Abstract
In this research, magnesium-based metal matrix composite (MMC) is fabricated through a novel powder metallurgy route, in which synthesized r-GO (reduced graphene oxide) nanosheet at different weight percentages (wt%) is used as reinforcement. The microstructural characterization along with X-ray mapping revealed the appropriate dispersion of r-GO nanosheets into Mg matrix. Effect of r-GO addition on wear loss is studied under dry-sliding condition with constant influencing parameter and electrochemical corrosion behavior of developed MMC is studied at 3.5% NaCl solution. The addition of r-GO improves the hardness value up to 64 HV, likewise wear loss of MMC decreases up to 0.38 g with respect to r-GO addition. Formation of tribolayer over worn out surface is visualized by means of SEM micrographs. Even though the addition of r-GO improvises the wear behavior, the corrosion resistance of MMC reduces after a certain level of r-GO addition; Mg composites with 0.3 wt% of r-GO depicts low corrosion rate of 3.57 × 10−7 mpy and further addition of r-GO results in increment in their corrosion rate (4.07 × 10−5 mpy). Investigation over the SEM micrographs on corroded surface marked the occurrence of galvanic corrosion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Magnesium and its alloys are amongst the well-known materials for being light in weight and have enough potential to replace alumnum-based material used in structural applications [1]. Mg-based alloys possess many unique properties like that of low density, better damping resistance, etc., which eventually increases their range of applications. However, these materials have some limitations such as poor tribological behavior and low corrosion resistance. Among certain Mg-based alloys, AZ31 alloy has inferior corrosion resistance while comparing with pure magnesium (Mg) [2] and the same limits are not suitable for fabricating component used in tribological applications such as cylinder liner, piston rings, and also for marine environment [3]. This fact makes the researchers to focus on property enhancement on the Mg-based material. There are different methods to improve the corrosion and wear resistance of the materials such as surface treatment, protective coatings, and composite fabrication [4]. Amid this, composite fabrication is one of the mostly accepted and widely acknowledged method to improve the mechanical and tribological properties of material by the addition of hard ceramic particle. Uniform dispersion and size of the reinforcements are considered as the influential factors to attain metal matrix composite (MMC) with better properties. In the present days, nanoparticle-reinforced composites have attained keen interest in the field of material science for increments in material properties and another reason is that small addition of nanoparticle exhibits more desired properties when compared with micro-sized reinforcements [5]. The addition of ceramic material results in brittleness of the developed composite material, but, however, reduces its life. The above-said flaw can be solved to a great extent with the introduction of nanocarbon material as it improves the hardness besides possessing self-lubricating nature which helps greatly to enhance the properties of developed material. Hence, this research is focused on a new and emerging nanocarbon material, namely, graphene, a two-dimensional carbon layer material with fascinating properties such as mechanical strength, chemical inertness, thermal stability, and high surface area [6, 7]. In addition, it is renowned as an anti-corrosive material because of its hyperbolic nature and water repellent capacities. These engineering facts endorse graphene as a strong candidate for improving wear and corrosion resistance of the developed composite [8, 9].
MMCs are fabricated by methods like that of newer casting techniques, powder metallurgy route etc. As stated earlier, the properties of developed MMCs are mainly based on reinforcement size and uniform distribution of the reinforcement. However, carbon-based material exhibits poor wettability and density difference between matrix, and reinforcement material results in floating of the carbon particles during the casting routes and thus converts proper dispersion of reinforcement particles into the matrix a difficult task. This improper mixing of reinforcement leads to poor bonding between matrix and reinforcement, thereby leading to poor load transferability and also cluster formation of nanoparticles results in decrement of mechanical strength. Based on the above-said consideration, casting-based method is not suitable for developing carbon nanoparticle-reinforced MMC, and hence, to overcome such difficulties, the conventional powder metallurgy route is adopted for nanoparticle-reinforced composite fabrication in which uniform dispersion of reinforcement can be achieved.
Only limited literatures are available for graphene-based material usage as reinforcement and as coating material and, thereby, improve the tribological properties of the substrate material. Selvam et al. [10] fabricated graphene-coated AZ91D Mg alloy and studied the corrosion behavior of the coating in different aqueous electrolytes. The results justified that graphene coating acts as an active barrier to restrict the flow of electron from the base material into the electrolyte and at the same time reduces the chance of corrosion due to its chemical inertness. Bimal et al. [11] developed graphene-reinforced composite coating over the copper strip and studied the corrosion behavior of coating in 3.5% NaCl solution. It was concluded that graphene layer has the tendency to act as a protective layer against hard environment and improves corrosion resistance of the base metal. Liu et al. [12] developed graphene–cobalt composite coat over a copper-based material and tribological behavior of composite coating was investigated. Observations based on obtained results revealed that incorporation of graphene oxide upshots hardness, self-lubricating nature of graphene results in reduction of wear rate apart from improvement in corrosion rate due to its chemical inertness. Dhirajprasai et al. [13] developed graphene coating over copper and found improvement in corrosion resistance of copper material. Kumar et al. [14] deposited graphene coating over mild steel and found improvement in corrosion resistance. Algul et al. [15] developed nickel–graphene coating in copper and studied the wear behavior; results interpreted that the addition of graphene improved the tribological behavior of base material. Rashad et al. [16] developed magnesium-based MMC with varying weight percentage of graphene nanoplatelets(GNP) and observed that the addition of GNP results in the increment of mechanical properties. Effect of GNP and MWCNT as reinforcement (0.3%) was investigated and it was concluded that GNP-based MMC showcased better mechanical strength when compared with MWCNT-based composite [17]. J.LI et al. [18] developed graphene-reinforced MMC and the tribological behavior of developed MMC was studied. It was confirmed based on the study that the addition of graphene improves hardness and wear resistance of the base material.
A detailed survey over literatures confirmed that until date, no evidential research has been reported on the tribological and corrosion studies of reduced graphene oxide (r-GO)-reinforced Mg-MMC, and likewise, the addition of graphene nanoplates (Gnps) is limited to 0.3% for Mg-based MMC. Hence, this research focuses on the development of Mg-MMC reinforced with r-GO for varying wt% (0.2, 0.3, 0.4), respectively. Tribological behavior of the developed composites was studied with constant wear test conditions such as load, sliding distance, and velocity. Tafel polarization method is adopted to investigate the corrosion behavior of a developed set of composites with 3.5% NaCl solution as electrolyte which is similar to the sea water.
2 Experimental
In this study, solvent-based powder metallurgy route is adapted to attain uniform dispersion of the reinforcement particles. AZ31 alloy powder particles with average size of 400 µm are chosen as base material, since it possess inferior corrosion resistance. The chemical composition of the purchased sample is shown in Table 1. Reduced graphene oxide (r-GO) nanosheet of 5.8 nm average sheet thickness is used as reinforcement.
The morphology of chosen alloy powder is showcased in Fig. 1a based on which the particle size was determined and Fig. 1b depicts the micrograph of synthesized r-GO nanosheet via chemical reduction method called improved Hummer’s method [19]. Here, AZ31 alloy powder and synthesized r-GO nanosheet are dispersed with the help of hexane solvent solution individually and consequently ultrasonicated for 2 h duration. Then, the well-sonicated AZ31 slurry powder is stirred at 900 rpm for 1 h and dispersed r-GO is added in drops; after r-GO addition, the mixture is stirred briskly at 1600 rpm speed for 2 h. Now, the consequential mixture is well dried at room temperature using linear hot oven. Obtained composite powder was ball milled at a semi-dried circumstance using stainless steel balls usually maintained at 1:10 powder ratio. The ball milled powder is then dried at 80 °C in the existence of argon atmosphere. Dried out composite powder is cautiously filled into a hollow cylinder of 40 mm diameter for compaction and then pressed at 580 Mpa pressure for prevailing room temperature (28 °C) so as to develop the green compact sample specimens. Made-up specimens are buried in fine silica sand and sintered at 560 °C under argon atmosphere.
Micro-hardness of the developed composite is measured based on ASTM standard E384-88. In this study, Mitutoyo micro-hardness tester is utilized for micro-hardness testing in which a load of 50 g for 15 s dwell time is applied at four regions over the polished surface of the fabricated MMC. Surface morphology of the worn out surface and corroded surface of fabricated composite specimens was investigated using JEOL JSM-6610LV scanning electron microscope (SEM). To study the tribological behavior of the developed set of composites, dry sliding wear behavior was studied using a pin on disc apparatus, namely, DUCOM TR. Wear samples from the composite specimens were cut based on ASTM G99-05 standard through wire-cut EDM and the same was allowed to slide over hardened EN 31 steel-made rotating disc with a load of 10 N for a sliding distance of 1000 m at a velocity of 1 m/s. The weight loss of the sample was measured using Eq. (1) and compared with its prior weights so as to calculate the wear loss value. The experiments were repeated thrice and the average value is noted.
Wear loss of the developed MMC can be calculated from the Eq. (1):
where WL = wear loss, X = initial weight of pin before wear test, and Y = final weight of pin after wear test.
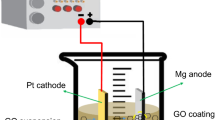
Corrosion resistance of the MMC is exploratory using CHI604C electrochemical workstation with the electrolyte, 3.5% NaCl solution equivalent to sea water. Developed MMC is prepared for corrosion characterization through wire-cut EDM and the specimens yield a surface area of 1 cm2, which was then mirror polished with SiC abrasive paper; the specimens were cleaned with acetone for every step to remove the impurities. A standard three electrode setup is adapted to investigate the electrochemical behavior of fabricated composite specimens. Saturated calomel electrode (AgCl) and platinum wire are used as counter electrode and reference electrode, respectively. Tafel polarization is used to determine the corrosion rate of MMC, the potential applied is between 0.5 and −1.5 V at a scan rate of 0.01 V/s, and all the experiments were repeated for four times so as to obtain accurate results. A pictorial view of the experimental setup for wear test and electrochemical studies is illustrated in Fig. 2a, b, respectively.
3 Results and discussion
3.1 Microstructural characterization
Figure 3a–d shows the SEM micrograph of developed MMC with varying wt% of reinforcements. Clear surface of AZ31 alloy shown in Fig. 3a states that the adopted solvent-based powder metallurgy method can be used to fabricate Mg-based metal products with less surface defects. Microgrooves are visualized in Fig. 3b and this may be due to the weak bonding between matrix material and r-GO sheets. Lower wt% addition of r-GO sheet results in poor dispersion which further affects the properties of developed MMC. Nil presence of porosity is envisioned in Fig. 3c and this is mainly because of proper dispersion of r-GO with better bonding between Mg matrix. Agglomeration of r-GO sheets could not be evidenced in Fig. 3d and could further be stated that flawless surface morphology is obtained. Unlike other nanocomposites, the presence of reinforcement cannot be observed for the reason being the nanometer size of r-GO sheets besides its lower percentage of reinforcement addition. However, it is vital to confirm the presence of reinforcement particles in the composite material as it provides hardness, wear resistance along with certain other vital bulk properties. Hence, the confirmation of particles and its distribution in the developed composites carried out by the way of this research is affirmed with the help of elementary mapping and the results are showcased in Fig. 4a–e.
Here, the orange region in mapping area evidents the distribution of oxygen element, whereas green, pink, and pale green are supposed to be the relevant colors of magnesium, aluminum, and carbon, respectively. EDS results from Fig. 4g illustrate the absence of carbide formation in developed MMC, since the presence of carbon-based material in the metals initiates carbide formation during the sintering process. From X-ray mapping, uniform dispersion of r-GO sheets over the Mg matrix is evident. Hence, it is obvious that the adopted powder metallurgy method has the viability to fabricate Mg-based nanocomposite that too with uniform dispersion of reinforcement particles.
3.2 Influence of r-GO over hardness of fabricated MMC
Micro-hardness results (Fig. 5) revealed that the addition of strong r-GO nanosheet into matrix improvises the micro-hardness of fabricated composite up to 64.6 HV. Incorporation of strong r-GO into the Mg matrix material can be attributed to the increase in hardness of the developed MMC.
The r-GO nanosheets found dispersed into the Mg matrix restrains localized deformation engendered during the indentation, thereby increasing the hardness value. Apart from this, it can be declared that the presence of r-GO particle found dispersed in the Mg matrix may reduce the grain growth increasing the strength and hardness value of the developed set of composites. The high surface area of synthesized r-GO nanosheets tends to improve the bonding structure between matrix and reinforcement particles while sintering process; this enhances the hardness of the developed set of composite specimens [23]. Whatsoever, increase in hardness attributes for a good range of mechanical bonding between Mg and r-GO nanosheets.
3.3 Influence of r-GO on wear behavior of developed MMC
As notified in Fig. 6, the wear loss decreases with respect to the addition of r-GO nanosheet. The reason behind this is the assimilation of reinforcement nanosheets, viz., r-GO particles are supposed to be a strong particle with self-lubricating nature due to the presence of graphite structure (since graphene is an exfoliated form of graphite). During wear condition, the r-GO particles form a self-lubricating layer on pin surface and this results in a decrement in the contact area between pin and counter disc; this phenomena initiates for wear loss reduction. From the X-ray mapping, uniform dispersion of r-GO nanosheets is observed. Dispersion of reinforcement is the main strengthening mechanism that takes place in the composite material which initiates for increment in hardness value of the developed MMC. Likewise, it is understandable that drastic decrement of wear loss with increase in hardness has happened and such occurrences support Archard’s law which states that hardness is proportional to wear loss. The type of wear mechanism occurred is justified from the specimen’s worn out surface, as shown in Fig. 7a–d.
From Fig. 7b–d, smoother surface is visualized for composite specimens when compared with the unreinforced alloy and this may be due to the addition of r-GO nanosheet. Wornout surface of AZ31 alloy shown in Fig. 7a notifies wear debris and microcracks which may be due to the occurence of deformation hardening mechanism; in addition, several damages and groves on the surface evidence the occurrence of abrasive wear. Figure 7b shows the worn out morphology of MMC with 0.2 wt% of r-GO sheet. Thin plastic deformation observed in the worn out surface is supposed to have happened as an effect of sliding speed. Increment in sliding speed increases the surface temperature which results in the occurrence of plastic deformation. Load is the key parameter in wear mechanism, since rise in load increases the pressure in pin which again increases the contact area between pin and disc that may lead to thermal softening and cause plastic deformation. Due to plastic deformation, the bonding between reinforcement and matrix material gets reduced and leads to ploughing out of reinforcement.
Figure 5c shows the worn out surface of the MMC containing 0.3 wt% of r-GO, and formation of tribolayer is clearly viewed which reduces the wear loss. During sliding, the r-GO particle present in the MMC forms a thin-lubricating layer which reduces the contact surface between the pin (specimen) and disc (steel); this results in a drastic reduction of wear loss. Figure 5d implies the surface of MMC with 0.4 wt% of r-GO, wherein smoother surface is observed due to the addition of strong r-GO nanosheets, which again coincides with the acquired data that wear loss decreases with respect to the addition of r-GO. These nanosheets penetrate deep into the surface of pin and thereby reduce the contact surface of the counter disc, thus improving the wear resistance of developed MMC [18, 24].
3.4 Electrochemical studies
Open circuit potential (OCP) measurement:
Open circuit potential (OCP) variations taken in the range of −1 to +1 V for 30 min obtained for the base matrix material and developed set of composites are portrayed in Fig. 8. It can be observed that OCP results for AZ31 alloy show a negative behavior for the first 5 min and then shift towards the positive trend indicating active behavior of oxidation into Mg2+ ion. It has to be noted that the standard value of Mg electrode is 2.37 V, but in this research, it shifts to −0.09 V which indicates the formation of Mg(OH)2 layer over cathodic nature. Decrease in potential with respect to time implies the anodic dissolution on the surface, and in addition, higher OCP is notified for MMC while compared with pure alloy; this denotes that the addition of r-GO nanosheets increases the resistance to Mg dissolution in aqueous media. The fluctuations in OCP values are observed due to the passive film formation and its breakdown due to exposure to corrosive environment. The high OCP denotes least tendency to corrosion. From OCP values, it could be identified that the addition of r-GO up to 0.3% increases the OCP. Further addition of r-GO shifts OCP towards more negative value. Potentiodynamic polarization is conducted for further corrosion behavior analysis of developed MMC.
Electrochemical corrosion studies are carried out for AZ31 alloy and it developed MMCs’ strips in 3.5% aqueous NaCl solution. Figure 9 shows the Tafel plot of fabricated composite in which corrosion parameters are attained. The extrapolated values of Tafel plots such as corrosion current density (icorr), anodic Tafel constant (β a), and cathodic Tafel constant (β c) are shown in Table 2.
In aqueous electrolyte, pure Mg undergoes the following reaction [20]:
Mg ion present in the MMC strip reacts with electrolyte and forms Mg2+ ions and the metal dissolves in solution and forms the corrosion product Mg (OH)2, which is the passive layer formed on the surface of composite. However, some of the Mg2+ ions react with chlorine ions which lead to MgCl2 formation in electrolyte and undergo the following reaction:
Corrosion rate (CR) was calculated using corrosion current density:
where K is corrosion rate constant (milli-inch per year), EW is equivalent weight of Mg, and d is material density [21].
From the Tafel plot, the anodic Tafel constant (β a) implies the ion dissolution and cathodic Tafel shows the hydrogen evolution. If the hydrogen formation is high, then the cathodic current potential is more negative when compared to corrosion potential; likewise, if metal oxidation is more, then the anodic current potential is more positive when compared with corrosion potential.
When AZ31 alloy strip is immersed in NaCl solution, the corrosion potential is −0.54 V, and corrosion current density value is 1.29 × 10−5µA/cm−2, as noticed from Fig. 9.
When MMC contains 0.2% of r-GO particles dipped in aqueous electrolyte, the corrosion potential moves towards the cathodic region; this implies the occurrence of hydrogen evolution. The current density decreases from 1.29 × 10−5 to 2.61 × 10−6 µA/cm−2, as because these r-GO nanosheets combine with the Mg(OH)2 over the surface of MMC and form a strong inhibiting layer which reduces the formation of metal ion. When 0.3% of r-GO particles reinforced Mg alloy strip is immersed in electrolyte, the corrosion potential shifts towards the anodic region and usually such happening reasons for the anodic dissolution and less corrosion rate. The current density decreases from 2.61 × 10−6 to 4.01 × 10−7µA/cm−2, and this is due to the unique structure offered by r-GO that in turn leads to good impermeability thus avoiding the metal ion formation. Further, in case of MMC containing 0.4% of r-GO showed again the shifting of corrosion potential towards the negative region, i.e., the cathodic region. Here in, the current density value increases from 4.01 × 10−7 to 4.49 × 10−5µA/cm−2, the reason behind this increament is breaking up of passive layer over the MMC surface which initiates the metal ion flow, and also increase in the amount of r-GO wt% that further results in increment of nanosheet thickness and thereby improve the electrical conductivity of MMC. It could be well said that corrosion current density is inversely proportional to the corrosion rate (CR). The variation of CR with respect to MMC samples is illustrated in Fig. 10.
From Fig. 10, it can be observed that a gradual decrement of CR values with respect to r-GO addition takes place. Above 0.3% of r-GO results in increment in corrosion rate which may be explained as an effect of cathode nature. Pure AZ31 alloy has higher corrosion rate of 1.18 × 10−5 mpy and MMC strip that contains 0.3% of r-GO delivering a low corrosion rate of 3.58 × 10−7mpy.
Inhibiting efficiency of the coating can be calculated based on the corrosion current density [11], as shown in Eq. (8):
Inhibiting efficiency for 0.3% r-GO-reinforced MMC approximates to 96.9%, and thus exposes high-inhibiting efficiency while compared with other compositions like 0.2%, which has only 79% of inhibition efficiency. However, 0.4% of r-GO exhibited a poor inhibition towards corrosion.
From Fig. 11a, large measure of cracks and pores is seen over the corroded surface (AZ31) and this evidents for the occurrence of pitting due to the corrosive electrolyte. During corrosion test, after few minutes of immersion, formation of a passive layer over metal surface takes place and this in turn acts as the barrier for corrosion product that reduces the flow of electrons. As the immersion time increases, the passive layer formed over the surface starts to break up and another fact is that the Mg alloy gets eroded fast in the aqueous solution due to galvanic corrosion effect. From Fig. 11b, a trace of voids is notified which acts as the active site for corrosion instigation. The homogenous dispersion of r-GO sheets reduces chances of the Cl− ion penetration. From Fig. 11c, the observed white layer states for the formation of Mg(OH)2 layer over the surface. SEM results also exhibited pits developed due to the replacement of absorbed oxygen on the surface with Cl− ions from the solution. Cl− ions with small atomic radii penetrate through the developed oxide layer and replace the oxygen whereever metal–oxygen bond is minimal [22]. Again, microcracks are notified in Fig. 11d and this is due to increase in the wt% of r-GO sheet, resulting in cluster formation within them, and forms a graphite structure. This increases the electron flow because of which the corrosion current density increases, thereby resulting in corrosion rate increments, and hence, r-GO with 0.3 wt% is optimal for better corrosion resistance. Further to investigate the corrosion mechanism and corrosion products, EDS mapping studies have also been performed. X-ray mapping of AZ31/0.3 wt% of r-GO is shown in Fig. 12a–h.
Formation of microcracks is clearly visible in the mapping surface of developed MMC (Fig. 12a) and this may be due to the action of corrosive electrolyte over Mg composite. Formation of oxide layer is shown in Fig. 12b, in which oxygen has the major contribution, and in addition, it is present only in the corroded surface. The presence of oxygen element is exposed as orange color in the scanned region (Fig. 12c), whereas dark green, pink, pale green, and violet are the respective colors of magnesium, aluminum, chlorine, and carbon. In this, magnesium and aluminum existing at the corroded surface are blurred in X-ray due to the hindrance of oxide layer formation. EDS spectra from Fig. 12 indicate the presence of respective elements in Mg, C, Al, Na, Cl, and O over the corroded surface. From overall research, it can be stated that Mg composite with 0.3 wt% of r-GO has the potential to have better corrosion resistance, and hence, it may be used in fabricating components for marine application which further supports weight reduction.
4 Conclusion
The following potential observations were made out of the investigations carried over by the current research:
-
Uniform dispersion of r-GO nanosheets into Mg matrix is achieved by adopting a novel powder metallurgy route.
-
Hardness value of developed MMC increases from 45 HV to 64 HV when compared with that of matrix metal.
-
Wear loss shows decremental trends (0.71–0.38 g) with respect to r-GO addition.
-
Occurrence of both abrasive and adhesive wear is notified from the wornout surface analysis.
-
MMC with 0.3 wt% of r-GO poses good inhibition efficiency (96%) and lower corrosion rate of 3.57 × 10−7 mpy when compared with base metal.
-
Investigation over the SEM micrographs of corroded specimen marked the formation of passive layer over the corroded surface and the presence of oxygen element is more nearer to the cracked surface which initiates corrosion.
References
K.S. Prakash, P. Balasundar, S. Nagaraja, P.M. Gopal, V. Kavimani, J. Magnes. Alloy. 4, 197 (2016). doi:10.1016/j.jma.2016.08.001
I.B. Singh, M. Singh, S. Das, J. Magnes. Alloy. 3, 1 (2015). doi:10.1016/j.jma.2015.02.004
J. Rams, C. Taltavull, P. Rodrigo, B. Torres, A. J. Lo, Mat. Des., 56, 549 (2014)
M.H. Fini, A. Amadeh, Trans. Nonferrous Met. Soc. China 23, 2914 (2013). doi:10.1016/S1003-6326(13)62814-9
B. Selvam, P. Marimuthu, R. Narayanasamy, V. Anandakrishnan, K.S. Tun, M. Gupta, M. Kamaraj, J. Mater. 58, 475 (2014). doi:10.1016/j.matdes.2014.02.006
M.A. Almomani, W.R. Tyfour, M.H. Nemrat, J. Alloys Compd. 679, 104 (2016). doi:10.1016/j.jallcom.2016.04.006
J. Ou, J. Wang, S. Liu, B. Mu, J. Ren, H. Wang, S. Yang, Lang. 26, 15830 (2010). doi:10.1021/la102862d
D. Berman, A. Erdemir, A.V. Sumant, Car. 59, 167 (2013). doi:10.1016/j.carbon.2013.03.006
Y. Yaghoubinezhad, A. Afshar, J. Sol. St. Elec. Chem, 19, 1367–1380 (2015). doi:10.1007/s10008-015-2754-6
M. Selvam, K. Saminathan, P. Siva, P. Saha, V. Rajendran, Mater. Chem. Phys. 1, 1–8 (2016). doi:10.1016/j.matchemphys.2016.01.051
B.P. Singh, S. Nayak, K.K. Nanda, B.K. Jena, S. Bhattacharjee, L. Besra, Car. 61, 47 (2013). doi:10.1016/j.carbon.2013.04.063
C. Liu, F. Su, J. Liang, P. Appl, Surf. Sci. 351, 889 (2015). doi:10.1016/j.apsusc.2015.06.018
D. Prasai, J.C. Tuberquia, R.R. Harl, G.K. Jennings, K.I. Bolotin, ACS Nano 6, 1102 (2012). doi:10.1021/nn203507y
C.M.P. Kumar, T.V. Venkatesha, R. Shabadi, Mater. Res. Bull. 48, 1477 (2013). doi:10.1016/j.materresbull.2012.12.064
H. Algul, M. Tokur, S. Ozcan, M. Uysal, T. Cetinkaya, H. Akbulut, A. Alp, App. Sur. Sci. 359, 340 (2015)
M. Rashad, F. Pan, H. Hu, M. Asif, S. Hussain, J. She, Mater. Sci. Eng. A 630, 36 (2015). doi:10.1016/j.msea.2015.02.002
M. Rashad, F. Pan, A. Tang, M. Asif, M. Aamir, J. Alloys Compd. 603, 111 (2014). doi:10.1016/j.jallcom.2014.03.038
J. Li, L. Zhang, J. Xiao, K. Zhou, Trans. Nonferrous Met. Soc. China 25, 3354 (2015). doi:10.1016/S1003-6326(15)63970-X
J. Chen, B. Yao, C. Li, G. Shi, Carbon 64, 225 (2013). doi:10.1016/j.carbon.2013.07.055
B.Y. Yoon, H.S. Kwon, J. Nanosci. Nanotechnol. 15, 8837 (2015). doi:10.1166/jnn.2015.11527
M.C. Turhan, Q. Li, H. Jha, R.F. Singer, S. Virtanen, Electrochim. Acta 56, 7141 (2011). doi:10.1016/j.electacta.2011.05.082
F.H. Latief, E.S.M. Sherif, A.A. Almajid, H. Junaedi, J. Anal. Appl. Pyrolysis. 92, 485 (2011). doi:10.1016/j.jaap.2011.09.003
V. Kavimani, K. Soorya Prakash, T. Thankachan, Surf. Int. 6, 143 (2017). doi:10.1016/j.surfin.2017.01.004
H.J. Song, N. Li, Appl. Phys. A, 105, 827 (2011). doi:10.1007/s00339-011-6636-1
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kavimani, V., Soorya Prakash, K. & Arun Pandian, M. Influence of r-GO addition on enhancement of corrosion and wear behavior of AZ31 MMC. Appl. Phys. A 123, 514 (2017). https://doi.org/10.1007/s00339-017-1118-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-017-1118-8