Abstract
La or W-doped lead zirconate titanate thin films (PLZT or PZTW) were prepared on platinized silicon substrates by sol–gel process. The effects of La or W dopant on the phase development, microstructure, dielectric and ferroelectric characteristics of films were studied. For PLZT films, the optimum doping concentration was found to be 2 mol%. While for PZTW films, the dielectric and ferroelectric properties were found to be improved as the doping concentration increased. The fatigue properties of PLZT and PZTW thin films were also investigated, the results showed that A- or B-site donor doping could improve the fatigue properties of PZT thin films. The theory of oxygen vacancy was used to explain the performance improvement caused by donor doping.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Lead zirconate titanate (PZT) which has perovskite structure with the chemical formula of PbZr1−x Ti x O3 is the solid solution of lead titanate (PbTiO3) and lead zirconate (PbZrO3) [1]. In the phase diagram of PZT, Ti-rich tetragonal phase and Zr-rich rhombohedral phase coexist around Zr/Ti = 52/48, which is called as morphotropic phase boundary (MPB). Special attention has been given to the compositions near MPB [2]. This composition shows good piezoelectric, pyroelectric, dielectric and ferroelectric properties. Owing to these superior properties, PZTs are promising candidates for various applications such as ferroelectric random access memory (FeRAM) [3], micro-electromechanical systems [4,5,6,7] and dynamic random access memory (DRAM) [8,9,10].
Many preparation techniques have been employed to produce PZT thin films, such as MOCVD, RF magnetron, sol–gel, pulse laser deposition (PLD), ion beam sputtering and so on [11]. Nowadays, as the manufacture of high-quality pure PZT films has been reported by many researchers, improving the intrinsic deficiencies of PZT films has become a major trend in current research. On one hand, the dielectric constant and remnant polarization need to be improved as far as possible. On the other hand, the most imperative problem that needs to be solved in PZT is the problem of fatigue which refers to the phenomenon that the remnant polarization of the PZT film is significantly reduced after a number of read and write operations [12]. Several mechanisms have been proposed to explain the phenomenon of ferroelectric fatigue in PZT films, and the most widely accepted theory is the oxygen vacancy. Oxygen vacancy is the only mobile ionic species within the crystal lattice at room temperature which can lead to the space charge segregation at film/electrode interfaces or can be pinned at the domain walls during repeated switching [13,14,15,16,17,18]. In addition, Miura and Tanaka et al. [19] reported that another cause of fatigue is that Ti 3d states are occupied by the electrons released from oxygen vacancies, which lead to the weakening of the bonds between oxygen ions and titanium ions. A lot of methods have been put forward to solve the fatigue of PZT films, one of which is the alteration of electrodes. Using hybrid metal-oxide or oxides such as (La, Sr)CoO3 (LSCO), LaNiO3(LNO), IrO2, and RuO2 as electrode instead of Pt has been found to reduce the fatigue of PZT. This is because these oxides can reoxidize or reduce repeatedly and reversibly without degradation [20,21,22]. However, this method causes electrical leakages and makes the process more complicated and expensive [23]. Doping is another method that can effectively be used to improve the fatigue performance of PZT. Introducing aliovalent metal ions into PZT to modify its structure and properties has attracted a lot of researchers to study it [24, 25]. According to the valence state of dopant and host ions, doping can be divided into three types: isovalent, acceptor and donor doping. The donor doping of PZT has been found to increase the dielectric constant, enhance the remnant polarization, reduce the coercive field, as well as improve the fatigue properties of PZT [26,27,28,29,30]. Several mechanisms have been put forward to explain the performance improvement induced by donor doping. According to previous studies [31, 32], donor dopants like La (at A site) and Nb (at B site) in PZT can result in the lead vacancies for charge compensation, and reduce the concentration of oxygen vacancies simultaneously. Defect dipoles such as \({\text{Nb}}_{{{\text{Ti}}}}^{ \cdot } - {\text{V}}_{{{\text{Pb}}}}^{\prime \prime }\) may be produced by doping. Since both \({\text{Nb}}_{{{\text{Ti}}}}^{ \cdot }{\text{ and V}}_{{{\text{Pb}}}}^{\prime \prime }\) are immobile near room temperature, such defect dipoles should be immobile if they really exist. Zhang et al. [33] considered that random fields would be produced by such defect dipoles, which could locally influence the domain structures. They considered that the stability of the domain structure against electrical fields or external mechanical would be lowered in the donor-doped PZT, leading to the increased mobility of domain walls. This is in accord with the results observed by Li [34], in which high domain wall mobility was reported to be related to the chaos of defect dipoles in donor-doped PZT. However, Eichel et al. [35] proposed that the lead vacancies and the dopant ions were more likely to act as isolated defects rather than a defect dipole, which was at least suitable for the case of Gd-doped PbTiO3. Gerson [36] has reported that the key function of donor doping is merely to increase the concentration of lead vacancies. He hypothesized that the local stresses in PZT lattice could be minimized by lead vacancies. Consequently, the domain wall motion was facilitated.
Now in the present study, La3+ and W6+ were chosen as A-and B-site donor dopants to study the influence of donor doping on the dielectric and ferroelectric properties of PZT films. La-doped and W-doped PZT films near morphotrophic phase boundary were prepared by sol–gel method, and the doping effects of lanthanum and tungsten in PZT thin films on the ferroelectric and fatigue properties for ferroelectric memory applications were studied.
2 Experimental
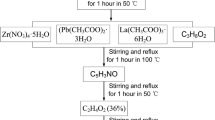
La- and W-doped PZT films near MPB were prepared by the sol–gel method. The La and W doping concentrations were calculated according to the equation of Pb1.1−x La x (Zr0.52Ti0.48)O3 (abbreviated as PLZT, where x = 0.01–0.04) and Pb1.1(Zr0.52Ti0.48)1−y W y O3 (abbreviated as PZTW, where y = 0.005–0.01), respectively. To prepare the PLZT precursor solution, lead acetate trihydrate [Pb(CH3COO)2·3H2O], lanthanum acetate [La(NO3)3·6H2O], titanium tetrabutoxide [Ti(OC4H9)4] and zirconium nitrate [Zr(NO3)4·5H2O] were used as the source materials for Pb2+, La3+, Ti4+ and Zr4+ ions. 2-Methoxyethanol was used as the solvent. In addition, acetic acid was used as the catalyst and acetyl acetone was used as the chelating agent. Solutions were prepared with 10 mol% Pb excess to compensate for the volatilization of Pb during the annealing process and suppress the formation of pyrochlore. All the chemical reagents used were of analytical pure. The reagents were fully dissolved and stirred sufficiently, then clear straw-yellow PLZT precursors with concentration of 0.4 mol/L were obtained. Different from the preparation of PLZT precursor solution, the chemical mixing order was important in fabricating PZTW precursor solution. Tungsten chloride was used as the source material for W6+. To avoid precipitation, tungsten chloride was added into 2-methoxyethanol and stirred for about 30 min. Meanwhile, lead acetate trihydrate with 10 mol% lead excess was added into acetic acid and fully stirred. Two kinds of solution were mixed, afterwards titanium tetrabutoxide and zirconium nitrate were added into the mixed solution, respectively. Finally, ethanolamine was added as the chelating agent. The concentration of the PZTW precursor solution was adjusted to 0.2 mol/L to also avoid precipitation.
The precursor solution was deposited onto Pt(111)/Ti/SiO2/Si(100) substrates by spin coating at 3500 rpm for 40 s to form the thin coating layer, the wet films were then dried on a hot plate of 450 °C for 300 s to remove the residual organic. To obtain the desired film thickness, the process was repeated four times. The following annealing and crystallization processes were performed at 650 °C for 1 h in a muffle furnace. It should be noted that the samples were put directly into the furnace reaching at a temperature of 300 °C. We found that the crystal quality can be improved using this modified annealing treatment.
The crystal structure and orientation of the thin films were characterized using an X-ray diffractometer (XRD, Rigaku D/MAX-2500) with CuK\({\upalpha }\) radiation source. The surface morphologies of thin films were observed by field emission scanning electron microscopy (FESEM, ZEISS MERLIN Compact). The gold top electrodes were sputtered on the films’ surface through a shadow mask. Dielectric properties were characterized using an Agilent precision impedance analyzer (Agilent E4981A). The ferroelectric and fatigue properties were measured using a ferroelectric material parameter tester with a UNI- TUTD2052CL oscilloscope.
3 Results and discussion
3.1 Phase and microstructure
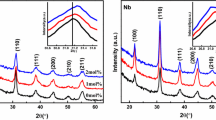
Figure 1 shows the XRD patterns of the PZT, PLZT and PZTW thin films deposited on Pt(111)/Ti/SiO2/Si(100) substrates. Figure 1a shows the XRD patterns of PLZT films and Fig. 1b shows the XRD patterns of PZTW films. All the films have the same structure of perovskite, with a slight difference in the relative strength of diffraction peaks, indicating the slight difference in the orientation of the films. The reason for the similarity of XRD images of the doped PZT films may be that the dopant ions are sufficiently combined to the lattice of PZT and have no significant effect on the crystal structure of PZT due to the low doping amount. The three main diffraction peaks can be indexed as (100), (111), and (200), respectively. The orientation change of the thin films may be caused by the lattice distortion, which could be due to the difference of ionic radius between doped ions and host ions.
SEM images of the PZT, PLZT and PZTW thin films are shown in Fig. 2. It can be seen that both surfaces of the PLZT and PZTW films are smooth and crack-free. The average surface grain size for La-doped PZT films increased as there was an increase in doping concentration. However, it seems to decrease when the La content is over 2 mol%. The average grain size of the PZT film is approximately 90 nm, while that of the 4 mol% PLZT film is slightly smaller, of about 75 nm. The changing trends of grain size of PLZT films can be explained as follows: when the doping concentration was below 2 mol%, the lanthanum ions were homogeneously dissolved in the lattice structure of PZT, but the further addition of La ions would in turn inhibit the grain growth because of the accumulation of lanthanum ions at the grain boundary. On the other hand, the average surface grain size for W-doped PZT films was measured to be between 90 and 110 nm, independent of the W6+ concentration within the doping range, which implies the uniform distribution of W6+ in the film. Figure 2i shows the cross-sectional image of PZT films, from which we know that the film thickness of PZT films of four coating layers is approximately 250 nm. When the number of spin coating layers is too many, the crystal quality of the film will deteriorate, which would affect its performance. The films of four coating layers are of good quality and are suitable for testing-related properties.
3.2 Dielectric properties
Figure 3 shows the dielectric constant (ε γ) and loss tangent (tanδ) values as a function of concentration of doping ions, which were measured at 1 kHz. In Fig. 3, 0 mol% doping concentration corresponds to the dielectric properties of pure PZT thin film. For La-doped PZT films, the dielectric constant increased from 800.87 to 822.76 and 896.23 as the doping concentration of La3+ increased from 0 to 2 mol%, meanwhile the loss tangent decreased from 0.087 to 0.080 and 0.070. However, the dielectric constant decreased and loss tangent increased, as the doping concentration of La3+ further increased from 2 to 4 mol%.
Figure 3b shows the dielectric constant (ε γ) and loss tangent (tanδ) as a function of doping concentration of W6+. As shown in the figure, the dielectric constant increased from 800.87 to 1010.46, as the W doping increased from 0 to 1 mol%, and the loss tangent decreased from 0.087 to 0.052.
3.3 Ferroelectric properties
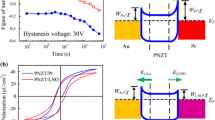
Ferroelectric properties of PLZT and PZTW thin films were showed in Fig. 4. The data of Fig. 4c, d were extracted from the P-E hysteresis loops of undoped and doped PZT films. For the undoped PZT film, the remnant polarizations P r = 20.05 μC/cm2 and the coercive fields E c = 51.36 kV/cm. In comparison, the P r reached the maximum value of 23.62 μC/cm2 for 2 mol% La doping. The coercive field E c fluctuated around 46.65 kV/cm. For W-doped PZT films, P r of 0.5–1 mol% PZTW films are 22.35, 23.62 and 24.50 μC/cm2 with E c of 43.46, 40.82 and 38.05 kV/cm, respectively. Therefore, there was an increase in remnant polarization and decrease in coercive field with W doping.
From the results above, we can draw a conclusion that appropriate doping can improve the dielectric and ferroelectric properties. The improvement in dielectric and ferroelectric properties can be explained from the perspective of domain wall movement. It can be pinned by the planes of oxygen vacancies, resulting in the reduction of domain wall mobility. The formation of oxygen vacancies are expected to be reduced by the La3+ and W6+ dopants, consequently, the domain wall mobility is increased. Therefore, better dielectric and ferroelectric properties were observed in the donor-doped PZT films. For PLZT films with doping concentration above 2 mol%, the decrease in dielectric and ferroelectric properties may be due to the fact that excessive doping reduces the grain size and crystal quality of the films.
3.4 Fatigue properties
Based on the studies above, the effect of donor doping on the fatigue properties of PZT films were further studied. We focused on the fatigue property of 2 mol% PLZT and 1 mol% PZTW thin films because of the better ferroelectric performance. Figure 5 shows the fatigue behavior of PZT, 2 mol% PLZT and 1 mol% PZTW thin films as a function of polarization switching cycles. For the PZT films, the remnant polarizations P r decreased after 105 cycles and reduced about 20% at 108 cycles. For the 2 mol% PLZT, a reduction of P r after 106 cycles was observed, and the P r reduced about 5% at 108 cycles as shown in Fig. 5. For the 1 mol% PZTW, P r decreased after 106 cycles and reduced about 6% at 108 cycles. From Fig. 5, it is clear that the appropriate donor-doped PZT films possess a lower fatigue rate than the undoped films.
In our opinion, the mechanism of the fatigue of PZT films can be explained by the formation and the redistribution of oxygen vacancies in the PZT films. It is easy for PbO to evaporate during heat treatment process because of the high partial pressure of Pb, which leads to the formation of oxygen and lead vacancies [37] as shown in Eq. (1). The oxygen vacancies gradually move to the interfaces between the electrode and the PZT film during electric switching. This results in the formation of a space charge layer which could be described by a Schottky barrier. Due to the separation of the space charges at electric interfaces, the electric field in the ferroelectric is reduced by the internal field. This may be the cause which activates the fatigue behavior in PZT thin films.
For PLZT films, the A-site substitution of La3+ for Pb2+ resulted in a reduction of oxygen vacancies, which could be explained by Eq. (2). Due to the lowered concentration of oxygen vacancies, it was expected to reduce the fatigue phenomenon:
In the PZTW films, the B-site substitution of W6+ for Ti4+ (or Zr4+) resulted in a reduction of oxygen vacancies which could be expressed by Eq. (3).
Equation (3) actually resulted in the consumption of oxygen vacancies. Consequently, the fatigue behavior can also be improved by doping W6+.
4 Conclusions
Crack-free films of PLZT and PZTW were successfully prepared by a modified sol–gel method. The effects of La/W doping on PZT [Zr/Ti:52/48] thin films were studied. The optimum doping concentration of La3+ for PZT films was determined to be 2 mol%. For 2 mol% La-doped PZT, grain size of PLZT was a little larger than that of the undoped specimen. The maximum dielectric constant of 896.23 was achieved for 2 mol% PLZT film. While as the doping concentration of La3+ further increased to 4%, the dielectric constant decreased to 558.6. Ferroelectric measurements have the similar variation trend, where the maximum remnant polarization was found to be 23.62 μC/cm2 for 2 mol% PLZT film. For W-doped PZT films, the dielectric constant and the remnant polarization increased with the doping concentration of W6+ in the scope of our experiments. The maximum dielectric constant of 1010.46 and remnant polarization of 24.50 μC/cm2 were achieved for 1 mol% W-doped PZT films. Both PLZT and PZTW films revealed improved fatigue behavior over undoped PZT specimen. The theory of oxygen vacancy was used to explain the performance improvement caused by donor doping, showing that PZT films doped with donors such as La and W can be good candidates for FeRAM applications.
References
B. Jaffe, R.S. Roth, S. Marzullo, J. Appl. Phys. 25(6), 809 (1954)
M. Prabu, I.B.S. Banu, S. Gobalakrishnan et al., J. Mater. Sci. Mater. Electron. (5), 1 (2016)
A. Chaipanich, G. Rujijanagul, T. Tunkasiri, Appl. Phys. A Mater. 94(2), 329 (2009)
P. Jegatheesan, N.V. Giridharan, J. Mater. Sci. Mater. Electron. 23(5), 1103 (2012)
R. Moazzami, C. Hu, W.H. Shepherd, IEEE. Trans. Electron. Dev. 39(9), 2044 (1992)
W.C. Goh, K. Yao, C.K. Ong, Appl. Phys. A Mater. 81(5), 1089 (2005)
I. Bretos, R. Jiménez, A. Wu et al., Adv. Mater. 26(9), 1405 (2014)
J.P.B. Silva, S.A.S. Rodrigues, K.C. Sekhar et al., J. Mater. Sci. Mater. Electron. 24(12), 5097 (2013)
T. Zhang, S.Y. Zhang, K. Wasa et al., Phys. Status Solidi 208(10), 2460 (2011)
Y.B. Jeon, R. Sood, J.H. Jeong et al., Sens. Actuators A Phys. 122(1), 16 (2005)
M.C. Rodríguez-Aranda, F. Calderón-Piñar, F.J. Espinoza-Beltrán et al., J. Mater. Sci. Mater. Electron. 25(11), 4806 (2014)
J.F. Scott, Ferroelectrics 236(1), 247 (2000)
H.M. Duiker, P.D. Beale, J.F. Scott et al., J. Appl. Phys. 68(11), 5783 (1990)
J.F. Scott, C.A. Araujo, B.M. Melnick et al., J. Appl. Phys. 70(1), 382 (1991)
A. Gruverman, O. Auciello, H. Tokumoto, Appl. Phys. Lett. 69(21), 3191 (1996)
E.L. Colla, S. Hong, D.V. Taylor et al., Appl. Phys. Lett. 72(21), 2763 (1998)
C.H. Park, D.J. Chadi, Phys. Rev. B 57(22), 13961 (1998)
S. Shannigrahi, K. Yao, J. Appl. Phys. 98(3), 797 (2005)
K. Miura, M. Tanaka, Jpn. J. Appl. Phys. 35(5A), 2719 (1996)
T. Tseng, R.P. Yang, K. Liu et al., Appl. Phys. Lett. 70(1), 46 (1997)
B. Yang, S. Aggarwal, A.M. Dhote et al., Appl. Phys. Lett. 71(3), 356 (1997)
K.S. Liu, T.F. Tseng, I. Lin, Appl. Phys. Lett. 72(10), 1182 (1998)
J.H. Jang, K.H. Yoon, Appl. Phys. Lett. 75(1), 130 (1999)
S. Dutta, A.A. Jeyaseelan, S. Sruthi, Thin Solid Films 562(562), 190 (2014)
H. Zhao, K. Zhang, L. Xu et al., J. Appl. Phys. 115(7), 2965 (2014)
Z.G. Liu, J. Yin, Z.C. Wu, Appl. Phys. A Mater. 69(1), 659 (1999)
W.Y. Choi, J.H. Ahn, W.J. Lee et al., Mater. Lett. 37(3), 119 (1998)
M.V. Raymond, J. Chen, D.M. Smyth, Integr. Ferroelectr. 5(1), 73 (1994)
T. Kijima, T. Aoyama, H. Miyazawa et al., Jpn. J. Appl. Phys. 44(1A), 267 (2005)
S.R. Shannigrahi, H.M. Jang, Appl. Phys. Lett. 79(7), 1051 (2001)
K.K. Shung, Proc. SPIE 3, 174 (2008)
G.H. Haertling, C.E. Land, J. Am. Ceram. Soc. 54(1), 1 (2010)
Q.M. Zhang, J. Zhao, K. Uchino, J. Zheng, J. Mater. Res. 12(1), 226 (1997)
J. Li, in Broadband Dielectric Response in Hard and Soft PZT: Understanding Softening and Hardening Mechanisms.” Epfl (2011)
R.A. Eichel, J. Electroceram. 19(1), 11 (2007)
R. Gerson, J. Appl. Phys 31(1), 188 (1960)
T. Sreesattabud, B.J. Gibbons, A. Watcharapasorn et al., Ceram. Int. 39, S521 (2013)
Acknowledgements
The authors gratefully acknowledged the support from the Key Laboratory of Advanced Ceramics and Machining Technology, Ministry of Education (Tianjin University).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xiao, M., Zhang, Z., Zhang, W. et al. Effect of La and W dopants on dielectric and ferroelectric properties of PZT thin films prepared by sol–gel process. Appl. Phys. A 124, 8 (2018). https://doi.org/10.1007/s00339-017-1428-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-017-1428-x