Abstract
This work is concerned with the use of TiO2-coated ZnS films as a photoanode in dye-sensitized solar cell (DSSC). The ZnS layer has been coated on TiO2 films via immersion technique. The influence of ZnS precursor concentration on the properties of the sample has been investigated. The effect of the precursor concentration on the performance of the device has also been studied. The XRD analysis reveals that anatase and ZnS phase exists in TiO2-coated ZnS sample. The samples absorb light in UV region but reflect more light in the visible region. The FESEM analysis reveals that the morphology of the TiO2-coated ZnS is nanograss. The device utilizing 0.03 M ZnS precursor demonstrates the highest η of 1.323% due to the smallest bulk and charge transfer resistance and also due to longest carrier lifetime.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Titania or TiO2 is well known as a popular wide band-gap metal oxide as photoanode of dye-sensitized solar cell (DSSC). This is due to TiO2 in anatase phase possesses good optical, electronic, and photocatalytic properties [1]. To modify these properties, various modifications have been attempted such as introducing metal oxide or chalcogenide composite material into TiO2 host material or coating another metal oxide or chalcogenide material onto the TiO2. Hu and his group reported that the recombination of electrons with holes was depressed by introducing strontium oxide (SrO) shell coating on TiO2 and consequently increased the power conversion efficiency of the device [2]. Zhu and his co-workers found that the efficiency of the device was improved upon utilizing graphene-TiO2 composite photoanode due to the increase in photocurrent density and voltage [3]. Dong and his co-researchers employed silver-loaded TiO2 composite as a photoanode of DSSC and found a significant increase in the efficiency of the device [4]. TiO2-coated ZnO has been applied as photoanode of DSSC and demonstrated the power efficiency of 5.65% [5]. TiO2-coated ZrO2 has been employed as a photoanode of the device and the short-current density (Jsc) has been improved by twofold compared with the control device [6]. The DSSC employing TiO2-coated Nb2O5 demonstrated the efficiency of 7.23% due to the significant increase in the short-circuit current density [7].
However, in this work, TiO2 has been coated with chalcogenide semiconductor that is zinc sulphide (ZnS). The objective of this work is to investigate the influence of ZnS content by varying the zinc nitrate hexahydrate precursor concentrations on the properties of TiO2 films such as structure and optical properties. These properties are then correlated with the performance parameters of DSSC utilizing TiO2-coated ZnS photoanode such as short-circuit current density and power conversion efficiency. The new idea of the work is the utilization of TiO2-coated ZnS films prepared via liquid-phase deposition assisted with immersion techniques as photoanode of DSSC.
2 Experimental
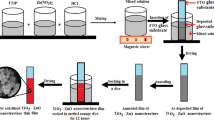
First, ITO substrates were cleaned using acetone and ethanol in the right sequence in an ultrasonic bath. TiO2 films were grown twice on ITO substrates via liquid-phase deposition technique [8]. The ITO substrates were immersed in a solution containing 0.2 M boric acid, H3BO3, and 0.1 M ammonium hexafluorotitanate, (NH4)2TiF6 for 25 h. The growth temperature was maintained at 30 °C. After 25 h, the TiO2 samples were left to dry. ZnS solution was prepared separately by dissolving zinc nitrate hexahydrate and thiourea in ethanol with molarity ratio of 1:1. Then, the TiO2 samples were immersed in 0.01 M ZnS solution for 30 min. Finally, the sample was annealed at 400 °C for 1 h. These procedures were repeated for preparing TiO2-coated ZnS with other precursor concentrations, namely, 0.03, 0.05, 0.07, and 0.10 M. The samples were characterized by XRD, UV–Vis, and FESEM to study its phase structure, optical absorption and reflection, and morphology, respectively. The crystallite size of the sample was estimated using Scherrer equation.
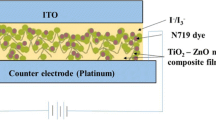
0.5 mM of N719 dye solution was prepared as the photoanode sensitizer. TiO2-coated ZnS films were immersed in the dye solution for 15 h at room temperature to ensure that sufficient amount of dye molecules were adsorbed on the surface of the samples. The prepared samples were employed as the photoanode for DSSC. The counter electrode of the device was platinum film grown on FTO substrates. Redox electrolyte containing iodide/triiodide was injected into the space between TiO2/ZnS-coated N719 and platinum counter electrode. The devices with an active area of 0.23 cm2 were tested in dark and under the illumination of 100 mW cm−2 light using Gamry Interface 1000 Potentiostat instrument. The light source used was tungsten halogen lamp. The electrochemical impedance spectroscopy (EIS) was performed on the devices to determine the charge transport properties and carrier lifetime.
3 Results and discussion
Figure 1 shows the XRD spectra of ZnS-coated TiO2 films with different ZnS precursor concentrations. The presence of anatase phase of TiO2 can be confirmed from five major peaks in the spectra. The peaks at the diffraction angles of 25°, 37°, 48°, 54°, and 55° corresponding to the planes (101), (004), (200), (105), and (211) belong to anatase phase. These peaks agrees well with the XRD pattern for TiO2 anatase phase studied by Umar and his group [8]. ZnS phase was also detected from the XRD patterns. However, the ZnS peak intensity is much smaller compared to that of anatase peaks. The ZnS phase was detected at the diffraction angles of 29°, 48°, and 57° corresponding to the planes (002), (001), (110), and (112), respectively. These results agree well with the XRD results analyzed by Damm and his co-workers who studied the mechanically induced phase transformation of ZnS [9]. The crystallite size of TiO2 for the 0.01, 0.03, 0.05, 0.07, and 0.10 M samples are 101.2, 103.7, 134.7, 170.2, and 89.7 nm, respectively. From these data, it is found that the crystallite size increases with precursor concentration and then decreases at 0.10 M precursor concentration.
Figure 2 illustrates the reflection spectra for the ZnS-coated TiO2 samples with various concentrations of the precursor. It is found that the samples reflect more light in the visible región (400–700 nm) rather than in ultra-violet (UV) región (300–400 nm). It is also found that the reflection varies with ZnS content. The 0.05 and 0.07 M samples possess the highest reflection in the visible light region, while 0.10 M sample displays the lowest reflection in the visible light region. This indicates that 0.05 and 0.07 samples have the highest light scattering light ability in this region. In other words, these samples possess high-light harvesting efficiency when incorporated with sensitizer resulting in high photocurrent in dye-sensitized solar cell [10]. Figure 3 depicts the optical absorption spectra of the samples with various concentrations of the precursor. Opposite to the reflection spectra illustrated in Fig. 3, the samples absorbed more light in the UV region ranging from 300 to 350 nm. The samples absorb less light in the visible light región, since they are not coated with dye which serves as the photosensitizer. It is also observed that the reflection varies with ZnS content. The 0.07 M sample possesses the highest absorption in the UV region, while the 0.01 and 0.10 M samples display the lowest reflection in the same region.
The FESEM micrographs in Fig. 4 show the morphology of pure TiO2 and TiO2-coated ZnS prepared with 0.03 M ZnS precursor. From the FESEM images, the morphology of pure TiO2 is nanograss. The morphology of pure TiO2 is porous, providing large surface area for dye adsorption and fast charge transfer in the device [11]. The formation of ZnS particles on TiO2 layer can be seen clearly from Fig. 4b upon deposition of ZnS layer on TiO2 film. The ZnS particles do not change the nanograss structure of TiO2 but appear to be agglomerated on the TiO2 nanograss.
Figure 5 shows the dark current curves for all device utilizing TiO2-coated ZnS samples. From the figure, it can be seen that there is no increasing or decreasing trend of leak current with the variation of ZnS content. The lowest leak current is observed in the device with 0.10 M precursor. On the other hand, the highest leak current is obtained in the device with pure TiO2 sample. From Fig. 5, it is clear that the devices do not exhibit rectification property, since the leak current curves represented by the reverse bias current curves are almost symmetry with forward current curves. The highest forward current belongs to the device with 0.10 M sample.
Figure 6 demonstrates the J–V curves of the DSSC utilizing pure TiO2 and TiO2-coated ZnS photoanodes under 100 mW cm−2 light illumination. The photovoltaic parameters are analyzed from the figure and illustrated in Table 1. From the table, it is found that the device utilizing 0.03 M sample has the highest ŋ, which is 1.323% compared to the device utilizing pure TiO2 which exhibits the efficiency of 1.176%. However, the ŋ of the device with pure TiO2 is higher than that of the devices utilizing the TiO2-coated ZnS samples with other concentrations. The device utilizing 0.03 M sample demonstrates the highest ŋ, since it posseses the lowest Rb and Rct. Furthermore, this device has the longest carrier lifetime, τ observed from the Bode plot indicating that the electron transfer in the device is faster than the electron–hole recombination [12]. The decrease in the electron–hole recombination causes higher device efficiency. The device employing pure TiO2 has higher ŋ than that of the devices utilizing other TiO2-coated ZnS samples due to the lower Rb than that of the devices with other TiO2-coated ZnS samples. In general, the highest ŋ obtained from this work that is 1.323% is comparable with that of the DSSC utilizing TiO2-coated CdS which demonstrated the ŋ of 1.44% [13]. The highest efficiency of the device from this work is lower than the moderate efficiency for the N719-sensitized device that is 5%. This might be due to the N719 dye dipping time chosen in this work, that is, 15 h is still insufficient time for the dye adsorption on the TiO2 film surface. Moreover, the dye concentration of 0.05 mM and room temperature as dipping temperature are not the optimum concentration and dipping temperature of the N719 dye.
Figure 7 shows the Nyquist plots of the DSSC utilizing TiO2-coated ZnS photoanode with various contents of ZnS. The bulk resistance (Rb) represented by bigger semicircle and charge transfer resistance (Rct) denoted by smaller semicircle are determined from the plots and presented in Table 1. From Table 1, the device utilizing 0.03 M sample has the smallest Rb. Three devices have the lowest Rct, namely, the devices utilizing 0.03, 0.05, and 0.07 M samples. Figure 8 shows the Bode plots of the DSSC utilizing TiO2-coated ZnS photoanode with various contents of ZnS. The carrier lifetime, τ, is computed from the resonant frequency located at the peak of the Bode curves. The τ for each device is presented in Table 1. From the table, it is found that the device utilizing the 0.03 and 0.01 M samples possesses the longest and shortest τ, respectively.
4 Conclusions
TiO2 has successfully been coated with ZnS via liquid-phase deposition assisted with immersion technique. TiO2-coated ZnS has been utilized as photoanode of the dye-sensitized solar cell. The effect of ZnS content on the structure, optical properties, and the performance of DSSC has been investigated. The morphology of the TiO2-coated ZnS film is nanograss but agglomerated with TiO2 nanograss particles. The device utilizing the 0.03 M ZnS precursor demonstrates the highest power efficiency of 1.323% due to its lowest resistance and longest carrier lifetime. Coating TiO2 with ZnS was found as an effective way of improving the performance of DSSC.
References
S.K.M. Maarof, M. Rusop, S. Abdullah, Adv. Mater. Res. 832, 763–766 (2014)
Q. Hu, C. Wu, L. Cao, B. Chi, J. Pu, L. Jian, J. Power Sources 226, 8–15 (2013)
M. Zhu, X. Li, W. Liu, Y. Cui, J. Power Sources 262, 349–355 (2014)
H. Dong, Z. Wu, Y. Gao, A. El-Shafei, S. Ning, J. Xi, B. Jiao, Org. Electron. 15, 2847–2854 (2014)
C. Xu, J. Wu, U.V. Desai, D. Gao, Nano Lett. 12, 2420–2424 (2012)
T.C. Li, M.S. Góes, F. Fabregat-Santiago, J. Bisquert, P.R. Bueno, C. Prasittichai, J.T. Hupp, T.J. Marks†, J. Phys. Chem. C 113 (2009) 18385–18390
H.-N. Kim, J.H. Moon, ACS Appl. Mater. Interfaces 4, 5821–5825 (2012)
A.A. Umar, M.Y.A. Rahman, S.K.M. Saad, M.M. Salleh, M. Oyama. Appl. Surface Sci. 270, 109–114 (2013)
C. Damm, P. Armstrong, C. Robkopf, S. Romeis, W. Peukert. Particuology. 18, 1–10 (2015)
V. Dhas, S. Muduli, S. Agarkar, A. Rana, B. Hannoyer, R. Banerjee, S. Ogale, Sol. Energy 85, 1213–1219 (2011)
S.K. Md. Saad, A.A. Umar, S. Nafisah, M.M. Salleh, B.Y. Majlis. RSM2013 Proc. 2013, (2013) 402–405
X. Guo, G. Lu, J. Chen, Front. Energy Res. 3, 1–15 (2015)
H.-J. Kim, J.-H. Kim, I.K. Durga, D. Punnoose, N. Kundakarla, A.E. Reddy, S.S. Rao, New J. Chem. 40, 9176–9186 (2016)
Acknowledgements
This work was supported by Universiti Kebangsaan Malaysia (UKM) under research Grant GUP-2016-013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahman, M.Y.A., Sadikin, S.N. & Umar, A.A. TiO2-coated ZnS films as photoanode for dye-sensitized solar cell: effect of zinc nitrate hexahydrate concentration on the performance. Appl. Phys. A 124, 460 (2018). https://doi.org/10.1007/s00339-018-1879-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-018-1879-8