Abstract
In this communication, the effect of gadolinium (Gd) substitution on structural, microstructural, electrical and dielectric properties of bismuth ferrite BiFeO3 (i.e. Bi(Fe0.95Gd0.05)O3 abbreviated as BFGO5) has been reported. The development of an environment-friendly lead-free multiferroic material by substituting a rare earth element at the uncommon site of BiFeO3 (BFO) (i.e. Gd at the Fe site rather than commonly preferred Bi site) for the tailoring of its multiferroic properties has been attempted in this study. The present studied material has been fabricated through a conventional standard solid-state reaction (SSR) method using carbonates and high-quality oxides in a stoichiometric amount. The phase formation and basic crystal data were analysed by X-ray diffraction technique which shows a single-phase formation of BFGO5 material in orthorhombic symmetry. The average crystallite size was calculated using Scherrer’s formula and found to be 84 nm. The surface morphology and compositions examined by FE-SEM, EDX, FT-IR and TEM show the formation of highly compact sample with uniform distribution of grains. Detailed studies of dielectric parameters (dielectric constant and tangent loss) in a selected frequency range (1–1000 kHz) at different temperatures (273–773 K) clearly exhibit enhancement on dielectric properties of BFO. Studies of its impedance spectroscopy, electrical modulus and electrical conductivity confirm the semiconductor behaviour [negative temperature coefficient of resistance (NTCR)] and non-Debye type relaxation process of the material. The polarization versus electric field (P–E) analysis of BFGO5 shows an improvement in remnant polarization as compared to the parent compound BFO. Therefore, based on the several investigations of results, the BFGO5 material could be considered as a favourable candidate for electronic device applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Materials which can exhibit ferroelectric and ferromagnetic features at the same time in a single phase are known as multiferroic. During the last few decades, researchers have exploited and paid much attention to develop an eco-friendly multiferroic material for a wide range of multiple/multifunctional applications, such as information storage media, sensors, spintronics, actuators, transducers, and optoelectronic devices [1,2,3,4]. The demand of multiferroic materials is rapidly increasing day by day for electronic applications. Such kind of materials is not found in nature, as it needs to possess empty and partially filled orbitals to show ferroelectric and ferromagnetic properties, respectively [5]. Among all the multiferroics known today, BiFeO3 (BFO) is the most promising single-phase compound as it has a Curie temperature (T C) and Neel temperature (T N) of 1103 K and 643 K, respectively. It has a magnetic arrangement of G-type anti-ferromagnetism. Since its multiferroic behaviour was discovered in 1960, several extensive investigations in the study of its structure and multiferroic properties have been carried out [6]. It possesses an ABO3 type distorted perovskite structure with a polar R3c space group with a lattice parameter of a r = 3.96 Å and α r = 89.4° at room temperature [7]. The ferroelectricity and ferromagnetic behaviour of BFO are occurring due to the presence of a lone electron pair (6s 2) of Bi3+ ions and partially filled d-orbital of Fe3+ ions [8]. In spite of its multiferroic properties at room temperature, BFO has been restricted for practical uses due to some inherent problems in its properties, such as, large leakage current density, high tangent loss, structural distortion, existence of some unknown defects. The main reason of high current leakage is due to the oxidation–reduction of Fe ions (\( {\text{Fe}}^{ + 2} \to {\text{Fe}}^{ + 3} + {\text{e}}^{ - } \)), which creates oxygen vacancies and effects the potential of its applications in multifunctional devices [9]. The impurity phases formed during the sample preparation may result in high current leakage on the sample. The literature survey shows that several attempts have already been made by substituting at the Bi/Fe sites and/or forming solid solution/composites with perovskite materials for eliminating the above shortages and minimizing high tangent loss, lowering of high current leakage density, reduction of the structural distortion, removal of the formation of secondary phase and enhancement of ferromagnetic and ferroelectric properties of bismuth ferrite [10,11,12,13]. In this process, several attempts were made to increase the properties of BFO materials by replacing and/or adding at Bi or Fe site with other rare earth ions such as La3+ [14, 15], Nd3+ and Sm3+ [16]. Furthermore, Mukherjee et al. [17] reported that the substitution at the Bi site of BFO by rare earth ions (≤ 5%) reduced the oxygen vacancy concentration, which in turn results in decreasing the current leakage density to 10−6 A cm−2 and improves the ferroelectric properties. The ionic substitution is found to be one of the most adopted strategies for suppressing a high current leakage, enhancing ferroelectricity and ferromagnetism by destroying the cycloidal modulation. Some recent studies of nanocomposite-based materials which are synthesized through a different approach such as sonochemical reaction and hydrothermal reaction methods have also been explored and investigated for multifunctional applications [18,19,20,21].
The effect of Gd doping at the Bi site exhibits gradual transition of phase from rhombohedral to pseudo-tetragonal structure. Moreover, Gd is considered as a good dopant for bismuth ferrite at the Fe site to improve the magnetic properties, as it has a large magnetic moment (8.0 µ B) which cooperated with Gd+3 ions [22]. It was evident that the dielectric constant increases much more as compared to that of pure BFO, whereas the tangent loss decreases after partially substituted by Gd3+ [23]. Furthermore, gadolinium has some added advantages in terms of toxicity comparing with some conventional catalyst, such as mercury (Hg), lead (Pb) and cadmium (Cd). These elements are defined as a toxic agent as they induced hypertension in humans and animals, affecting endothelial function, raised cardiovascular risk and associated with environmental pollution. However, interestingly, gadolinium has not been categorized as a toxic agent [24, 25]. A comprehensive study has also been carried out to adjust the optical band gap of bismuth ferrite material by single and co-substitution approach. Sm- and Mn co-doped of BFO expands the optical band gap of bismuth ferrite [26]. A similar nature of increase of band gap and photocatalytic activity is also reported for Gd (< 15% at Bi-site) [27] doping, and Y and Co co-doping [28] of BFO while Mn doping [29] decreases. Irfan et al. have reported that decrease of BFO band gap from 2.10 to 2.03 eV for co-doping of Gd3+ and Sn4+ initially and then an increase up to 2.06 eV on increasing Sn4+ concentration were confirmed, and the increment in band gap may improve the photocatalytic activity of Gd3+ and Sn4+ co-doped BFO nanomaterial [30]. Few recent studies based on nanoparticles/nanocomposite are also performed via several methods that explored multifunctional utility [31,32,33]. Some controversial issues are arising from the previously published literature which demands more works in this area.
From on the above several remarkable points of view, planning has been made to develop a new material, based on a lead-free (non-toxic) and environment-friendly multiferroic material associated with gadolinium (rare earth family). Additionally, it is observed that not much investigation has been done in the similar theme. Therefore, for exploring the above-mentioned deficiencies, a multiferroic material of Bi(Fe0.95Gd0.05)O3 was prepared through a conventional solid-state reaction method from a solid solution of BiFeO3 and Gd2O3 and studied its various properties with the help of various advanced methods. The Gd-doped BFO at the Fe site offers improvement in its essential physical properties as well as a great reduction of leakage current. In this work, the value of crystallite size and lattice strain, characteristics of electrical parameters (dielectric, impedance, electrical modulus, ac conductivity) of Gd (5%) substituted at the Fe site of bismuth ferrite material (BFGO5) have been reported.
2 Experimental details
2.1 Synthesis
The Gd-modified bismuth ferrite polycrystalline material, Bi(Fe0.95Gd0.05)O3 (BFGO5), was synthesized via a standard solid-state reaction (SSR) method. The high quality of oxide powders of Loba Chemie Pvt Ltd. such as bismuth oxide (Bi2O3, purity 99%), iron oxide (Fe2O3, purity 98%), and gadolinium oxide (Gd2O3, purity 99.9%) were weighted as per appropriate stoichiometry using high-precision digital electronic balance. The ingredients were ground manually with the help of agate mortar and pestle in the air atmosphere for 2 h, and wet atmosphere (methanol as solvent) for 3 h to get a homogeneous mixture. The BFGO5 compound was successfully prepared using the following chemical reaction formula:
The mixed compound was calcined with a heating rate of 2.5° min−1 at 1023 K for 8 h using a high-temperature muffle furnace. The calcined sample powder was mixed with 2% of PVA (polyvinyl alcohol) as a binder and the disc-shape pellets (10 mm diameter, 1–2 mm thickness) was fabricated under iso-static pressure of \( 4 \times 10^{{6_{\,} }} \) Nm−2 using a KBR press. The pellets so obtained are sintered at a temperature of 1073 K for 6 h to achieve a very high density compact pellets for the use of electrical measurements. For the synthesis process of the BFGO5 compound, a high-quality alumina crucible, boat and disc were used.
2.2 Measurement and characterization techniques
The basic structural details and phase formation were studied using the XRD data obtained from X-ray powder diffractometer (D8 Advance; Bruker). The X-ray structural data were collected with a 2° min−1 scan speed in a wide range of Bragg’s angles \( (20{^\circ } \le 2\theta \le 80{^\circ }) \) with CuKα radiation. The basic crystal structure and lattice parameters were analysed by a specially designed software for X-ray data analysis called ‘POWDMULT’. The microstructure morphology of the synthesized compound was examined by scanning the pellet surface thoroughly with the help of FE-SEM at room temperature (298 K). The microstructure composition (grain distribution, grain size, voids) and elements concentration in the sintered pellet were analysed using energy-dispersive X-ray spectroscopy (EDXS). For particle size analysis, transmission electron microscope (TEM) and a high-resolution transmission microscope (HRTEM) images were obtained. To understand the molecular structure of the studied material, FT-IR (Shimadzu), spectrum was obtained in a wide frequency range (400–1600 cm−1). Then a sintered pellet was polished on both the surfaces to make it flat and smooth, and then coated with a high-standard quality silver paste. The silver-coated pellet was dried at 423 K for 1 h to eliminate moisture from the pellet surface, if any. For measuring the electrical and dielectric parameters, a highly accurate phase-sensitive multimeter (PSM-1735) was used in a wide range of frequencies (1–1000 kHz) and temperatures (273–773 K). The electrical polarization versus applied electric field was measured with the help of PE-Loop tracer (M/S Marine India).
3 Results and discussion
3.1 Structural and microstructural studies
3.1.1 Crystal system and data
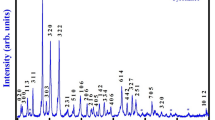
Figure 1 shows the XRD pattern of the calcined BFGO5 powder recorded at room temperature. A single-phase formation was confirmed from the diffraction peaks of XRD data. The narrow and sharp peak’s width confirmed a good crystalline nature of the material. The standard indexing software “POWDMULT” was utilized for indexing the major reflection peaks of the XRD pattern [34]. The orthorhombic symmetry of the material was also confirmed by comparing the observed and calculated lattice spacing (d values) with the diffraction angle for each reflection. The selected lattice parameters in the orthorhombic system are a = 3.9297 Å, b = 22.2849 Å, c = 13.5115 Å. The unit cell volume and the lowest estimated standard deviation are V = 1183.23 Å3 and SD = 0.049 Å, respectively. The average crystallite size (S) was calculated taking some widely scattered peaks using Scherrer’s equation:
where k is a constant (= 0.89), \( \lambda \) is the X-ray wavelength (= 1.5406 Å), \( \beta \) is the full width at half maximum of peak intensity and \( \theta \) is the Bragg’s angle of diffraction is found to be 84 nm. Detailed information of the prepared BFGO5 material is summarized in Table 1. Additionally, some characteristics of our earlier work on La, Gd and co-substitution effects (Ni, Co, Cd with Ti) on bismuth ferrite have been compared in Table 2.
3.1.2 Microstructure
The FE-SEM commonly used flexible instruments for investigating the surface morphology of solid materials. Figure 2a illustrates the FE-SEM micrograph of the sintered BFGO5 compound (1073 K for 6 h), which depicts the high density of the grains with a compact nature, less formation of holes, regular distribution of grains throughout the surface. The grains and their boundaries are also clearly visible in the FE-SEM image. The grain size of the studied material was found to be in the range of 300–400 nm. Most of the grains are a combination of elements and are smaller in shape and size, which suggested the presence of polycrystalline nature in the prepared sample. Figure 2b shows the corresponding energy-dispersive X-ray (EDX) microanalysis pattern, which clearly exhibits the presence of BFGO5 constituent elements, such as Bi, Fe, Gd and O with their atomic ratios and without any impurity phase.
3.1.3 Transmission electron microscopy
Figure 3a shows the TEM image of Bi(Fe0.95Gd0.05)O3. For preparing the TEM sample, BFGO5 powder was disseminated using methanol (as solvent) with the help of sonicator and then dropped on a copper grid, so that different-shaped sample can be observed in the TEM image. A low dispersion of particle was observed in the TEM image as shown in Fig. 3a. Since the particles are in agglomerated form, accurate measurement of the boundary limit of each particle is not possible. However, the approximate size of the particles may be estimated in the range of 80–90 nm. This approximate sample image size is nearly equal to the particle size calculated using Scherrer’s formula from the powder XRD data. In Fig. 3b, the bright diffraction spots are seen in the selected area electron diffraction image, which clearly confirms the formation of a high-quality single-crystal lattice structure of perovskite BFGO5 nanoparticles with high crystallinity. The HRTEM image shown in Fig. 3c exhibits a monocrystalline nature of the particle in the sample powder.
3.1.4 Fourier transform infrared spectroscopy
Figure 4 shows the room temperature FT-IR spectra of Bi(Fe0.95Gd0.05)O3 powders in the range of 400–1600 cm−1. FT-IR spectroscopy is an essential characterization technique for obtaining the information on the arrangement of the structural units of as-prepared ceramics. The strong absorptive peaks of 412, 437 and 457 cm−1 are ascribed to the Fe–O–Fe bending vibration and 536 and 692 cm−1 are ascribed to Fe–O stretching vibration [38], which confirm the formation of metal–oxygen bond. It is the characteristic behaviour of FeO6 octahedra in the perovskites. The absorption bands at 846 and 1043 cm−1 exhibited the presence of trap nitrates in the sample [39]. The strong band at 1303 and 1417 cm−1 may be due to the bending of Bi–O bond vibration [40].
3.2 Dielectric measurements
The dielectric measurement is very important for studying internal properties of the material. Some of the factors, which affect the internal behaviour of a compound, are elemental compositions, particle size, temperature, frequency, applied electric field, etc. Thus, it is essential to study the dielectric characteristics of a prepared compound for best understanding and applications.
3.2.1 Temperature-dependent dielectric study
Figure 5a, b displays the temperature dependence of dielectric constant and tan δ at some selected frequency (25–1000 kHz) for Bi(Fe0.95Gd0.05)O3. Initially, the dielectric behaviour shows a partially independent of frequency and temperature (i.e. up to 560 K). The main reason for this nature is the presence of different types of polarizations, such as dipole, atomic, ionic, and interfacial [41]. However, a strong dispersive nature is observed in the high-temperature region (i.e. 560 ≤ ɛ r ≥ 773 K) and then reached the maximum value for all the frequencies. Such higher values of dielectric constant confirm the presence of ferroelectric nature in the BFGO5 compound. Figure 5a shows that the decreasing trend of dielectric constant with increasing frequency due to the gradual dissipation of polarization.
Figure 5b shows the variation of tangent loss (tan δ) as a function of frequency (25–1000 kHz) for the BFGO5 compound. It also follows a similar curve to that of dielectric constant from the initial point to 430 K, showing that the independent nature of both the frequency and temperature. Nevertheless, there is an abrupt increase in the value of tan δ in the temperature range of 520–745 K at all the selected frequencies which may be due to the effect of sample defects, oxygen vacancies and scattering of thermally activated charge carriers existing in the sample. Generally, dielectric loss has an increasing trend at all the frequencies with the rise of temperature, especially in iron (Fe)-containing compounds and then gradually decreases with further rise in temperatures (Fig. 5b) [37].
3.2.2 Frequency-dependent dielectric study
Figure 5c, d exhibits the frequency dependence of dielectric parameters of Bi(Fe0.95Gd0.05)O3 at some selected temperatures (298, 373, 473, 573, 673, 773 K). It clearly shows the gradual decreasing characteristics of both dielectric parameters (dielectric constant and tangent loss) with the rise in frequency at all the temperatures. The value of dielectric constant is comparatively high (ɛ r = 234 at 273 K) in the low-frequency region which starts decreasing gradually with the rise in frequency, which is due to the collection of charge at the grain boundaries. Therefore, it is evident that only a few dipoles follow the applied electric field in the high-frequency region, which confirms the existence of polar characteristics of BFGO5.
Figure 5d shows the variation of dielectric loss (tan δ) with respect to frequency at some selected temperatures (298–773 K) which shows a rapid increase in the low-frequency region and decreases with increasing the frequency by following the dielectric constant characteristics due to the existence of iron (Fe) in BFGO5 compound. The scattering of temperature-dependent charge carriers and a small imperfection occur during the synthesizing process which results in a small change in dielectric loss in the low- and medium-temperature range (i.e. 298–573 K). However, the dielectric loss remains almost constant at the higher temperatures (673–773 K).
3.3 Electrical impedance study
The impedance spectroscopy is a powerful technique which has been widely used for the study of electrical characteristics of developing materials. This experimental technique is commonly used to examine the effect of grains and their boundaries. Moreover, it is also used to examine the inter-relationship between dielectric and ionic conductors, and analysing the ionic movement in the prepared solid material [42]. In this study, a detailed analysis of different impedance parameters and their characteristics will be discussed.
Figure 6a shows the characteristics of real part of impedance (Z′) versus a frequency at some selected temperatures (298–773 K) of BFGO5. Initially, the value of Z′ rapidly falls with the rise of frequency, whereas it grows with the rise of temperature (up to 573 K) and then remains constant at higher temperatures of 673 and 773 K (as shown in the Fig. 6a). In the low-frequency range, the magnitude of Z″ gradually decreases with increasing temperature, which clearly indicates the NTCR behaviour of the compound. Moreover, the value of real impedance, Z′, continuously falls with the rise of frequency as well as temperature, and then all the impedance curves combined to get almost a constant value in the high-frequency region (i.e. 430–1000 kHz). This characteristic is probably due to the release of space charges.
Figure 6b displays the imaginary part of impedance (Z″) characteristics with respect to frequency at some of the selected temperatures (298–773 K). The magnitude of imaginary impedance sharply decreases on increasing the operating frequency and temperature. On increasing the temperature, there is a considerable decrease in peak broadening which suggests the occurence of phenomenon of temperature dependence of electrical relaxation process in the BFGO5 material. Such different types of peak broadening occur with rise of temperature clearly depicting the existence of a different electrical relaxation time in the studied material. The immobile charge carriers accumulated at the low temperatures may be attributed to this relaxation behaviour and make oxygen vacancies and defects at higher temperatures [43]. The relaxation time and frequency can also be calculated with the help of peak position in x-axis using of general relation of τ = 1/2πF r (where τ is the relaxation time; ω r RC = ω r τ = 1 and thus F r = 1/2πRC). Generally, the movement of electric charges is much dependent on the relaxation types of the material. The transmission of charges occurs through ion vacancies, oxygen voids and hopping of electrons [44].
Figure 6c shows the spectrum of real and imaginary impedance (Z′ vs Z″) through Nyquist plot at a selected temperature over a broad range of frequency (1–1000 kHz) for the BFGO5 compound. It is clearly observed from the different curves of the BFGO5 compound that the radius of a semi-circular arc irregularly decreases with the rise of intermediate temperatures (≥ 523 K). The impedance properties are distinguished by the emergence of semi-circular arcs whose curve depends on the change of temperatures. The semi-circular arc depicts a particular degree of curve which suggests that the centre of semicircles lying below the x-axis will confirm the existence of non-Debye-type relaxation process in the material. Since the Nyquist plot satisfied the said condition of semicircle (as shown in Fig. 6c), the relaxation process of the BFGO5 material is of a non-Debye type of relaxation process.
3.4 Electrical modulus analysis
The electrical modulus analysis is a specific technique used for the study of inside electrical relaxation process and the electrical characteristics of a polycrystalline material [45]. It is one of the most precise techniques for analysing the grain boundary conduction mechanism and detection of the electric polarization of the studied compound. Figure 7a, b displays the variation of real and imaginary parts of electrical modulus versus frequency (1–1000 kHz) of the BFGO5 compound at selected temperatures (between 298 and 773 K). Using the following mathematical equations, the estimated value of real (M′) and imaginary (M″) parts of complex electrical modulus can be calculated:
where symbols have their usual meaning.
The impedance data can be used for calculating the value of complex impedance modulus using general relations:
where \( M^{{\prime }} = \omega C_{0} Z^{{{\prime \prime }}} \) and \( M^{{{\prime \prime }}} = \omega C_{0} Z^{{\prime }} \); \( \omega \) is the angular frequency = \( 2\pi f \); \( C_{0} = \varepsilon_{0} \); A/t is the capacitance; A is the area of electrode sample surface; \( \varepsilon_{0} \) is the permittivity of free space; t is the thickness of the sample.
In Fig. 7a, it is observed that the value of M′ decreases on increasing temperature in the low range of frequency (i.e. 1–7 kHz) and then gradually increases at all the selected temperatures with a dispersive spectrum in the high-frequency range (7–1000 kHz). It clearly confirmed the existence of short-range mobility of charge carriers and conduction phenomenon in the present studied material. This implies the insufficiency of restoring force for moving the charges under the influence of constant electric field [46].
Figure 7b shows M″ characteristics for selected temperatures (298–773 K) within the frequency region of (1–1000 kHz). The M″ peaks are shifted accordingly towards higher temperature with increasing both the frequency and temperature. It is evident from the figure that the rate of relaxation process also gradually increasing with rise of temperature in the high-frequency region. The systematic shift of M″ peak position suggests that there is a thermally activated relaxation mechanism in the material. Moreover, the regular broadening of peak refers to an equal distribution of relaxation time, which shows the existence of non-Debye-type relaxation process in the studied material [47].
3.5 Electrical conductivity
3.5.1 Frequency-dependent ac conductivity
Figure 8a shows the characteristics of a frequency-dependent ac conductivity (\( \sigma_{\text{ac}} \)) of BFGO5 in a wide range of frequency (1–1000 kHz) at selected temperatures (298–773 K). The \( \sigma_{\text{ac}} \) vs frequency measurement has been carried out to examine the effect of frequency on the electrical properties of the studied material. The conductivity characteristic shows the rise in the value of \( \sigma_{\text{ac}} \) with the rise of frequency. This nature is due to the irregular arrangement of cations between the neighbouring sites and the presence of space charges [48]. Figure 8a shows the dispersive curves in the low-frequency region, whereas it gradually increases with the rise of operating frequency. Then they become closer and approach each other at higher frequencies. The conductivity characteristics clearly show a continuously increasing trend with respect to frequency at all the temperatures in BFGO5. Therefore, the nature of ac conductivity indicates the presence of NTCR behaviour in the studied compound. The value of ac conductivity is calculated from the dielectric data using a mathematical relation:
where symbols have their usual meaning [37, 38]. Jonscher’s power law [49] is the most commonly used method for studying the frequency dependence of conductivity behaviour of a material, it is given by
where \( \sigma_{\alpha } \) is a frequency-independent dc conductivity value, \( \sigma_{\beta } (\omega ) \) is a frequency-dependent ac conductivity having a power law characteristics, \( Y \) is the temperature dependent pre-exponential factor which controls the polarizability strength and \( \eta \) is the power law exponent which determines the correlation of mobile ions with its lattice around them. The value of \( \eta \) may change between 0 and 1 (zero for random hopping and around 1 is for correlation between charge carriers).
3.5.2 Temperature-dependent ac conductivity
Figure 8b shows the variation of ac conductivity of the BFGO5 compound as a function of inverse of absolute temperatures at some selected frequencies. The spectrum of ac conductivity has been divided into three regions depending on the temperature and slope, i.e. region I, region II and region III. In the low-temperature region (i.e. region I), the \( \sigma_{\text{ac}} \) conductivity value gradually decreases on rising the frequency, which exhibits the frequency-dependent conductivity in this region. In region II, the \( \sigma_{\text{ac}} \) conductivity spectrum continuously increases with the rise of temperature at all the frequencies. In region III, a sharp increase of ac conductivity (\( \sigma_{\text{ac}} \)) on increasing the temperature has been observed. The nature of \( \sigma_{\text{ac}} \) characteristics in this region confirms the frequency-independent region and it suggests an appropriate Arrhenius type. Therefore, based on the observations, the activation energy (E a) for all the selected frequencies is almost constant at low temperatures, whereas it increases as the temperature increases, which confirms the negative temperature coefficient of resistance (NTCR) behaviour of the material. It is also observed that the \( \sigma_{\text{ac}} \) conductivity of the studied material is much influenced by both frequency and temperature. The temperature-dependent activation energy (E a) at all the frequencies in the different regions has been calculated from the conductivity vs inverse of absolute temperature T with the help of Arrhenius relation, \( \sigma_{\text{ac}} = \sigma_{\text{o}} \cdot \exp ( - E_{\text{a}} /K_{\text{B}} \cdot T) \), where \( \sigma_{\text{o}} \) is the pre-exponential factor and \( K_{\text{B}} \) Boltzmann constant.
The calculated (from the slope) activation energy of the compound is summarized in Table 3. This result clearly shows that the value of activation energy decreases with increasing the frequency in the studied material.
3.6 Ferroelectric study
Figure 9 shows the room temperature (298 K) polarization vs electric field (P–E) characteristics of the Bi(Fe0.95Gd0.05)O3 material at the selected applied electric field (i.e. 10, 15 and 20 kV cm−1).
The presence of proper ferroelectric nature in the studied material has been confirmed by the ferroelectric hysteresis loop (Fig. 9). The unsaturated hysteresis loop may arise due to the result of current leakage made by small oxygen vacancies in the material. The high-temperature synthesis process of the BFGO5 compound results in a loss of small amount of oxygen which adds to the leaky trend of the studied compound [36, 38]. The values of the remanent polarization of the prepared material at the applied electric field 10, 15, and 20 kV cm−1 are 0.018, 0.022 and 0.031, respectively. The ferroelectric parameters (i.e. remnant polarization, coercive field) increase with the rise of applied electric field (10–20 kV cm−1) at room temperature (298 K). Based on the study of ferroelectric features of the BFGO5 compound, it has been observed that remanent polarization and coercive field are higher than that of parent compound, BFO [50]. Therefore, the observations of the present study clearly confirm BFGO5 compound which has significant advantages for making a model electronic device over most of the multiferroic compounds.
4 Summary and conclusions
Gadolinium (Gd)-substituted bismuth ferrite i.e. Bi(Fe0.95Gd0.05)O3 multiferroic material, referred as BFGO5, was successfully synthesized through a solid-state reaction technique. The basic structural, microstructural and particle size analyses of the BFGO5 compound were carried out through powder X-ray diffraction (XRD), energy-dispersive X-ray (EDX) and transmission electron microscope (TEM), respectively. The analysis of XRD peaks confirms the formation of a monophase orthorhombic symmetry. The microstructural analysis and grain size estimation of the studied material have been carried out using FE-SEM which confirms the average grain size is in the range of 300–400 nm. The average crystallite size calculated through Scherrer’s formula is found to be 84 nm. The small particle size has been estimated using TEM. The FT-IR spectrum has provided some molecular characteristics of the material. The dielectric study shows that both the dielectric parameters increase with a rise in temperature whereas it decreases with increasing frequency for the BFGO5 compound. The electrical characteristics of the material studied through complex impedance spectroscopy data of LCR impedance analyzer reveal the existence of grain and its boundary effects and NTCR behaviour in the studied material. Based on the observations of impedance and electric modulus, it has been shown that the BFGO5 has the non-Debye type relaxation process and follows non-exponential type of conductivity relaxation. The study of ac conductivity plot shows that the conductivity of the present study material is much influenced by both the temperature and frequency. Therefore, based on the significant improvement in the electrical and dielectric properties of the BFGO5 compound, it can be assumed that the material is a promising candidate for device applications.
Change history
08 September 2021
Editor's Note: Readers are alerted that the reliability of the data presented in this article is subject to criticism that is being considered by the editors. We will update readers once we have further information and all parties have been given an opportunity to respond in full.
19 May 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s00339-022-05625-7
References
S.W. Cheong, M. Mostovoy, Multiferroics: a magnetic twist for ferroelectricity. Nat. Mater. 6, 13–20 (2007)
W. Prellier, M. Singh, P. Murugavel, The single-phase multiferroic oxides: from bulk to thin film. J. Phys. Condens. Matter 17, R803 (2005)
N. Kumar, A. Shukla, N. Kumar, R.N.P. Choudhary, Structural, electrical and magnetic properties of eco-friendly complex multiferroic material: Bi(Co0.35Ti0.35Fe0.30)O3. Ceram. Int. 45, 822–831 (2019)
N. Kumar, A. Shukla, C. Behera, R.N.P. Choudhary, Structural, electrical and magnetic properties of Bi(Ni0.45Ti0.45Fe0.1)O3. J Alloy Compd. 688, 858–869 (2016)
N.A. Spaldin, S. Cheong, R. Ramesh, Multiferroics: past, present, and future. Phys. Today 63, 38 (2010)
E.A.V. Ferri, I.A. Santos, E. Radovanovic, R. Bonzanini, E.M. Girotto, Chemical characterization of BiFeO3 obtained by Pechini method. J. Braz. Chem. Soc. 19, 1153–1157 (2008)
S.K. Pradhan, J. Das, P.P. Rout, S.K. Das, D.K. Mishra, D.R. Sahu, A.K. Pradhan, V.V. Srinivasu, B.B. Nayak, S. Verma, B.K. Roul, Defect driven multiferroicity in Gd doped BiFeO3 at room temperature. J. Magn. Magn. Mater. 322, 3614–3622 (2010)
A.K. Pradhan, K. Zhang, D. Hunter, J.B. Dadson, G.B. Louts, Magnetic and electrical properties of single-phase multiferroic BiFeO3. J. Appl. Phys. 97, 093903 (2005)
Y.P. Wang, L. Zhou, M.F. Zhang, X.Y. Chen, J.M. Liu, Z.G. Liu, Room-temperature saturated ferroelectric polarization in BiFeO3 ceramics synthesized by rapid liquid phase sintering. Appl. Phys. Lett. 84, 1731–1733 (2004)
J. Wei, R. Haumont, R. Jarrier, P. Berhtet, B. Dkhi, Nonmagnetic Fe-site doping of BiFeO3 multiferroic ceramics. Appl. Phys. Lett. 96, 102509 (2010)
N. Kumar, A. Shukla, R.N.P. Choudhary, Structural electrical and magnetic properties of (Cd, Ti) modified BiFeO3. Phys. Lett. A 381, 2721–2730 (2017)
A. Shukla, N. Kumar, C. Behera, R.N.P. Choudhary, Structural and electrical characteristics of (Co, Ti) modified BiFeO3. J. Mater. Sci. Mater. Electron. 27, 7115–7123 (2016)
A. Mukherjee, S. Basu, G. Chakraborty, M. Pal, Effect of Y-doping on the electrical transport properties of nanocrystalline BiFeO3. J. Appl. Phys. 112, 014321–014328 (2012)
V.V. Lazenka, A.F. Ravinski, I.I. Makoed, J. Vanacken, G. Zhang, V.V. Moshchalkov, Weak ferromagnetism in La-doped BiFeO3 multiferroic thin films. J. Appl. Phys. 111, 123916 (2012)
H. Uchida, R. Ueno, H. Funakubo, S. Koda, Crystal structure and ferroelectric properties of rare-earth substituted BiFeO3 thin films. J. Appl. Phys. 100, 014106 (2006)
Z.X. Cheng, X.L. Wang, S.X. Dou, H. Kimura, K. Ozawa, Enhancement of ferroelectricity and ferromagnetism in rare earth element doped BiFeO3. J. Appl. Phys. 104, 116109 (2008)
A. Mukherjee, S. Basu, P.K. Manna, S.M. Yusuf, M. Pal, Enhancement of multiferroic properties of nanocrystalline BiFeO3 powder by Gd-doping. J. Alloy Comp. 598, 142–150 (2014)
D. Ghanbari, M. Salavati-Niasari, M. Ghasemi-Kooch, A sonochemical method for synthesis of Fe3O4 nanoparticles and thermal stable PVA-based magnetic nanocomposite. J. Ind. Eng. Chem. 20(6), 3970–3974 (2014)
A. Abbasi, D. Ghanbari, M. Salavati-Niasari, M. Hamadanian, Photo-degradation of methylene blue: photocatalyst and magnetic investigation of Fe2O3–TiO2 nanoparticles and nanocomposites. J. Mater. Sci. Mater. Electron. 27, 4800–4809 (2016)
D. Ghanbari, M. Salavati-Niasari, Synthesis of urchin-like CdS-Fe3O4 nanocomposite and its application in flame retardancy of magnetic cellulose acetate. J. Ind. Eng. Chem. 24, 284–292 (2015)
A. Sobhani, M. Salavati-Niasari, Synthesis and characterization of FeSe2 nanoparticles and FeSe2/FeO(OH) nanocomposites by hydrothermal method. J. Alloy Compd. 625, 26–33 (2015)
G.S. Lotey, N.K. Verma, Structural, magnetic, and electrical properties of Gd-doped BiFeO3 nanoparticles with reduced particle size. J. Nanopart. Res. 14, 742 (2012)
F. Chang, S. Guilin, F. Kun, Q. Ping, Z. Qijun, Effect of gadolinium substitution on dielectric properties of bismuth ferrite. J. Rare Earth 24, 273–276 (2006)
D.V. Vassallo, M.R. Simões, L.B. Furieri, M. Fioresi, J. Fiorim, E.A.S. Almeida, J.K. Angeli, G.A. Wiggers, F.M. Peçanha, M. Salaices, Toxic effects of mercury, lead and gadolinium on vascular reactivity. Braz. J. Med. Biol. Res. 44, 939–946 (2011)
T.W. Clarkson, L. Magos, G.J. Myers, The toxicology of mercury-current exposures and clinical manifestations. N. Engl. J. Med. 349, 1731–1737 (2003)
W. Zhou, H. Deng, H. Cao, J. He, J. Liu, P. Yang, J. Chu, Effects of Sm and Mn co-doping on structural, optical and magnetic properties of BiFeO3 films prepared by a sol–gel technique. Mater. Lett. 144, 93–96 (2015)
R. Guo, L. Fang, W. Dong, F. Zheng, M. Shen, Enhanced photocatalytic activity and ferromagnetism in Gd doped BiFeO3 nanoparticles. J Phys. Chem. C 114, 21390–21396 (2010)
D. Kuang, P. Tang, X. Wu, S. Yang, X. Ding, Y. Zhang, Structural, optical and magnetic studies of (Y, Co) co-substituted BiFeO3 thin films. J. Alloy. Compd. 671, 192–199 (2016)
S. Chauhan, M. Kumar, S. Chhoker, S.C. Katyal, H. Singh, M. Jewariya, K.L. Yadav, Multiferroic, magnetoelectric and optical properties of Mn doped BiFeO3 nanoparticles. Solid State Commun. 152, 525–529 (2012)
S. Irfan, S. Rizwan, Y. Shen, L. Li, S. Butt, C.W. Nan, The gadolinium (Gd3+) and tin (Sn4+) co-doped BiFeO3 nanoparticles as new solar light active photocatalyst. Sci. Rep. 7, 42493 (2017)
M. Esmaeili-Zare, M. Salavati-Niasari, A. Sobhani, Simple sonochemical synthesis and characterization of HgSe nanoparticles. Ultrason. Sonochem. 19, 1079–1086 (2012)
S. Moshtaghia, D. Ghanbarib, M. Salavati-Niasari, Characterization of CaSn(OH)6 and CaSnO3 nanostructures synthesized by a new precursor. J. Nanostruct. 5, 169–174 (2015)
S. Zinatloo-Ajabshir, M.S. Morassaei, M. Salavati-Niasari, Facile fabrication of Dy2Sn2O7–SnO2 nanocomposites as an effective photocatalyst for degradation and removal of organic contaminants. J. Colloid Interface Sci. 497, 298–308 (2017)
B. Park, An interactive powder diffraction data interpretations and indexing program version 2.1 (E. WU School of Physical Sciences, Flinders University of South Australia, Bedford Park, 1989), p. 5042
N. Kumar, A. Shukla, N. Kumar, S. Sahoo, S. Hajra, R.N.P. Choudhary, Structural, bulk permittivity and impedance spectra of electronic material: Bi(Fe0.5La0.5)O3. J. Mater. Sci. Mater. Electron. 30, 1919–1926 (2019)
N. Kumar, A. Shukla, N. Kumar, S. Sahoo, S. Hajra, R.N.P. Choudhary, Structural, electrical and ferroelectric characteristics of Bi(Fe0.9La0.1)O3. Ceram. Int. 44, 21330–21337 (2018)
A. Shukla, N. Kumar, C. Behera, R.N.P. Choudhary, Structural, dielectric and magnetic characteristics of Bi(Ni0.25Ti0.25Fe0.50)O3 ceramics. J. Mater. Sci. Mater. Electron. 27, 1209–1216 (2016)
N. Kumar, A. Shukla, R.N.P. Choudhary, Structural, dielectric, electrical and magnetic characteristics of lead-free multiferroic, Bi(Cd0.5Ti0.5)O3–BiFeO3 solid solution. J Alloy. Compd. 747, 895–904 (2018)
A.V. Zalesskii, A.A. Frolov, T.A. Khimich, A.A. Bush, Composition-induced transition of spin-modulated structure into a uniform antiferromagnetic state in a Bi1−xLaxFeO3 system studied using 57Fe NMR. Phys. Solid State 45(1), 141–145 (2003)
I.O. Troyanchuk, A.N. Chobot, O.S. Mantytskaya, N.V. Tereshko, Magnetic properties of Bi(Fe1−xMx)O3 (M = Mn, Ti). Inorg. Mater. 46, 424–428 (2010)
N. Kumar, A. Shukla, R.N.P. Choudhary, Structural, electrical and magnetic characteristics of Ni/Ti modified BiFeO3 lead free multiferroic material. J. Mater. Sci. Mater. Electron. 28, 6673–6684 (2017)
J.R. Macdonald, W.B. Johnson, Impedance spectroscopy theory, experiments and applications (Wiley, Hoboken, 2005)
A.K. Jonscher, The ‘universal’ dielectric response. Nature 267, 673–679 (1977)
S. Dash, R.N.P. Choudhary, A. Kumar, Impedance spectroscopy and conduction mechanism of multiferroic (Bi0.6K0.4)(Fe0.6Nb0.4)O3. J. Phys. Chem. Solids 75, 1376–1382 (2014)
N. Hirose, A.R. West, Impedance spectroscopy of undoped BaTiO3 ceramics. J. Am. Ceram. Soc. 79, 1633 (1996)
A. Sinha, A. Dutta, Microstructure evolution, dielectric relaxation and scaling behaviour of Dy for-Fe substituted Ni-nanoferrites. RSC Adv. 5, 100330–100338 (2015)
G. Singh, V.S. Tiwari, P.K. Gupta, Role of oxygen vacancies on relaxation and conduction behaviour of KNbO3 ceramic. J. Appl. Phys. 107, 064103 (2010)
N.K. Karan, D.K. Pradhan, R. Thomas, B. Natesan, R.S. Katiyar, Solid polymer electrolytes based on polyethylene oxide and lithium trifluoro-methane sulfonate (PEO–LiCF3SO3): ionic conductivity and dielectric relaxation. Solid State Ion. 179, 689 (2008)
A.K. Jonscher, Universal relaxation law (Chelsea Dielectrics Press, London, 1996)
B. Dhanalakshmi, P. Kollu, B. Chandra Sekhar, B. Parvatheeswara Rao, P.S.V. Subba Rao, Enhanced magnetic and magneto-electric properties of Mn doped multiferroic ceramics. Ceram. Int. 43, 9272–9275 (2017)
Acknowledgements
The authors are grateful to National Physical Laboratory, New Delhi, for providing some TEM characterization facility. Author Alok Shukla gratefully acknowledges the financial support received from SERB-DST, Government of India, New Delhi, in the form of Research Project no. EMR/2015/002420.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Thansanga, L., Shukla, A., Kumar, N. et al. RETRACTED ARTICLE: Study of effect of Gd substitution at the Fe site on structural, dielectric and electrical characteristics of BiFeO3. Appl. Phys. A 125, 764 (2019). https://doi.org/10.1007/s00339-019-3058-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-019-3058-y