Abstract
In this work, ethylenediaminetetraacetic acid (EDTA) surface grafting and copper(II) doping of superparamagnetic iron oxide nanoparticles (EDTA/Cu-SPIONs) were simultaneously performed during their formation by a simple electrochemical procedure. This method was achieved by applying a constant current of 10 mA/cm2 to the two-electrode electrochemical cell containing aqueous electrolyte of iron(II) chloride (0.75 g), iron(III) nitrate (2 g), copper(II)chloride (0.5 g) and 0.2 g EDTA. The prepared EDTA/Cu-SPION samples are specified by TEM, VSM, IR, EDS, FE-SEM, TGA and XRD analyses. The magnetite (i.e., Fe3O4) crystal structure (verified by the XRD results), 10 nm particle size (proved by TEM observations), 11 wt% doped copper (revealed though EDS data), and 10 wt% surface coat (calculated from the thermogravimetric data) were specified for the deposited SPION powder. IR data showed all the absorptions related to the chemical bonds (i.e., C–C, C–N, N–H, C–O) of EDTA and proved the EDTA-grafted configuration of the deposited SPIONs. VSM measurements exhibited the superparamagnetic behavior of the EDTA-grafted EDTA/Cu-SPION sample, where relative high saturation magnetization (Ms = 31.38 emu g−1), low remanence (Mr = 0.31 emu g−1) and negligible coercivity (Hci = 6.6 Oe) were observed. Based on the results, the developed route is introduced as a facile procedure for the preparation of EDTA-grafted metal ion-doped magnetite fine particles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, iron oxide nanoparticles (IONs) have received tremendous attention in biomedicine, nanoengineering, biology, catalysis and environmental applications [1,2,3,4,5]. These oxide particles are a class of excellent nano-medical materials due to their superparamagnetism, high magnetic moments, extra magnetic anisotropy contribution, irreversibility of high-field magnetization, high surface area, magnetothermal effect, and ideal biocompatibility [6,7,8,9]. Three most common structures of IONs are magnetite (Fe3O4), hematite (α-Fe2O3) and maghemite (γ-Fe2O3), among which, superparamagnetic iron oxide nanoparticles (SPIONs) are of great interest for clinical, environmental and energy storage applications due to their mixed oxidation states, natural abundance, low cost, as well as the environmental friendliness, low toxicity, colloidal stability, superparamagnetic property and surface engineering capability [10, 11]. The applications of SPIONs include heavy metal removal [12,13,14], super-capacitors [15,16,17,18], magnetic resonance imaging (MRI) [19, 20], hyperthermia [21, 22], and drug delivery [23, 24]. Hence, many efforts have been paid to prepare SPIONs with proper properties. In this regard, some chemical synthetic methods including co-precipitation [25, 26], thermal decomposition [27, 28], hydrothermal [29] and solvothermal [30] have been developed for the fabrication of SPIONs with suitable physico-chemical characters (e.g., crystalline nature, controlled particle size, narrow distribution and spherical shape), and desirable magnetic properties (e.g., high saturation magnetization (Ms), negligible remanence and low coercivity). Furthermore, different surface engineering strategies including direct nanoparticle conjugation, covalent linkage, click chemistry, covalent linker chemistry, and physical interactions have been introduced for preparing surface-grafted SPIONs with proper surface charge, stability, solubility and biocompatibility [31,32,33]. Surface functionalization has also resolved the unavoidable problems associated with the naked SPIONs after their synthesis, i.e., (i) loss of magnetism and (ii) dispersibility loss, where the former issue is due to the easy oxidation of bare IONPs in air, and the latter results from the tendency of small NPs to aggregate and form large particles [32, 33]. In this regard, various bio-compatible coating agents including saccharides [34], PVP [35, 36], amino acids [37], PEI [38], chitosan [39] and EDTA [40, 41] have been coated onto the surface of SPIONs and their biomedical performances investigated.
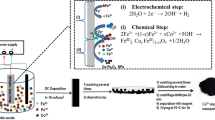
Electrochemical synthesis (i.e., cathodic electrodeposition) represents a highly efficient method for the fabrication of nanostructured materials with various nanostructures (e.g., nanoparticles [42], nanoplates [43], nanosheets [44], and composite nanostructures [45, 46]), with advantages of low cost, low synthetic temperature, and simplicity. In this method, the product is formed through an electrochemical/chemical mechanism; first, base ions are electrochemically generated onto the cathode surface and then reacted with metal cations [47]. In the case of SPIONs, cathodic electrochemical synthesis (CES) has been applied as alternative and simple technique for their synthesis and in situ surface functionalization [48, 49]. Herein, we report ethylenediaminetetraacetic acid (EDTA)-grafted Cu2+-doped SPIONs (i.e., EDTA/Cu-SPIONs) prepared through one-step and simple electrochemical deposition route for the first time. The fabricated EDTA/Cu-SPIONs are characterized via TEM, XRD, FT-IR, FE-SEM, EDS, DSC–TGA and VSM techniques.
2 Experimental procedure
2.1 Materials
Copper (II) chloride hexahydrate (Sigma-Aldrich, 99.5%), iron(III) nitrate nonahydrate (Sigma-Aldrich, 99.9%), iron(II) chloride hexahydrate (Sigma-Aldrich, 99%) and ethylenediaminetetraacetic acid disodium salt dehydrate (EDTA-Na2, Sigma-Aldrich, 99.9%) were purchased and used as received. Stainless steel 316L and graphite plates were provided from local companies.
2.2 Preparation of iron oxide samples
The iron oxide samples were prepared through cathodic electrochemical synthesis (CES) method. A two-electrode electrochemical cell connected to an external power supply (PROVA 8000) was used in the CES experiments. The cathode and anode electrodes were stainless steel sheet (316L, size = 5 cm × 5 cm) and graphite plate (size = 7 cm × 7 cm), respectively. The deposition bath was 1 g Fe(NO3)3·9H2O, 0.5 g FeCl2·4H2O, 0.25 g CuCl2·6H2O and 0.2 g EDTA dissolved in 500 cc deionized water. For the synthesis of EDTA-grafted Cu2+-doped SPIONs, dc current of 0.5 A was applied to the electrochemical cell for 20 min and a dense black film was formed onto the steel cathode. The cathode was then removed from the deposition solution and washed several times with ethanol. The deposited film was collected from the steel sheet and the obtained wet powder was dispersed in 200 cc ethanol solution. This solution was then centrifuged at 3000 rpm for 10 min to remove the weakly bounded EDTA onto the iron oxide particles. In the final step, the dispersed particles were collected from the ethanol solution by magnet and dried at 70 °C for 1 h.
2.3 Characterization analyses
The morphological feature of the prepared iron oxides was analyzed via field-emission scanning electron microscopy (FE-SEM, Mira 3-XMU with accelerating voltage of 100 kV) and transmission electron microscopy (TEM, Zeiss EM900 with an accelerating voltage of 80 kV). The elemental analysis was also provided by energy-dispersive diffraction X-ray analyzer connected to the FE-SEM instrument. The crystal structure and crystallite size (D) of the prepared sample was provided through X-ray diffraction (XRD, model: Phillips PW-1800). Thermogravimetric analysis (DCS-TG) was done in N2 atmosphere between room temperature and 500 °C at a heating rate of 5 °C min−1 using a thermoanalyzer, model STA-1500. The FT-IR analysis was done at a resolution of 4 cm−1 from 400 to 4000 cm−1 using a Bruker Vector 22 Fourier transform infrared spectroscope. The magnetic properties of the prepared sample were specified in the range of − 20,000 to 20,000 Oe by vibrating sample magnetometer (VSM, model: Lake shore 7400, USA).
3 Results and discussion
3.1 X-ray diffraction
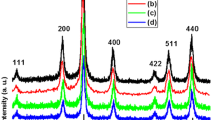
Figure 1 shows XRD pattern of the synthesized EDTA/Cu-SPIONs. As noted in Fig. 1, all the observed diffractions are easily ascribed to the magnetite crystal phase of iron oxide with cubic spinel structure (i.e., Fe3O4, JCPDS 01-088-0315). No extra diffraction is detected, revealing the pure magnetite crystal phase for the deposited EDTA/Cu2+-doped SPIONs sample. Using the Scherrer’s equation (D = 0.9λ/β cos (θ)) and diffraction peak of (311), an average crystallite size (D) of 7.6 nm was calculated for the prepared EDTA/Cu2+-doped SPIONs.
3.2 Morphology and particle size
Morphological properties and chemical composition data of the prepared EDTA/Cu2+-doped SPIONs are shown in Fig. 2. The FE-SEM images (Fig. 2a, b) presented particle features with spherical shapes for the prepared sample. Notably, the prepared particles have rather agglomerated forms as seen in the FE-SEM images (Fig. 2a, b). TEM observations exhibited complete spherical form of the prepared Cu2+-doped SPIONs with narrow size distribution and 10 nm size particle (Fig. 2c).
The elemental analysis of the prepared sample through energy-dispersive X-ray spectroscopy (EDAX) confirmed the presence of oxygen, carbon, nitrogen, copper and iron in its composition (Fig. 2d). The C, O and N presence deals with the EDTA content in the sample composition, and proved the EDTA-capped layer on the surface of the deposited Cu2+-doped SPION particles. The copper element with weight percentage of 11.17% verified the doping of iron oxide with this element. Hence, from the TEM and EDS analyses, the configuration of EDTA-grafted Cu2+-doped iron oxide particles with size 10 nm was specified for the electrodeposited sample.
3.3 FT-IR
The chemical composition of the prepared EDTA/Cu-SPION sample was also studied through IR spectroscopy. In this regard, the IR spectrum of the prepared EDTA/Cu-SPION sample was recorded in the wavenumbers of 400–4000 cm−1 and the obtained data are presented in Fig. 3. The IR absorptions at the wavenumbers below 700 cm−1 are due to the stretching vibrations of M–O–M bonds [49, 50]. In the case of EDTA/Cu2+-doped SPIONs, two observed IR bands at 577 cm−1 and 658 cm−1 originated from the splitting of ν1 vibration mode of the Fe2+–O2−/Cu2+–O2− bonds [50, 51], IR band at 427 cm−1 also resulted from the ν2 vibration mode of Fe3+–O2− bond [49, 51]. As listed in Table S1, there are various IR bands at higher ranges, which could be assigned to the stretching and bending vibrations of N–H, C–O, N–C–H, C–H, C–C and C–N bonds. Hence, EDTA-grafted layer onto the surface of the electrodeposited Cu2+-doped SPION particles is verified.
3.4 Thermogravimetric analysis
Figure 4 shows thermal behavior of the prepared EDTA-grafted Cu2+-doped SPIONs in the range of 25–700 °C. In the DSC profile (Fig. 4a), there is an endothermic peak at T < 150 °C, which is related to removing the OH− groups attached onto the surface of Cu2+-doped SPIONs [32,33,34]. For this step, the sample exhibits 3.76% weight loss on TG profile (Fig. 4b). After this step, two successive endothermic peaks are observed at 200 °C < T < 400 °C with peaks at the temperatures of 215 °C and 274 °C (Fig. 4a), which are consistent with the reported data for EDTA decomposition, i.e., 200–400 °C [40, 52, 53]. The prepared sample showed the main weight loss for this temperature range on its TG profile (i.e., 9.12 wt%), as denoted in Fig. 4b. In the range 400–700 °C, there is one peak on the DSC curve at a temperature of 482 °C, which is due to the oxidation of Fe3O4 into α-Fe2O3 phase [35,36,37]. For this step, 1.01 wt% is measured with the TG profile (Fig. 4b). The total weight loss of sample was observed to be 13.88%. These results confirmed the successful electrochemical synthesis of EDTA-grafted iron oxide particles.
3.5 Magnetic evaluation
To characterize the magnetic behavior of the prepared EDTA-grafted Cu2+-doped iron oxide nanoparticles, magnetization data at the applied fields of − 20,000 Oe to + 20,000 Oe were recorded and are presented in Fig. 5a. Magnetization profile of sample was also provided under the condition of applied field → 0 to determine the remanence (Mr) and coercivity (Hci) values. From Fig. 5a, it is verified that the prepared nanoparticles show superparamagnetic properties due to the absence of any hysteresis loop in the applied fields and also the S shape of VSM curve. The obtained magnetic data from Fig. 5a, b including Ms, Mr and Hci are listed in Table 1. For comparison, the magnetic data reported for the naked SPIONs and Cu2+-doped SPIONs are also provided from Ref. [54]. The prepared EDTA-grafted Cu2+-doped SPIONs exhibited Ms, Mr and Hci values of 31.38 emu g−1, 0.31 emu g−1 and 6.6 Oe, respectively. These data showed the superparamagnetic nature of the prepared sample and comparable with the reported ones for the naked SPIONs and naked Cu-SPIONs [15,16,17,18]. Comparison to the naked iron oxides, the EDTA-grafted particles showed lower Ms value, which is related to the non-magnetic organic layer and also the reduced magnetite part in the prepared sample. However, the EDTA-grafted Cu2+-doped SPIONs exhibited lower remanence and coercivity (Mr = 0.31 emu/g and Hci = 6.6 Oe) as compared to the naked SPIONs (Mr = 0.95 emu/g and Hci = 14.6 Oe), which revealed its better superparamagnetic behavior and hence improvement of superparamagnetism of iron oxide nanoparticles as the results of copper cations doping on their crystal structure and EDTA grafting onto their surfaces.
4 Conclusion
In summary, a novel and simple procedure based on the cathodic electrodeposition technique was developed for fabrication of the EDTA-grafted Cu2+-doped iron oxide nanoparticles. The XRD and TEM analyses verified the magnetite crystal structure and 10 nm particle size of the prepared sample. Copper cations doping (about 11 wt%) into the magnetite structure was also confirmed through IR and EDS data. Both FT-IR and TG analyses revealed the EDTA-grafted surface feature for the deposited iron oxide nanoparticles. An improvement in the superparamagnetic behavior of iron oxide nanoparticles was observed as a result of metal cations doping on the Fe3O4 crystal structure and surface capping with EDTA.
References
X. Li, J. Wei, K.E. Aifantis, Y. Fan, Q. Feng, F.Z. Cui, F. Watari, J. Biomed. Mater. Res. 104A, 1285–1296 (2016)
R.K. Sharma, S. Dutta, S. Sharma, R. Zboril, R.S. Varma, M.B. Gawande, Green Chem. 18, 3184–3209 (2016)
L.S. Arias, J.P. Pessan, A.P. Miranda Vieira, T.M.T. de Lima, A.C.B. Delbem, D.R. Monteiro, Antibiotics 7, 46–77 (2018)
Y.L. Pang, S. Lim, H.C. Ong, W.T. Chong, Ceram. Int. 42, 9–34 (2016)
N.V.S. Vallabani, S. Singh, 3 Biotech 8, 279–301 (2018)
W. Li, J. Zaloga, Y. Ding, Y. Liu, C. Janko, M. Pischetsrieder, C. Alexiou, A.R. Boccaccini, Sci. Rep. 6, 23140 (2016)
G. Muzio, M. Miola, S. Ferraris, M. Maggiora, E. Bertone, M.P. Puccinelli, M. Ricci, E. Borroni, R.A. Canuto, E. Verné, A. Follenzi, Mater. Sci. Eng., C 76, 439–447 (2017)
M. Aghazadeh, I. Karimzadeh, M.R. Ganjali, Curr. Nanosci. 15, 169–177 (2019)
Y. Ha, S. Ko, I. Kim, Y. Huang, K. Mohanty, C. Huh, J.A. Maynard, A.C.S. Appl, Nano Mater. 1, 512–521 (2018)
A.R. Nochehdehi, S. Thomas, M. Sadri, S.S.S. Afghahi, S.M.M. Hadavi, J. Nanomed. Nanotechnol. 8, 423–455 (2017)
S. Sabale, P. Kandesar, V. Jadhava, R. Komorekb, R.K. Motkuri, X.Y. Yu, Biomater. Sci. 5, 2212–2225 (2017)
S. Lin, M. Xu, W. Zhang, X. Hu, K. Lin, J. Hazard. Mater. 335, 547–555 (2017)
C. Tamez, R. Hernandez, J.G. Parsons, Microchem. J. 125, 97–104 (2016)
S. Lin, C. Lian, M. Xu, W. Zhang, L. Liu, K. Lin, Appl. Surf. Sci. 422, 675–681 (2017)
M. Aghazadeh, M.R. Ganjali, Ceram. Int. 44, 520–529 (2018)
M. Aghazadeh, I. Karimzadeh, M.R. Ganjali, A. Behzad, J. Mater. Sci.: Mater. Electron. 28, 18121–18129 (2017)
M. Aghazadeh, I. Karimzadeh, M.R. Ganjali, Mater. Lett. 209, 450–454 (2017)
M. Aghazadeh, M.R. Ganjali, J. Mater. Sci.: Mater. Electron. 29, 2291–2300 (2018)
X. Liu, S. Lu, D. Liu, L. Zhang, L. Zhang, X. Yu, R. Liu, Brain Res. 1707, 141–153 (2019)
V. Gómez-Vallejo, M. Puigivila, S. Plaza-García, B. Szczupak, R. Piñol, J.L. Murillo, V. Sorribas, G. Lou, S. Veintemillas, P. Ramos-Cabrerg, J. Llop, A. Millán, Nanoscale 10, 14153–14164 (2018)
A. Espinosa, R. Di Corato, J. Kolosnjaj-Tabi, P. Flaud, T. Pellegrino, C. Wilhelm, ACS Nano 10, 2436–2446 (2016)
J. Mosayebi, M. Kiyasatfar, S. Laurent, Adv. Healthc. Mater. 6, 1700306 (2017)
S. Ullah, K. Seidel, S. Türkkan, D.P. Warwas, T. Dubich, M. Rohde, H. Hauser, P. Behrens, A. Kirschning, M. Köster, D. Wirth, J. Control. Release 294, 327–336 (2019)
M. Magro, D. Baratella, E. Bonaiuto, J.A. Roger, G. Chemello, S. Pasquaroli, L. Mancini, I. Olivotto, G. Zoppellaro, J. Ugolotti, C. Aparicio, A.P. Fifi, G. Cozza, G. Miotto, G. Radaelli, D. Bertotto, R. Zboril, F. Vianello, Biomacromol 20, 1375–1384 (2019)
L.B. Mello, L.C. Varanda, F.A. Sigoli, I.O. Mazali, J. Alloy. Compd. 779, 698–705 (2019)
J. Wan, G. Tang, Y. Qian, Appl. Phys. A 86, 261–264 (2007)
W. Glasgow, B. Fellows, B. Qi, T. Darroudi, O.T. Mefford, Particuology 26, 47–53 (2016)
N.J. Orsini, B. Babić-Stojić, V. Spasojević, M.P. Calatayud, G.F. Goya, J. Magn. Magn. Mater. 449, 286–296 (2018)
S. Phumying, S. Labuayai, C. Thomas, V. Amornkitbamrung, E. Swatsitang, S. Maensiri, Appl. Phys. A 111, 1187–1193 (2013)
D.A. Brewster, D.J. Sarappa, K.E. Knowles, Polyhedron 157, 54–62 (2019)
K. Hola, Z. Markova, G. Zoppellaro, J. Tucek, R. Zboril, Biotechnol. Adv. 33, 1162–11761 (2015)
W. Xie, Z. Guo, F. Gao, Q. Gao, D. Wang, B. Liaw, Q. Cai, X. Sun, X. Wang, L. Zhao, Theranostics 8, 3284–3307 (2018)
M. Peng, H. Li, Z. Luo, J. Kong, Y. Wan, L. Zheng, Q. Zhang, H. Niu, A. Vermorken, W. Van de Ven, C. Chen, X. Zhang, F. Li, L. Guo, Y. Cui, Nanoscale 7, 11155–11162 (2015)
I. Karimzadeh, M. Aghazadeh, M.R. Ganjali, P. Norouzi, T. Doroudi, P.H. Kolivand, Mater. Lett. 189, 290–294 (2017)
S. İşçi, Y. İşçi, M.G. Bekaroğlu, Appl. Phys. A 123, 534 (2017)
M. Aghazadeh, I. Karimzadeh, M.R. Ganjali, Mater. Lett. 228, 137–140 (2018)
I. Karimzadeh, M. Aghazadeh, M.R. Ganjali, T. Dourudi, Curr. Nanosci. 13, 167–174 (2017)
I. Karimzadeh, H.R. Dizaji, M. Aghazadeh, J. Magn. Magn. Mater. 416, 81–88 (2016)
M. Aghazadeh, I. Karimzadeh, Curr. Nanosci. 14, 42–49 (2018)
E. Shah, P. Upadhyay, M. Singh, M.S. Mansuri, R. Begum, N. Shethd, H.P. Soni, New J. Chem. 40, 9507–9519 (2016)
A.G. Magdalena, I.M.B. Silva, R.F.C. Marques, A.R.F. Pipi, M. Jafelicci, J. Phys. Chem. Solids 113, 5–10 (2018)
M. Aghazadeh, A.N. Golikand, M. Ghaemi, Int. J. Hydrogen Energy 36, 8674–8679 (2011)
M. Aghazadeh, I. Karimzadeh, M.R. Ganjali, J. Mater. Sci.: Mater. Electron. 28, 13532–13539 (2018)
M. Aghazadeh, J. Mater. Sci.: Mater. Electron. 28, 3108–3117 (2017)
M. Aghazadeh, I. Karimzadeh, A. Ahmadi, M.R. Ganjali, J. Mater. Sci.: Mater. Electron. 29, 14567–14573 (2018)
M. Aghazadeh, I. Karimzadeh, A. Ahmadi, M.R. Ganjali, P. Norouzi, J. Mater. Sci.: Mater. Electron. 29, 14378–14386 (2018)
M. Aghazadeh, Anal. Bioanal. Electrochem. 11, 211–266 (2019)
M. Aghazadeh, J. Mater. Sci.: Mater. Electron. 28, 18755–18764 (2017)
M. Aghazadeh, I. Karimzadeh, M.R. Ganjali, J. Electron. Mater. 47, 3026–3036 (2018)
M. Aghazadeh, M.R. Ganjali, J. Mater. Sci.: Mater. Electron. 29, 4981–4991 (2018)
M. Aghazadeh, Mater. Lett. 211, 225–229 (2018)
Y. Huang, A.A. Keller, Water Res. 80, 159–168 (2015)
P.R. Desai, N.J. Jain, R.K. Sharma, P. Bahadur, Colloid Surf. A 178, 57–69 (2001)
M. Aghazadeh, M.R. Ganjali, J. Mater. Sci. 53, 295–308 (2018)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aghazadeh, M., Karimzadeh, I., Ganjali, M.R. et al. EDTA-grafted Cu2+-doped superparamagnetic nanoparticles: facile novel synthesis and their structural and magnetic characterizations. Appl. Phys. A 125, 506 (2019). https://doi.org/10.1007/s00339-019-2803-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-019-2803-6