanoparticles using magnetization versus magnetic field (M-H) loops and zero field cooled (ZFC) and field cooled (FC) curves, respectively. The results revealed that NMF and NCF nanoparticles exhibited superparamagnetic (SPM) nature at room temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In general, ferrite nanoparticles are the class of magnetic nanoparticles which acquired significant attention in different scientific, medical and industrial fields owing to their electrical, magnetic, and optical properties [1]. These ferrite nanoparticles are normally the metal oxides having the general chemical formula of AB2O4, where ‘A’ tends to divalent elements such as Ni, Mg, Zn, Mn, Co, Cu and Fe, and ‘B’ is associated with the trivalent Fe element [2]. Thus, the above chemical formula can be written as AFe2O4. Moreover, the ferrites showed variety of applications to the scientific community, viz., waste water treatment, drug delivery system, magnetic resonance imaging (MRI), transformer and inductor core devices, photocatalysis, magnetic tapes, magnetic recording media, magnetic memory devices, multilayer chip inductors (MLCI), electromagnetic shields, soft magnets fabrication, microwave absorbers, antenna devices, magneto-hyperthermia treatment, superparamagnetism, green anodes, magnetic refrigeration, magneto-optical devices, spintronics, sensors, etc., [1,2,3,4,5,6,7,8,9].
However, it was an observed fact that many researchers synthesized magnetic nanoparticles such as NiFe2O4, MgFe2O4, and CoFe2O4 via different preparation techniques and further investigated the structural, morphological, electrical, magnetic (M–H loops), superparamagnetic, piezoelectric, and optical properties [10,11,12,13,14,15,16,17]. In addition, some other scientists focussed on doping various elements to the above-said parent materials to enhance the electrical and magnetic properties for the required applications. In view of this, NiMg ferrites, LiNi ferrites, NiCuZn ferrites, Co-based ferrites, rare earth-based spinel ferrites [18,19,20,21,22,23,24,25] etc., were studied for electrical and magnetic properties. Nevertheless, these properties were confined to room (RT) or high temperatures. In particular, the electrical properties were measured from RT to high temperatures for finding the Curie transition temperatures (Tc). On the other hand, the magnetic properties such as magnetic permeability (RT to beyond Tc) [18, 19] and magnetic hysteresis (M–H) loops at RT [18,19,20,21,22,23,24,25] were recorded. These results provided the advancement of magnetic moment of parent materials. As a whole, it can be understood that there were no reports available on low-temperature magnetic properties of Mg/Co-doped NiFe2O4 nanoparticles using ZFC and FC curves for evaluating the SPM nature. In this context, the authors interested to explore the low-temperature magnetic properties of Mg/Co-doped NiFe2O4 nanoparticles using M–H loops and ZFC and FC curves via citrate-gel method.
2 Experimental method
2.1 Preparation of NMF and NCF nanoferrites
For the synthesis of NMF nanoparticles, Ni(NO3)2·6H2O, Fe(NO3)3·9H2O, and Mg(NO3)2 6H2O (each of 99.9% purity, Sigma-Aldrich) were chosen as precursors in nitrate form. Further, these raw materials were dissolved in deionized water by considering 1:3 ratios. In next step, the citric acid (chelating agent) was added to the nitrate mixture solution to prepare the aqueous solution. The resultant solution was poured in a fresh beaker and further, it was stirred constantly using magnetic stirrer to obtain homogeneous mixture. Latter, the above solution was mixed with NH3 solution to keep the pH identical to 7. The resultant solution at this stage was heated up to 100 °C on the hot plate of magnetic stirrer. Thus, after some time, the gel-like material called the citrate precursor was formed during the evaporation process. Again, this citrate precursor was heated at 180–200 °C. At this stage, the water content and few gaseous products were eliminated from the resultant gel material. In the mean time, the autocombustion reaction took place. This was just like volcanic eruption and the final material was produced in dark gray color with elongated asymmetric fiber type of shape. Furthermore, the prepared materials were removed and ground as well in the agate mortars to achieve a fine powder. This powder was once again calcined at 500 °C in a conventional furnace for 4 h to form a spinel structured material. The photographs containing the three main steps in citrate-gel autocombustion technique are shown in Fig. 1. The similar method and steps were followed for the preparation of Ni1-xCoxFe2O4 (x = 0.0–1) nanoferrites as in the case of Ni1-xMgxFe2O4 (x = 0.0–1). Specifically, the Mg(NO3)2·6H2O was replaced by Co(NO3)2·6H2O (99.9% purity, Sigma-Aldrich). Afterwards, the materials were analyzed using the X-ray diffractometer (Bruker XRD, CuKα, λ = 0.15406 nm), High-Resolution Transmission Electron Microscope (HRTEM: Model Tecnai G20, FEI, USA), Scanning Electron Microscope (Ultra 55 SEM Carl Zeiss), FT-IR spectrophotometer (IR affinity-1, Shimadzu) and Vibrating Sample Magnetometer (EV-7 VSM with Max. applied field ± 15 kOe) for discussing the structural, morphological, functional groups and low-temperature magnetic properties, respectively.
3 Results and discussion
3.1 X-ray diffraction analysis of Ni1-xMgxFe2O4 nanoparticles
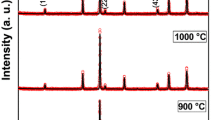
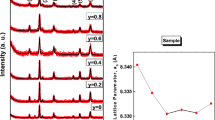
The diffraction patterns of conventionally heated (at 500 °C) Ni1-xMgxFe2O4 (x = 0.0–1) nanoparticles are shown in Fig. 2. The reflection planes were indexed using the Miller indices of (111), (220), (311), (400), (511) and (440) which in turn indicated the existence of single-phase cubic spinel structure. This confirmed that the prepared samples were of high phase purity in nature. These were consistent with the standard JCPDS: 71-1232. In addition, it was noted that the maximum intensity was noted in the (311) plane. From the diffraction patterns, it was clear that the cubic single phases were formed. Besides, the broad width of the peaks in the NMF materials revealed the small-sized nanoparticles. However, in case of the heated NMF samples, the cubic spinel structure remained the same. The average crystallite size was calculated for all heated NMF samples using Scherrer’s equation as mentioned in reference [26]. Specifically, this formula was applied to each diffraction peak of NMF (as indexed in Fig. 2). As a result, many crystallite size values were noted and further, the average crystallite size was evaluated. Hence, this can provide more reliability in achieving the average crystallite size. The obtained results (average crystallite size values) are reported in Table 1. It can be obviously seen from Table 1 that the heated NMF materials revealed the presence of sharp and narrow peaks. This evidenced that the heated samples expressed the high crystallinity in nature. The average crystallite size of the sintered samples was found to be in the range of 13.8–29.6 nm (shown in Table 1) for different compositions. The high microstrain produced during the reaction can be responsible for this behavior of acquiring low crystallite size values for all compositions [23]. According to literature survey, no other method showed the Ni–Mg ferrites with such a small average crystallite size except the citrate-gel autocombustion method [23, 24]. Normally, the conventional methods can consume very high temperatures and prolonged heating time [24, 27]. The structural parameters such as lattice constant (a = d(h2 + k2 + l2)1/2, where d is the inter-planar spacing and (hkl) are the Miller indices), X-ray density [Dx = ZM/Na3, where ‘Z’ is the no. of molecules per unit cell (Z = 8), ‘M’ is the molecular weight of the composition, ‘N’ is Avogadro’s number (6.023 × 1023) and ‘a’ is the lattice parameter] and volume of the unit cell (V = a3) of the individual composition were calculated and results are tabulated in Table 1. The data presented in Table 1 show the variation trend of different structural parameters as a function of Mg content. In this concern, the lattice parameter was noted to be increasing with an increase in Mg content and, therefore, it obeyed Vegard’s law [28]. This kind of manner can be well understood by means of ionic radii of nickel and magnesium cations. As per the Shannon ionic radii table [29], the Mg+2 cations will have the octahedral ionic radius of 0.72 Å which is larger in value than the octahedral ionic radius of 0.69 Å of Ni+2 ions. Thus, as the number of Mg cations is increased, the Mg+2 ions will preferably occupy the Ni+2-sites. Thus, the nickel cations of smaller ionic radii will be replaced by the magnesium cations of larger ionic radii. As a result, the volume of unit cell was increased which led to the enhancement of unit cell dimensions. Apart from these, the behavior of X-ray density (Dx) was seen with increase in ‘x’ value. In general, the theoretical (X-ray) density is a dependent parameter of molecular weight of compositions. It was observed that the molecular weight of NMF materials was decreasing with ‘x’ value. Consequently, the theoretical density was also decreased from 5.362 to 4.530 g/c.c., as a function of composition. These kinds of reports were seen earlier in the literature [30,31,32]. In addition, the density and atomic concentration of magnesium and nickel were noted to be 1.748 g/c.c, 8.905 g/c.c and 4.3 × 1022 cm−3, 9.1 × 1022 cm−3, respectively [18]. The increase of lattice constant from 8.342 to 8.372 Å for x = 0.0–1.0 contents was also one of the reasons for the decrease of the X-ray density. Moreover, the unit cell volume was observed to be increasing with increase in ‘x’ due to increase of lattice constants. Finally, the distance between magnetic ions (hopping length) in A site (tetrahedral) (dA = 0.25a√3) and B site (octahedral) (dB = 0.25a√2) was calculated (where the symbols have their usual meaning) [23, 24] and is listed in Table 1 as a function ‘x’.
3.2 X-ray diffraction analysis of Ni1-xCoxFe2O4 nanoferrites
The XRD patterns of heated NCF nanoparticles are depicted in Fig. 3. The crystalline phases were identified and compared with the standard JCPDS: 22-1086. This comparison evidenced that upon doping with the cobalt cations, the nickel ferrite showed the single-phase cubic spinel structure. The diffraction peaks were indexed using Miller indices as shown in Fig. 3. Similar crystallinity and diffraction peak width were observed for both NCF and NMF nanoparticles. The average crystallite size was calculated from all the diffraction peaks and is listed in Table 2. From Table 2, it was clear that nanosized Ni–Co ferrite powders can be directly synthesized by citrate-gel autocombustion method. The formed sharper and narrow peaks after calcination indicated the improvement of crystallinity. The measured average crystallite size from all the diffraction peaks of various NCF compositions was in the range of 16.4–32.1 nm (shown in Table 2). This kind of low crystallite size values can be normally attributed to the high magnitude of microstrain developed during the reaction process [23]. It was clear that the citrate-gel method can only result in obtaining such small average crystallite size of Ni–Co ferrites. In the literature, the small crystallite size was obtained for different ferrite materials using the present synthesis method [23, 24]. The structural parameters such as lattice constant (a), X-ray density (Dx) and volume of the unit cell of the individual composition were calculated and results are listed in Table 2. The lattice constants were found to be increasing from 8.342 to 8.362 Å with increase of Co+2 cations in the nickel ferrite system. The variation trend of lattice constants of NCF can be elucidated by means of ionic radii of the cations. It was known that the octahedral ionic radius of cobalt cations is ~ 0.82 Å which is larger than the octahedral ionic radius of nickel cations (0.78 Å). Therefore, if the cobalt cations are doped to the nickel ferrite system, the cobalt cations of high ionic radii can replace the nickel ions of small ionic radii. The X-ray density was noted to be decreasing from 5.363 to 5.328 g/c.c., with increase of cobalt content in the nickel ferrite–spinel ferrite system. It had happened due to the decrease of molecular weight as a function of composition. In addition, the density and atomic concentration of cobalt and nickel were noted to be 8.863 g/c.c, 8.905 g/c.c and 8.933 × 1022 cm−3, 9.1 × 1022 cm−3, respectively. This reinforced the decreasing trend of the resultant X-ray density. Besides, the increase of lattice constant from 8.342 to 8.362 Å for x = 0.0 to 1.0 contents was also one of the reasons for the decrease of the X-ray density. In addition, the distance between magnetic ions (hopping length) in A site (tetrahedral) (dA = 0.25a√3) and B site (octahedral) (dB = 0.25a√2) of NCF was calculated (where the symbols have their usual meaning) [23, 24] and is listed in Table 2 as a function ‘x’. It was observed that the hopping length was increased with increase of the Co content nickel ferrite system.
3.3 SEM and TEM analyses
The SEM images of NMF samples are shown in Fig. 4. It was obviously noticed that the surface morphology of all samples revealed the identical grains in shape. Besides, the well-defined and agglomerated grains were seen. This type of agglomeration among the nanosized grains was attributed to the existence of the proportional relationship between the permanent magnetic moment and volume of grain [33]. Moreover, the heating resulted to form solid bodies from the well-faceted grains. It was observed due to the porous network of sintered bodies. In addition, an enlarged mass of compound formation was observed due to the influence of magnesium ions. The SEM micrographs also indicated that the grain size of the sample was in the nanometer region. The TEM was performed for all the samples of Ni–Mg nanoferrites at 20 nm scale bar which is shown Fig. 5. The TEM results indicated the presence of nanoparticles having the size less than 20 nm for all samples, that is, the particle size was observed to be changing from 14.8 to 18.3 nm for all contents. The particles were seemed to be round in shape which formed loose aggregates. The presence of various compositional elements of NMF materials was examined by energy-dispersive spectra (EDS) including the At % (atomic) and Wt % (weight). These are presented in Table 3. The EDS of various compositions of NMF are shown in Fig. 6. The compounds revealed the presence of Ni, Mg, Fe and O without precipitating cations.
The SEM micrographs of NCF nanoparticles are presented in Fig. 7. It was clear from Fig. 7 that the NCF samples showed the presence of the homogeneous grains comprising of agglomeration in nature. This was confirmed by aggregation of grains found in nanometric region. In addition, the uniform nature of grains was evidenced from the good crystalline nature of NCF in the case of XRD patterns. It was a well familiar fact that there may be a favorable case of producing the iron oxide during the preparation of NCF and hence the pores will be developed. This kind of pore development can be almost removed by means of sintering process which can normally enhance the grain growth. This had happened in the morphology of NCF materials. It was also noticed that the agglomeration was increased upon increasing the cobalt content. Moreover, the apparent grain size was noted to be increasing with composition as it was evidenced in case of X-ray diffraction patterns of NCF. The TEM photographs of NCF were extracted at 20 nm scale bar and are shown in Fig. 8. The morphology suggested that the particles were round in shape and weak aggregates. The average particle size was observed to be altering from 13.6 to 19.4 nm. The existence of Ni, Co, Fe and O elements was confirmed using the EDS of NCF. The EDS photos of NCF materials are shown in Fig. 9. Besides, the atomic and weight percentages of elements are shown in Table 4.
3.4 FTIR spectral analysis
The FTIR spectra of NMF samples are recorded over the range of 200–800 cm−1 as depicted in Fig. 10. In general, the FTIR spectra of NMF were used to indicate the tetrahedral and octahedral positions of cubic spinel structure (Table 1). In the current investigation, the absorption bands ν1 (high frequency) and ν2 (low frequency) were noticed at ~ 600 cm−1 and ~ 400 cm−1. Thus, it exhibited the evolution of cubic spinel structure without any impurity by containing two sub-lattices of A and B [34, 35]. The ν1 was varying over the frequency of 606–618 cm−1 while ν2 was noted to be altering from 483 to 496 cm−1. Therefore, it was confirmed that there was a difference between tetrahedral and octahedral positions. This can be attributed to the distance between ferric and oxygen ions at A and B sites. Similar observation was seen in the FTIR spectral analysis for the Ni–Mg ferrite system reported by previous scientists [36, 37], the FTIR spectra of spinels showed ν1 at ~ 600 cm−1 which was assigned to the A site. On the other hand, ν2 observed at ~ 400 cm−1 was associated with the B site. Indeed, it was found that the addition of magnesium supported in broadening the absorption band at around 484 cm−1. This became more prominent at high concentration of Mg cations. It was seen that the positions of the absorption bands were composition dependent which may be due to the cation–oxygen bond distances. Likewise, the FTIR spectra of NCF nanoparticles were recorded over 200–800 cm−1 wavenumber range as shown in Fig. 11. The ν1 and ν2 were assigned to A and B sites at around 600 and 400 cm−1, respectively. These absorption bands for different compositions are listed in Table 2. It was evidently seen that ν1 was changed from 606 to 610 cm−1. On the other hand, ν2 was observed to be changing from 494 to 497 cm−1.
3.5 Magnetic properties of Ni1-xMgxFe2O4 nanoferrite system
3.5.1 Room temperature magnetization measurements using VSM
Figure 12 revealed the M–H loops (hysteresis loop) of NMF materials heated at 500 °C. The different magnetic parameters, viz., saturation magnetization—Ms, remanence magnetization—Mr, coercivity—Hc, and squareness ratio (Mr/Ms), were measured. The results are listed in Tables 5 and 6. From Fig. 12 and Table 5, it was obvious that both saturation magnetization and coercivity were decreased with increase of Mg content. In general, the magnetic properties of soft ferrites will be affected due to the concentration of dopant, type of dopant and morphology of the samples. However, the microstructure showed strong influence on the variation of magnetic parameters. In view of this, it was noted that the larger is the grain size, the higher is the saturation magnetization [18, 19]. From Table 5, it was observed that NiFe2O4 ferrites exhibited large grain size and hence high saturation magnetization and coercivity can be observed as depicted from hysteresis loop (Fig. 12). With increase in Mg content, the grain size was decreased and, therefore, the Ms values were also decreased. The Ms value (at room temperature) was decreased from 17.26 to 10.50 emu/g due to the doping of Mg ions in NMF system. This type of reduction of Ms value can be described owing to the fact that the ferric ions (~ 5 μB) can be occupied by magnesium cations (0 μB) at B sites. As the ‘x’ value was increased, the ratio between ferric ions at B site and ferrous ions at A site will be reduced. This implied that the A–B super-exchange interaction was decreased [18]. Consequently, the Ms was decreased. In addition, the variation of Ms with composition of NMF is shown in Table 5. The Hc was decreased from 273.79 to 100.68 Oe with increase of Mg content. It would have occurred as a result of reduction in anisotropy constant (K) (see Table 5). This can normally allow the decreasing trend of the domain wall energy [38]. The numerical values of Hc, Ms, and K were computed using the standard relations mentioned in references [39,40,41,42,43,44,45] and further these values are listed in Table 5. Besides, the magnetic moment per formula unit (μB/f.u.) was computed [40] and the values are listed in Table 5.
From Table 5, it was clear that magnetic moment values were decreased with increase in Mg content which in turn indicated the paramagnetic behavior of the ferrites. Furthermore, the variation of ‘K’ with Mg content is shown in Table 5. It can be understood that the ‘K’ value was decreased with increase in Mg content. Indeed, the M–H loops can be useful in evaluating the soft and hard kinds of magnetic specimen. This can be evidenced by means of the covered M–H loop area. That is, the large value of M–H loop area can predict the hard magnet nature while the small loop area can suggest the soft magnet nature of sample. However, the soft magnets can exhibit low coercive force. This was proved in the case of NMF system upon increasing the Mg content. That means, the soft magnetic behavior was increased upon increasing the dopant content. Therefore, these kinds of materials can be well suited for the transformers, inductor cores, recording heads, microwave devices, and magnetic shielding device applications.
3.5.2 FC and ZFC curves of MgFe2O4 and Ni0.2Mg0.8Fe2O4 nanoferrites
The magnetization curves of MgFe2O4 (MF) and Ni0.2Mg0.8Fe2O4 samples were recorded with varying magnetic field of ± 10 T and the input temperature from 5 to 300 K. The ZFC and FC plots were drawn for MF and Ni0.2Mg0.8Fe2O4 at 102 Oe magnetic field. Figure 13 indicates the ZFC and FC curves of MF nanomaterial. It was clear from the ZFC and FC plots that at temperatures below 300 K, both the curves got separated into two different directions. Hence, above 300 K [blocking temperature (TB)], the MF nanoparticles confirmed the SPM nature. It was also seen that the FC curve showed almost a constant manner with reduction in temperature. This can be attributed to the uncoupled ferrite system. However, this uncoupled system was evidenced by agglomerated nanoparticles in the surface morphology. Thus, the particle to particle interaction may become high which can in turn stabilize the magnetic spins leading to almost constant trend of FC curve with temperature.
Figure 14 depicts the M–H loops of MF at 5 and 310 K taken over the input magnetic field of ± 10 T. It was obvious from the M–H loops recorded at 5 and 310 K that the SPM nature of the sample was spoiled with decrease of temperature. The high coercive force observed in the M–H curves (at 5 K) indicated the soft ferrite nature. However, at 300 K, the M–H loop expressed almost zero value of coercivity. It would have occurred as a result of exponential variation of fluctuation time associated with the particle moment with input temperature. Therefore, the magnetization seemed to switch quickly to a stable state with decrease of temperature. It had happened at TB. It can basically depend linearly on the volume of material (V) and on the magnitude of ‘K’ [45].
The ZFC and FC plots of Ni0.2Mg0.8Fe2O4 at 100 Oe are depicted in Fig. 15. It was noted that the magnetization (M) was increased up to 45 K. Beyond 45 K, the M was decreased. In addition, M attained maximum value at 43 K. After crossing the 43 K temperature, the M started to decrease (as shown in inset plot of Fig. 15). This type of reduction in M will introduce the effective spin correlation between the spins or cluster in the ferrite system. Thus, it can lead to the reduction in M even at 100 Oe [33, 34].
The magnetization measurements of Ni0.2Mg0.8Fe2O4 were recorded over 5–310 K temperature at 100 Oe (input field). The plots were drawn between magnetization and applied magnetic field as shown in Fig. 16 at different low temperatures such as 5, 42, 75 and 310 K. Among these plots, huge value of coercivity, about 1200 Oe at 5 K (see inset Fig. 16), was noted which in turn reflected the existence of SPM nanoparticles [36]. In general, this would have happened in the case of single-domain nanoparticles which can contain strong anisotropic forces. To magnetize the materials comprising of single-domain nanoparticles, huge amount of work must be done against the strong anisotropic forces. At this moment, the strong magnetic fields may be required and, therefore, it can possess high coercive force [41]. Besides, the Mr and Hc values of NMF (x = 0.8) were evaluated at the four temperatures and listed in Table 6. It was obviously understood from Table 6 that huge value of coercivity was noted at 5 K while an abrupt reduction in Hc and Mr was observed as a function of temperature (see Table 6). With increase of temperature, the Hc and Mr parameters were diminished gradually and approached almost equal to zero. This established the formation of SPM nanomaterials including the TB. Hence, these kinds of nanoparticles can be used for biomedical applications such as magnetic resonance imaging (MRI).
3.6 Magnetic properties of Ni1-xCoxFe2O4 nanoferrite system
3.6.1 Room temperature magnetization measurements using VSM
Figure 17 revealed the M versus H loops (± 15 KOe) of NCF materials heated at 500 °C. The different magnetic parameters such as Ms, Mr, Hc and (Mr/Ms) were calculated to investigate the magnetic behavior. The results of magnetic parameters are reported in Table 7. It was understood from Fig. 17 and Table 7 that the Ms was increased upon increasing the cobalt concentration. However, the magnitude of Ms was increased from ~ 17 to 34.4 emu/g (at room temperature) with increase of cobalt content in NCF system. It can be attributed to the increase of cobalt cations at nickel lattice site. In particular, the nickel cations with low magnetic moment of 3 μB were replaced by the cobalt cations containing high magnetic moment of 5 μB at the B sites. Moreover, the increasing trend of cobalt cations can in turn enhance the ratio of ferric and ferrous ions at B and A sites, respectively. Thus, the magnetic exchange interaction between the two sites will be increased [34]. Therefore, the Ms was increased. It was noticed that the Hc value was increased from 273.79 to 2342.33 Oe with increase of Co content. This established a fact that the huge amount of coercive field can be required to change magnetic moment thereby overcoming the anisotropic forces with increase of Co content. In this way, the ‘K’ value will be increased and, furthermore, it can increase the magnetic domain wall energy as reported in the published work [38]. As a matter of fact, the Co-doping into the nickel ferrite system supported the evolution of hard magnet nature owing to high coercivity value.
From Table 7, it was clear that magnetic moment values were increased with increase in Co content which can be attributed to the less probability for the occupancy of Co cations at octahedral sites. The data revealed that the increasing nature of cobalt content increased the magnetization and further the material was converted into hard magnetic material. It can be understood that the ‘K’ value was increased with increase of Co content. From the M–H loops of NCF, it was clear that the hard magnetic Ni–Co nanoferrites were prepared which can be magnetized and demagnetized hardly.
3.6.2 FC and ZFC curves of CoFe2O4 and Ni0.2Co0.8Fe2O4 nanoferrites
The magnetization measurements of CoFe2O4 and Ni0.2Co0.8Fe2O4 nanomaterials were recorded with varying the applied magnetic field of ± 10 T at three different temperatures of 5, 25 and 310 K. Apart from these, the ZFC and FC plots were drawn (Figs. 18, 19) for the above-mentioned nanomaterials at 102 Oe. The usual behavior was observed in case of FC and ZFC curves of NCF. That is, the FC curves expressed almost a constant trend of M value while ZFC curves revealed the decreasing nature of M as a function of temperature. The similar observations were seen earlier in case of NMF nanomaterials. It was clear that the ZFC and FC curves of cobalt ferrite (CF) nanomaterials were merged at 355 K and below 355 K, both the curves were diverged (Fig. 18). This confirmed a fact that above 355 K, the CF nanomaterials acquired the SPM nature. However, this report established the TB ~ 355 K. The diverging manner of ZFC and FC curves in general can indicate the ferromagnetic behavior of nanomaterials.
The ZFC and FC curves of Ni0.2Co0.8Fe2O4 nanoparticles are shown in Fig. 19. It was found that the TB was ~ 353 K. Thus, the Ni0.2Co0.8Fe2O4 also exhibited the SPM nature above TB and below TB; the usual ferromagnetic behavior was noticed. In addition, the FC plot revealed the huge increase of magnetization as the temperature was decreased below TB. This would have happened owing to the presence of uncoupled system in Ni0.2Co0.8Fe2O4.
Figures 20 and 21 depict the M–H loops of CF and Ni0.2Co0.8Fe2O4, respectively, at 5, 25 and 310 K of NCF system. It was obviously noticed from the M–H loops that the above-said two samples performed the ferrimagnetic behavior at all the temperatures. But nevertheless, the extent of ferrimagnetism was altered. Moreover, the magnetic parameters were calculated and are listed in Table 8. The Ms values of the CF and Ni0.2Co0.8Fe2O4 materials at 310 K were about 43 and 12 emu/g, respectively. Herein, the saturation magnetization of CF was smaller than the published work for bulk CF [15]. From Table 8 and Figs. 20 and 21, it was noted that the Hc and Ms values were enhanced with increase of temperature. Specifically, the Hc of CF was increased from 1760 to 13,600 Oe. On the other hand, the Ni0.2Co0.8Fe2O4 showed the increasing value of the Hc from 338 to 10,364 Oe with decrease of temperature from 310 to 5 K. Moreover, the Mr was noticed to be increasing with decrease of temperature for all materials. As a whole, it was observed that at 310 K, the two nanomaterials showed soft ferrimagnetism in nature while for the rest of the temperatures, the sample revealed the hard magnetic nature. Therefore, at room temperature, these two samples may be used for transformer applications at high frequencies.
4 Conclusions
The NMF and NCF nanomaterials were prepared using citrate-gel method. The incorporation of magnesium ion into nickel ferrite system resulted in a decrease of crystallite size, saturation magnetization, coercivity and magnetic moment. The room temperature magnetization measurements indicated that NMF nano-ferrites showed narrow hysteresis loop with low saturation magnetization and low coercivity. This had happened due to the paramagnetic nature of the magnesium. Hence, these ferrites can be magnetically very soft materials. This kind of properties can be desirable for transformers, inductor cores, recording heads, microwave devices and magnetic shielding devices. The room temperature magnetization measurements of NCF revealed that the area in the hysteresis loop was more than NMF with moderate saturation magnetization and coercivity. This may be due to the ferromagnetic nature of cobalt. Hence, these ferrites can reveal the hard magnetic nature. These characteristics of ferrites may be desirable for magnetic recording applications. Low-temperature magnetization measurements (M–H loops and FC and ZFC measurements) revealed that at low temperature, i.e., at 5 K, the materials were ferromagnetic in nature with high coercivity. The MgFe2O4 showed SPM behavior above room temperature. At 5 K temperature, the MF revealed large Hc of 1200 Oe suggesting the ferrimagnetic behavior. Beyond the TB, the Ni0.2Mg0.8Fe2O4 nanomaterials showed anhysteresis curve and less than TB, it exhibited the lagging nature of M behind H and further suggested SPM behavior of nanomaterials. Therefore, the Ni0.2Mg0.8Fe2O4 nanoparticles can provide biomedical applications in drug delivery and in MRI. Low-temperature magnetization measurements (M–H loops and FC and ZFC measurements) revealed that below room temperature, i.e., at 5 and 25 K, the materials were ferromagnetic in nature with high coercivity. CoFe2O4 and CoFe2O4 nanoferrites showed SPM behavior above room temperature (at around 355 K) where coercivity and remanence became zero. Above blocking temperature, CoFe2O4 and Ni0.2Co0.8Fe2O4 nanoferrites showed anhysteresis and below TB, they showed large hysteresis which can be the criteria of a superparamagnetic behavior. Inclusion of Co in nickel ferrite decreased the coercivity from 1760 to 338 Oe at room temperature by making the material magnetically very soft favoring its application in high-frequency transformers.
References
N. Boda, K.C.B. Naidu, K.M. Batoo, G.H.R. Joice, J.L. Naik, D. Ravinder, Structural, morphological and electronic properties of cadmium cobalt ferrite nanoparticles. Biointerface Res Appl Chem 10, 4752–4763 (2020)
U. Naresh, R.J. Kumar, K.C.B. Naidu, Optical, magnetic and ferroelectric properties of Ba0.2Cu0.8-xLaxFe2O4 (x = 0.2 − 0.6) nanoparticles. Ceram Int 45, 7515–7523 (2019)
K.K. Kefeni, B.B. Mamba, T.A.M. Msagati, Application of spinel ferrite nanoparticles in water and waste water treatment: a review. Sep Purif Technol 188, 399–422 (2017)
N. Boda, G. Boda, K.C.B. Naidu, M. Srinivas, K.M. Batoo, D. Ravinder, A.P. Reddy, Effect of rare earth elements on low temperature magnetic properties of Ni and Co-ferrite nanoparticles. J Magn Magn Mater 473, 228–235 (2019)
K.C.B. Naidu, N.S. Kumar, G.R. Kumar et al., Temperature and frequency dependence of complex impedance parameters of microwave sintered NiMg ferrites. J Aust Ceram Soc 55, 541–548 (2019)
D. Sivakumar, K.C.B. Naidu, K.P. Nazeer, M.M. Rafi, G. Rameshkumar, B. Sathyaseelan, G. Killivalavan, A.A. Begam, Structural characterization and dielectric properties of superparamagnetic iron oxide nanoparticles. J Korean Ceram Soc 55, 230–238 (2018)
D.S. Kumar, K.C.B. Naidu, M.M. Rafi, K.P. Nazeer, A.A. Begam, G.R. Kumar, Structural and dielectric properties of superparamagnetic iron oxide nanoparticles (SPIONs) stabilized by sugar solutions. Mater Sci Pol 36, 123–133 (2018)
N.R. Ram, M. Prakash, U. Naresh, N.S. Kumar, T.S. Sarmash, T. Subbarao, R.J. Kumar, G.R. Kumar, K.C.B. Naidu, Review on magnetocaloric effect and materials. J Supercond Nov Magn 31, 1971–1979 (2018)
T. Ramaprasad, R.J. Kumar, U. Naresh, M. Prakash, D. Kothandan, K.C.B. Naidu, Effect of pH value on structural and magnetic properties of CuFe2O4 nanoparticles synthesized by low temperature hydrothermal technique. Mater Res Express 5, 095025 (2018)
D. Kumar, D.B. Das, R. Gopalan, Effects of surfactant on the structural and magnetic properties of hydrothermally synthesized NiFe2O4 nanoparticles. Mater Chem Phys 218, 70–76 (2018)
B. Aslibeiki, G. Varvaro, D. Peddis, P. Kameli, Particle size, spin wave and surface effects on magnetic properties of MgFe2O4 nanoparticles. J Magn Magn Mater 422, 7–12 (2017)
N. Kaur, M. Kaur, Envisioning the composition effect on structural, magnetic, thermal and optical properties of mesoporous MgFe2O4-GO nanocomposites. Ceram Int 44, 4158–4168 (2018)
R. Lamouri, O. Mounkachi, E. Salmani, M. Hamedoun, A. Benyoussef, H. Ez-Zahraouy, Size effect on the magnetic properties of CoFe2O4 nanoparticles: a Monte Carlo study. Ceram Int (2019). https://doi.org/10.1016/j.ceramint.2019.12.035
S. Munjal, N. Khare, B. Sivakumar, D.N. Sakthikumar, Citric acid coated CoFe2O4 nanoparticles transformed through rapid mechanochemical ligand exchange for efficient magnetic hyperthermia applications. J Magn Magn Mater 477, 388–395 (2019)
D. Hu, F. Zhao, Z. Zhang, L. Miao, R. Ma, W. Zhao et al., Synthesis and magnetic properties of monodisperse CoFe2O4 nanoparticles coated by SiO2. Ceram Int 44, 22462–22466 (2018)
S. Ramesh, D. Ravinder, K.C.B. Naidu, N.S. Kumar, K. Srinivas, D.B. Basha, B.C. Sekhar, A review on giant piezoelectric coefficient, materials and applications. Biointerface Res Appl Chem 9, 4205–4216 (2019)
N. Boda, K.C.B. Naidu, D.B. Basha, D. Ravinder, Structural and magnetic properties of CdCoFe2O4 nanoparticles. J. Supercond. Nov. Magn. 1–6 (2019). https://doi.org/10.1007/s10948-019-05242-1
K.C.B. Naidu, W. Madhuri, Microwave processed NiMg ferrites: studies on structural and magnetic properties. J Magn Magn Mater 420, 109–116 (2016)
K.C.B. Naidu, W. Madhuri, Ceramic nanoparticle synthesis at lower temperatures for LTCC and MMIC technology. IEEE Trans Magn 54, 2300808 (2018). https://doi.org/10.1109/TMAG.2018.2855663
M. Hashim, K.C.B. Naidu, G.H.R. Joice, J.L. Naik, D. Ravinder, Superparamagnetic and photocatalytic activity of CoCe0.02Dy0.02Fe1.96O4 nanoparticles synthesized by citrate-gel autocombustion technique. Biointerface Res Appl Chem 9, 4164–4167 (2019)
C.H. Vinuthna, K.C.B. Naidu, C.C. Sekhar, D. Ravinder, Magnetic and antimicrobial properties of cobalt zinc ferrite nanoparticles synthesized by citrate-gel method. Int J Appl Ceram Technol 16, 1944 (2019). https://doi.org/10.1111/ijac.13276
S. Joshi, M. Kumar, Effect of Ni2+ substitution on structural, magnetic, dielectric and optical properties of mixed spinel CoFe2O4 nanoparticles. Ceram Int 42, 18154–18165 (2018)
G. Aravind, M. Raghasudha, D. Ravinder, M.M. Raja, S.S. Meena, P. Bhatt, M. Hashim, Study of structural and magnetic properties of Li–Ni nanoferrites synthesized by citrate-gel auto combustion method. Ceram Int 42, 2941–2950 (2016)
M. Hashim, M. Raghasudha, J. Shah, S.E. Shirsath, D. Ravinder, S. Kumar, S.S. Meena, P. Bhatt, R. Kumar, R.K. Kotnala, High temperature dielectric studies of indium-substituted NiCuZn nanoferrites. J Phys Chem Solids 112, 29–36 (2018)
F.R. Mariosi, J. Venturini, A. da Cas Viegas, C.P. Bergmann, Lanthanum-doped spinel cobalt ferrite (CoFe2O4) nanoparticles for environmental applications. Ceram Int (2019). https://doi.org/10.1016/j.ceramint.2019.09.266
P. Scherrer, Bestimmung der Grosse und der Inneren Struktur von Kolloidteilchen Mittels Rontgenstrahlen, Nachrichten von der Gesellschaft der Wissenschaften, Gottingen. Math-Phys Kl 2, 98–100 (1918)
N.S. Kumar, R.P. Suvarna, K.C.B. Naidu, Grain and grain boundary conduction mechanism in sol-gel synthesized and microwave heated Pb0.8-yLayCo0.2TiO3 (y = 0.2 – 0.8) nanofibers. Mater Chem Phys 223, 241–248 (2019)
A.R. Denton, N.W. Ashcroft, Vegard’s law. Phys Rev A 43, 3161 (1991)
R.D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst A 32, 751–767 (1976)
N.S. Kumar, R.P. Suvarna, K.C.B. Naidu, G.R. Kumar, S. Ramesh, Structural and functional properties of sol-gel synthesized and microwave heated Pb0.8Co0.2-zLazTiO3 (z = 0.05 – 0.2) nanoparticles. Ceram Int 44, 19408–19420 (2018)
N.S. Kumar, R.P. Suvarna, K.C.B. Naidu, Sol-gel synthesized and microwave heated Pb0.8-yLayCo0.2TiO3 (y = 0.2 – 0.8) nanoparticles: structural, morphological and dielectric properties. Ceram Int 44, 18189–18199 (2018)
D. Kothandan, R.J. Kumar, M. Prakash, K.C.B. Naidu, Structural, morphological and optical properties of Ba1-xCuxTiO3 (x = 0.2, 0.4, 0.6, 0.8) nanoparticles synthesized by hydrothermal method. Mater Chem Phys 215, 310–315 (2018)
R. Chantrell, N.S. Walmsley, J. Gore, M. Maylin, Theoretical studies of the field-cooled and zero-field cooled magnetization of interacting fine particles. J Appl Phys 85, 4340 (1999)
U. Naresh, R.J. Kumar, K.C.B. Naidu, Hydrothermal synthesis of barium copper ferrite nanoparticles: nanofiber formation, optical, and magnetic properties. Mater Chem Phys 236, 121807 (2019)
L.J. Berchmens, R.K. Selvan, P.N.S. Kumar, C.O. Augustin, Structural and electrical properties of Ni1-xMgxFe2O4 synthesized by citrate gel process. J Magn Magn Mater 279, 103–110 (2004)
S.R. Shinde, S.D. Kulkarni, A.G. Banpurkar, R. Nawathey-Dixit, S.K. Date, S.B. Ogale, Magnetic properties of nanosized powders of magnetic oxides synthesized by pulsed laser ablation. J Appl Phys 88, 1566 (2000)
M.A. Gabal, Y.M. AlAngari, H.M. Zaki, Structural, magnetic and electrical characterization of Mg–Ni nanocrystalline ferrites prepared through egg white precursor. J Magn Magn Mater 363, 6–15 (2014)
E.C. Stoner, E.P. Wohlfarth, A mechanism of magnetic hysteresis in heterogeneous alloys. Phil Trans R Soc Lond A 240, 599 (1948)
C.P. Bean, J.D. Livingston, Superparamagnetism. J Appl Phys 30, 120 (1959)
N. Raghuram, T.S. Rao, K.C.B. Naidu, Magnetic properties of hydrothermally synthesized Ba1-xSrxFe12O19 (x = 0.0 − 0.8) nanomaterials. Appl Phys A 125, 839 (2019)
U. Naresh, R.J. Kumar, K.C.B. Naidu, Structural, morphological, optical, magnetic and ferroelectric properties of Ba0.2La0.8Fe2O4 nanofibers. Biointerface Res Appl Chem 9, 4243–4247 (2019)
D.R. Mane, D.D. Birajdar, S.E. Shirsath, R.A. Telugu, R.H. Kadam, Structural and magnetic characterizations of Mn-Ni-Zn ferrite nanoparticles. Phys Status Solidi A 207, 2355 (2010)
S. Singhal, K. Chanda, Cation distribution and magnetic properties in chromium-substituted nickel ferrites prepared using aerosol route. J Solid State Chem 180, 296 (2007)
C.M. Hurd, Varieties of magnetic order in solids. Contemp Phys 23, 469 (1982)
D.J. Craik, Magnetic oxides, part II (Wiley, London, 1975), p. 703
Acknowledgements
The authors (AG and DR) are very grateful to Prof. G. Prasad, Head, Department of Physics, Osmania University, Hyderabad, and also thankful to UPE-UGC-OU and DST-PURSE-OU. Authors K. M. Batoo, S. F. Adil and M. Khan are thankful to the Deanship of Scientific Research at King Saud University for its funding through the Research Group Project no. RG-1437-030.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gaffoor, A., Naidu, K.C.B., Ravinder, D. et al. Synthesis of nano-NiXFe2O4 (X = Mg/Co) by citrate-gel method: structural, morphological and low-temperature magnetic properties. Appl. Phys. A 126, 39 (2020). https://doi.org/10.1007/s00339-019-3225-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-019-3225-1