Abstract
In this work, we have employed a hydrothermal route for the synthesis of fullerene-type tungsten disulfide (WS2) nanoparticles. X-ray diffraction analysis signifies a hexagonal crystal structure of WS2 with the crystallites experiencing preferred orientations along (002) and (103) planes. The agglomerated nanoparticles and inorganic fullerene (IF)-type structures are apparently observable from the high-resolution electron micrographs. Raman spectrum shows prominent \( E^{1}_{{2{\text{g}}}} \) and A 1g modes emanating from the IF nano-WS2 system. The Tauc’s plot obtained from the optical absorption data predicts a direct band gap of ~1.91 eV for the nano-WS2 system; whereas, photoluminescence analysis reveals a broad emission peak located at ~638 nm and is ascribed to the associated transition from the indirect to direct nature of the band gap. The photocatalytic decomposition of malachite green (MG) solution (30 mg/l) by WS2 (100 mg/l) under UV and visible light irradiation has been evaluated. The latter condition exhibited a better photocatalytic response with the MG degradation as high as 71.2%, revealed for 120 min. Photocatalytic and optoelectronic features of IF-type nano-WS2 would bring new insights not only to resolve issues related to environmental hazards, but also in functional devices of technological relevance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The pioneering works on atomically thin graphene layers have paved the way for the investigation of 2D layered materials in recent years [1]. The recent research works in the field of graphene has shown some extraordinarily interesting properties including its applicability in the field of bioelectronics for artificial dynamic synapse [2]. Undoubtedly, graphene offers a unique platform in the sense that, with the use of electrical gating Fermi level can be easily tuned along with the band gap opening. The gate-controlled graphene electrode-based random access memory with an adaptable single-electron transistor window has been worked out [3]. Due to the lack of inherent band gap, the electronic components made from graphene cannot be switched on and off in an efficient manner, which gives a low turn on and off time ratios [4]. To overcome this technical limitation, there is an urgent need to exploit other 2D layered materials, known as transition metal dichalcogenide (TMDC) systems. TMDCs have generalized formula MX2, where M is a transition metal element (group IV or V), and X is a chalcogen [5]. In several applications, these materials are advantageous over graphene as they possess sizable band gap, better exfoliation and handling capability, low phonon energy, etc. These materials, in conjunction with other 2D materials, are capable of making high-performance optoelectronic components over a broad spectral range [3, 6]. On the other hand, monochalcogenides of group IV elements (like Ge and Si), which possess black phosphorus-type structure along with other low symmetry TMDC, such as rhenium disulphide (ReS2) and rhenium diselenide (ReSe2) exhibit unique physical properties for numerous applications in the field of nanoelectronics, nanophotonics, piezoelectric devices and thermo-electronics [7].
In the past decade, molybdenum disulfide (MoS2), which is an important member of the family of TMDCs, has received widespread interest among the researchers. MoS2 has been extensively studied compared to tungsten disulphide (WS2) counterpart [8,9,10]. However, WS2 has not been evaluated in great detail due to insufficient information as regards its physical properties and processibility conditions. WS2 has drawn research interest owing to exhibition of its matchless value in the field of hydrogen storage, micro-electrode material, solid lubrication, catalysis, etc. [11,12,13]. The crystal structure of WS2 consists of an S–W–S single-layer bonded Van der Waals force. WS2 can adopt a single graphite-like structure, or quantum dot nanoparticles, thus triggering the urge among the physicists to study this system [8]. WS2 is a semiconductor with a band gap of 1.35 eV, thus expanding the light absorption region to infrared (IR) region, i.e. 910 nm [8]. The band gap of WS2 can also be controlled from indirect to direct type by exfoliating the bulk WS2 to nanosheets [14]. Nanoscale WS2 has also demonstrated its potential use in advanced applications, such as photothermal therapy for destroying cancer cells by converting the absorbed near IR light into heat [8]. As a common, yet important application of WS2, its use as a nano-photocatalyst is tremendous. In this regard, a few reports have highlighted photocatalytic studies of pure and WO3-coupled WS2 systems [15, 16]. Under UV illumination for 10 min, the mesoporous WS2 nanosheets were shown to degrade rhodamine B (RhB) up to 21% [15]. In contrast, much enhanced photocatalytic degradation of phenol has been witnessed upon extended UV illumination of polycrystalline mixed WO3/WS2 systems [16].
The present work highlights hydrothermal synthesis of inorganic fullerene (IF)-type WS2 nanoparticles and their photocatalytic responses considering both visible and UV exposure and with malachite green (MG) as the target agent. Structural, morphological, optical and Raman analyses have also been discussed.
2 Experimental: materials and methods
2.1 Synthesis of inorganic fullerene-type nano-WS2 system
We have attempted a two-step hydrothermal process for the production of nano-WS2. The chemicals used in the synthesis were of analytical grade and have been used without further purification. While sodium tungstate (Na2WO4·2H2O) (Rankem, 98% pure) has been considered as the source of tungsten, thiourea (CH4N2S) (Merck, 99% pure) was used as the source of sulfur. First, 1.64 g of sodium tungstate, 0.69 g of hydroxylamine hydrochloride (NH2OH·HCl) (Merck, 98% pure) and 1.52 g of thiourea are dissolved in 30 ml of deionized Millipore® water. The mixture was kept under constant stirring for 25 min, with the pH value adjusted to 6 by adding few drops of ammonia water. The solution is transferred to a 50-ml teflon-lined stainless steel autoclave, which was properly sealed and then subjected to oven heating at a temperature of 180 °C, for 24 h. The collected product is washed several times with distilled water and then with ethanol under centrifugation (3500 rpm) for about 15 min. The precipitate obtained is oven dried at a temperature of 60 °C, for 10 h. The resultant product that appeared to be blonde yellow in color is labeled as S 1. The light colored powder obtained resembles that of an intermediate product (WO3−x ) in the formation of WS2 which may arise due to the inadequacy of sulphur in the reaction process [17].
In the second step, 0.58 g of S 1 (obtained through the first step) is mixed with 0.78 g of thiourea and 40 ml deionized water in a conical flask and subjected to vigorous stirring for 20 min. Then, the solution is poured in a 50-ml Teflon-lined stainless steel autoclave, sealed airtight and kept in an oven maintained at a temperature of 200 °C, for 36 h. The product is centrifuged (3500 rpm, 5 min), washed with distilled water and finally, cleansed with ethanol suitably. The product is then dried in an oven (80 °C) for about 10 h. The steps as regards formation of the final nano-WS2 product (S 2) is illustrated in Fig. 1. The chemical equations involved are as given below [17]:
2.2 Characterization techniques employed
To exploit the structural and crystallographic properties, X-ray diffraction (XRD) technique was employed using a MiniFlex Rigaku X-ray diffractometer equipped with a CuK α source (λ = 1.543 Å). The morphological details of the as-synthesized WS2 samples were revealed through scanning electron microscopy (JEOL, 6390LV) and transmission electron microscopy (FEI, Tecnai) imaging studies. The elemental composition of the samples was confirmed from the EDX spectra. On the other hand, luminescence characteristics and phonon-assisted vibrational modes are exploited through the luminescence spectrophotometer (Hitachi 2700 FL) and Raman spectrometer (Renishaw, Wottonunder-Edge); respectively. The optical absorption responses as obtained through UV–Vis spectroscopy (UV 2450, Shimadzu Corporation) are used for estimating energy band gap as well as photocatalytic degradation efficiency.
2.3 Execution of photocatalytic experiment
To analyze the photocatalytic efficiency of the as-prepared nano-WS2 samples, we have chosen malachite green (MG) as the target to be decomposed. MG is an organic compound that is used as a dyestuff and controversially as an antimicrobial in aquaculture [18]. All around the globe, researchers are trying hard to find suitable ways to degrade harmful organic dyes and pollutants. The photoactivity of the nano-WS2 samples has been investigated both under UV and day light illumination conditions. To perform the experiment, 100 mg/L of the WS2 nanoparticles is first added to 30 mg/L of the MG, and then subjected to stirring for about 1 h in dark. In the next step, the mixture is ultrasonicated for about half an hour. The collected sol is then placed inside a cabinet which has the provision for UV lamp as well as the incandescent lamp, the latter being used as the source of visible light. The samples are placed on the base of the chamber at a distance of 12 cm from the UV source (λ = 365 nm) and the polychromatic visible light source used independently. The WS2 nanocatalyst-loaded dye was irradiated for 15, 30, 45, 60 and 120 min. Next, 5 ml of the MG solution is taken for optical absorption analysis, knowing that the peak maxima for the initial absorption of MG appears at ~617 nm [19]. The percentage of decomposition of the MG under UV and visible light irradiation for various durations of UV exposure is given by:
where, C 0 and C t represent concentration of the MG solution before and after irradiation.
3 Results and discussion
3.1 Structural and morphological analyses
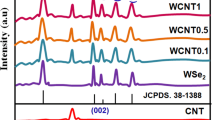
The XRD patterns of the as-prepared samples are shown in Fig. 2a, b. It can be seen that the sample S 1 exhibits diffraction peaks which is similar to that of WO3.5H2O (JCPDS File No. 44-0363) and WO3.33H2O (JCPDS File No. 35-1001) [17]. Attributed to (002) plane of hexagonal WS2, the diffraction peak at 2θ ~15.20° in sample S 1 is marginally shifted to 14.32° in system S 2 (JCPDS File No. 08-0237) [17]. The other peaks at 28.76°, 33.42°, 39.57°, 44.28°, 49.65° and 58.53° corresponded to (004), (101), (103), (006), (105) and (110) planes of the hexagonal WS2, respectively. The sharp peaks corresponding to the (002) and (103) planes suggest competitive growth of the crystallites along these directions. The sharp diffraction lines are also shown in the figure in accordance with respective JCPDS files. From the analyses of diffractograms, it is quite apparent that the synthesized product obtained through the additional step ensured the desired phase of the nano-WS2 system. The average crystallite size (D) of the WS2 nanoparticles was calculated using the Williamson–Hall (W-H) plot by choosing the most prominent diffraction peaks, such as (004), (101), (103), (006), (105) and (110). The W-H expression is given by:
where λ is the wavelength of the X-ray radiation (λ = 0.1543 nm for CuK α line), 2θ is the diffraction angle, β is the line width at half maximum height and ε is the mean lattice strain. As found in Fig. 2b, by plotting β cosθ vs. 4 sinθ, the size component can be obtained directly from the intercept of the straight line on the y-axis [20]. Similarly, microstrain ε can be evaluated from the desired slope. Using the W-H formula, the average crystallite size was calculated to be ~7.7 nm and the microstrain as, −1.3 × 103. The negative strain corresponds to relaxed nature of the crystallites, whereas positive strain indicates compaction of the crystallites [21]. Using conditions relevant to hexagonal structure, the crystal lattice parameters can be found as, a = b = 3.16 Å and c = 12.28 Å.
The EDX spectra of the two samples S 1 and S 2 are shown in Fig. 3. The presence of elements W, O and S is evident from the EDX spectrum of S 1. However, the amount of sulphur present in S 1 is quite small. The amount of sulphur present is significantly high in sample S 2, as compared to S 1. Since no oxygen peak is witnessed in the EDX spectrum of sample S 2, it substantiates the development of high-yield WS2 phase, free from oxide content. Moreover, the sample S 2 gave the atomic ratio of W to S as, 1:1.83. Since the formation of phase-pure WS2 is ascertained, further characterization wrt imaging was considered for the sample S 2.
Figure 4A shows the SEM micrographs of the desired WS2 nanosystem. Here, we could clearly notice formation of clustered nanoparticles similar to an earlier work [22]. In fact, the structural morphology of the WS2 nanostructures largely depends on the reaction time and processing temperature [23]. The agglomeration of particles is caused due to the multi-nuclei attachment, which serves as the possible mechanism of growth along (002) direction [24]. The low and high magnification micrographs are shown in Fig. 4A(a), A(b), respectively. Figure 4B(a–d) depicts the TEM imaging feature of the nano-WS2 system. Apparently, the TEM image reveals development of fullerene-shaped structures of nano-WS2 system [Fig. 4B(a), (c)]. The size distribution is shown in the inset of Fig. 4B(a). The formation of diffused rings as witnessed in the SAED micrograph [Fig. 4B(b)] suggests the polycrystalline nature of the synthesized product which could arise due to the presence of surface defects on nano-WS2 system [25]. The hollow core IF fullerene-type structures with bent lattice edges can be found in Fig. 4B(c). In the magnified image of the corner of an individual IF structure we noticed that a corner is essentially formed from a series of bent lattice planes [Fig. 4B(d)]. In this regard, strain relief mechanism has been proposed for exhibition of such unusual morphology/structures [26]. The average outer diameter of the IF nano-WS2 particles is estimated to be ~16 nm, with an interlayer spacing of 0.63 nm.
A SEM micrographs of the as-prepared nano-WS2 system: a low magnification, b high magnification. B a TEM micrograph depicting distributed view of IF-type WS2 nanoparticles with a histogram on size distribution (inset), b SAED pattern, c a single IF nano-WS2 structure and d enlarged view of a segment showing the bent lattice structure of the WS2 nanosystem
3.2 Vibrational analysis using Raman and FTIR spectroscopy techniques
Figure 5a represents the Raman spectrum of the synthesized nano-WS2 system measured with an excitation wavelength, λ ex ~514 nm. Raman spectroscopy is a non-invasive, yet important tool to reveal structural information as regards number of layers, defects, doping levels, strain effects and other factors. In fact, bulk WS2 shows two major bands corresponding to \( E^{1}_{2g} \) and A 1g modes essentially located at ~351 and 420 cm−1, respectively [27]. In our case, the two vibronic bands are witnessed at ~352.4 and 419.5 cm−1 which correspond to the first-order modes at the Brillouin zone; with first being an in-plane mode \( E^{1}_{2g} \) and second identified as out-of-plane mode A 1g [28]. These values are slightly different when compared to those of the bulk WS2 crystal. The slight shift of A 1g mode towards higher wavelength side may be due to the decrease in interlayer Van der Waals interaction which causes weaker restoring forces in the vibration as we move from bulk to few layers of WS2 [14]. On the other hand, the blue shift in \( E^{1}_{2g} \) is attributed to the reduced long-range Coulomb interaction between the effective charges caused by an increase in the dielectric screening on stacking-induced changes in the interlayer bonding [14]. The ratio of intensities of the \( E^{1}_{2g} \) and A 1g calculated stands at 0.61 which is higher than that of bulk WS2 but lower than the mono, bi and tri-layered WS2 nanosystems [29]. Thus our IF-type nano-WS2 must comprise sufficient number of layers which results in a dominant out-of-plane vibration over the in-plane one.
The FTIR spectroscopy essentially offers information regarding the nature of chemical bonding and the molecular structure of the system under study. Figure 5b depicts the FTIR spectrum of the synthesized nano-WS2 system in the wavenumber range 400–3200 cm−1. A distinct band at 454 cm−1 refers to the characteristic S–S bond of the elemental sulphur [30]. The bands positioned at ~580.2 and 978.6 cm−1 are ascribed to the W−S bond and S–S bond, respectively [31]. The bands found at ~1403.3 and 1625.5 cm−1 are ascribed to the stretching vibrations of the hydroxyl group [31]. Moreover, the vibrational bands at 2895.7 and 2924 cm−1 can be attributed to the atmospheric OH and that is available in the WS2 system [31].
3.3 Optical band gap calculation and luminescence response
The UV–Vis optical absorption spectrum of the sample S 2 is shown in Fig. 6a. It depicts presence of two weak absorption bands located at ~537 and ~649 nm. Even though these two bands represent characteristic absorption response of IF-WS2 nanostructures [32, 33], particularly the peak at ~649 nm corresponds to d–d type transitions of the WS2 at the center of the Brillouin zone [34]. To determine the band gap of the prepared WS2 nanoparticles, we have plotted the Tauc’s plot using the relation [31], αhν = (hν − E g)n, where h is the Planck’s constant, ν is the frequency of the incident light and α is the absorption coefficient. Putting n = ½ for the direct allowed transitions, the band gap of the prepared WS2 nanosystem is estimated to be 1.91 eV, which is in good agreement with an earlier report [8].
PL is a versatile tool to exploit the radiative emission and defect-mediated transitions. PL spectroscopy study is necessary to understand the carrier recombination processes to be understood for photocatalysis in latter studies. Figure 6b shows the PL spectrum of the synthesized WS2 nanosystem acquired under an excitation wavelength of λ exc = 460 nm. The broad peak at ~638 nm is the outcome of transition from the indirect band gap type to the direct band gap one when the bulk system is thinned down to a few nanometric layers [14]. This peak suggests the separation efficiency of the electron–hole pairs, with superior characteristics due to the presence of surface area and active edges [19]. Reports suggest that a direct gap exists at the K points of the Brillouin zone between the spin-orbit split valence band and the doubly degenerate conduction band. The indirect band gap is known to form between a local conduction band which is minimum at the midpoint between Γ and K and the valence band is maximum at the Γ point [14]. The existence of the direct transition can be explained from excitonic radiative relaxation and accordingly, the appearance of PL peak has been observed at energies slightly lower than the 2.05 eV direct band gap of WS2 [14]. The luminescence pattern is observed to be asymmetrically broadened and with a full-width half-maxima (FWHM) of approximately 183 meV. A broad emission peak suggests that, the transition from indirect to direct nature of the gap is not discrete, but continuous over an inseparable energy band.
3.4 Evaluation of photocatalytic performance of fullerene-type nano-WS2 system
To investigate photoactivity of the IF WS2 nanoparticles, we have chosen MG as the target agent under UV and visible light illumination conditions. The UV–Vis absorption response of the organic dye and nano-WS2 catalyst-loaded MG can be found in Fig. 7a. The WS2-loaded dye showed a steady fall in the absorbance with increasing the UV exposure time, exhibiting a minimal strength at ~617 nm. Conversely, the samples irradiated under visible light showed a rapid drop in the absorbance response as compared to the samples subjected to UV light (Fig. 7a, b). The IF WS2 nanoparticles tend to exhibit better catalytic efficiency under visible light because of the narrow band gap, which acts as a driving force to activate the redox reaction necessary for the production of hydroxyl radicals [8]. Also, it has been reported that irregular hexagonal-type structures with ample surface defects served the purpose of a good nanocatalyst under visible light illumination [35]. Also, IF-type particles can have enhanced photocatalytic responses because of the strong optical absorption, large surface area, and documented chemical inertness under illumination [36].
The schematic diagram as regards the mechanism of the photocatalytic activity of the synthesized WS2 nanosystem is shown in Fig. 8. The host WS2 nanocatalyst, upon light irradiation would release electrons and holes. The excited electrons are likely to move to the conduction band creating adequate holes in the valence band. These generated charge carriers are likely to be trapped in the active W4+ and S2− defect sites lest they might recombine with the counterparts to dissipate energy. These carriers would migrate to the nanocatalyst surfaces, resulting in the formation of reactive intermediates. The photoinduced holes and the reactive hydroxyl radicals are profoundly responsible for initiating the redox reaction required for decomposition of the harmful dye/organic pollutant. The hydroxyl radical is produced either by decomposition of water or by a hole reacting with the OH−. Since the target MG is in aqueous form; therefore, the water molecule is believed to come in contact with the surface of the nanocatalyst. The OH group or O2− is likely to be trapped by the active sites of the nanocatalyst [37]. The oxygen vacancy centers are easily de-trapped and the electrons are transferred to the adsorbed oxygen which produces the superoxide radicals [38]. Water and hydroxyl ions are transformed to the hydroxyl radicals by the photo-generated holes [39]. These superoxide and hydroxyl radicals being very strong oxidizing agents could easily degrade the complete dye to its constituent products [40]. We observed an increasing trend of degradation of the MG on increasing the irradiation time. As high as 45% decomposition has been witnessed on irradiating the catalyst-loaded dye under UV light for 120 min (Fig. 7c). Conversely, the IF-WS2 nanocatalyst gives a superbly enhanced decomposition of MG (~71.2%) when subjected to visible light irradiation for 120 min (Fig. 7c). For the sake of comparison, the photocatalytic responses of different MoS2 and WS2 nanosystems are presented in Table 1. To name a few, WS2 nanosheets possess excellent photocatalytic activity over the complete spectrum, from UV to NIR [8]. Also, mesoporous WS2 nanosheets are shown to be highly stable and efficient even after four cycles of RhB degradation [15]. In case of degradation of phenol by WO3/WS2 polycrystalline powders, the photoactivity is highly dependent on the molar ratio of the systems used [16]. Similarly, WS2/TiO2 has also shown an improved photocatalytic activity on harmful organic dyes, such as, target MO [41]. In this regard, IF-type WS2 systems, which are rarely discussed in the literature, find its importance in the list of nanocatalysts. The performance of IF-type WS2 nanosystem on the degradation of MG dye is evaluated through simple kinetics, as discussed below.
The kinetics of photocatalytic degradation of MG at the nanocatalyst surface can be understood through Langmuir–Hinshelwood (L-H) model [42]. For a dilute solution (mM) (C << 1), it can be expressed in the form of a first-order reaction given by [42]:
Here, C 0 is the initial concentration of the MG solution, t is the irradiation time and k a is the pseudo-first-order rate constant. At t = t 0, C = C 0, the initial concentration of the target before irradiation. The percentage degradation of MG is represented in Fig. 7c. The plots of C t /C 0 versus t, for UV and visible light conditions are shown in Fig. 7d. The plots essentially help to predict the pseudo-first-order rate constant, k a. The rate constants, as obtained directly from the graphs (Fig. 7d), are calculated to be 0.0239 and 0.0414 min−1 for IF nano-WS2 samples exposed to UV light and visible light, respectively. A higher rate constant for the latter case, suggests that our IF nano-WS2 are capable of displaying improved photoactivity under visible light illumination than that of UV exposure.
4 Conclusion
We have demonstrated a user friendly synthesis and photocatalytic activity of IF-type WS2 nanoparticles. The hexagonal crystal structure of WS2 is evident from XRD analysis. The presence of elements W and S has been confirmed from the EDX spectra. The direct band gap of the nano-WS2, as predicted from the Tauc’s plot, is ~1.91 eV. The single distinct peak at 630 nm corresponds to the transition of the band structures from the indirect to direct band gap type. On the other hand, Raman spectrum reveals the presence of one in-plane \( E^{1}_{2g} \) mode and one A 1g out-of-plane mode. The formation of IF-type nanoparticles with an average outer diameter of 16 nm has been observed through the electron micrographs. The photocatalytic efficiency of the synthesized IF WS2 nanocatalyst under visible light illumination gave a maximum degradation of 71.2% as compared to 45% when illuminated by UV light. Photoactivity studies of TMDCs with inclusion of suitable dopants and radiation effects are in progress.
References
K.S. Novoselov, A.K. Geim, S.V. Morozov, D. Jiang, Y. Zhang, S.V. Dubonos, I.V. Grigorieva, A.A. Firsov, Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004)
H. Tian, W. Mi, X.-F. Wang, H. Zhao, Q.-Y. Xie, C. Li, Y.-X. Li, Y. Yang, T.-L. Ren, Graphene dynamic synapse with modulatable plasticity. Nano Lett. 15, 8013–8019 (2015)
H. Tian, H. Zhao, X.F. Wang, Q.Y. Xie, H.Y. Chen, M.A. Mohammad, C. Li, W.T. Mi, Z. Bie, C.H. Yeh, Y. Yang, H.S. Philip Wong, P.W. Chiu, T.L. Ren, In situ tuning of switching window in a gate-controlled bilayer graphene-electrode resistive memory device. Adv. Mater. 27, 7767–7774 (2015)
Q.H. Wang, K.K. Zadeh, A. Kis, J.N. Coleman, M.S. Strano, Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat Nanotechnol 7, 699–712 (2012)
S. Bhattacharyya, A.K. Singh, Semiconductor-metal transition in semiconducting bilayer sheets of transition-metal dichalcogenides. Phys. Rev. B. 86, 075454 (2012)
H. Tian, M.L. Chin, S. Najmaei, Q. Guo, F. Xia, H. Wang, M. Dubey, Optoelectronic devices based on two-dimensional transition metal dichalcogenides. Nano Res. 9, 1543–1560 (2016)
H. Tian, J. Tice, R. Fei, V. Tran, X. Yan, L. Yang, H. Wang, Low-symmetry two-dimensional materials for electronic and photonic applications. Nano Today 11, 763–777 (2016)
Y. Sang, Z. Zhao, M. Zhao, P. Hao, Y. Leng, H. Liu, From UV to near-infrared, WS2 nanosheet: a novel photocatalyst for full solar light spectrum photodegradation. Adv. Mater. 27, 363–369 (2015)
S.V.P. Vattikuti, C. Byon, C.V. Reddy, R.V.S.S.N. Ravikumar, Improved photocatalytic activity of MoS2 nanosheets decorated with SnO2 nanoparticles. RSC Adv. 5, 86675–86684 (2015)
W. Zhang, X. Xiao, L. Zheng, C. Wan, Fabrication of TiO2/MoS2@zeolite photocatalyst and its photocatalytic activity for degradation of methyl orange under visible light. Appl. Surf. Sci. 358, 468–478 (2015)
D. Voiry, H. Yamaguchi, J. Li, R. Silva, D.C.B Alves, T. Fujita, M.W. Chen, T. Asefa, V.B. Shenoy, G. Eda, M. Chhowalla, Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat. Mater. 12, 850 (2013)
L. Rapoport, Y. Bilik, Y. Feldman, M. Homyonfer, S.R. Cohen, R. Tenne, Hollow nanoparticles of WS2 as potential solid-state lubricants. Nature 387, 791–793 (1997)
R. Levi, O. Bitton, G. Leitus, R. Tenne, E. Joselevich, Field-effect transistors based on WS2 nanotubes with high current-carrying capacity. Nano Lett. 13, 3736–3741 (2013)
H.R. Gutierrez, N. Perea-Lopez, A.L. Elias, A. Berkdemir, B. Wang, R. Lv, F. Lopez-Urias, V.H. Crespi, H. Terrones, M. Terrones, Extraordinary room-temperature photoluminescence in triangular WS2 monolayers. Nano Lett. 13, 3447–3454 (2013)
S.V.P. Vattikuti, C. Byon, C.V. Reddy, Preparation and improved photocatalytic activity of mesoporous WS2 using combined hydrothermal-evaporation induced self-assembly method. Mater. Res. Bull. 75, 193–203 (2016)
A. Di Paola, L. Palmisano, V. Augugliaro, Photocatalytic behavior of mixed WO3/WS2 powders. Catal. Today 58, 141–149 (2000)
Y. Shang, J. Xia, Z. Xu, W. Chen, Hydrothermal synthesis and characterization of quasi 1D tungsten disulfide nanocrystal. J. Disp. Sci. Technol. 26, 635–639 (2005)
S. Srivastava, R. Sinha, D. Roy, Toxicological effects of malachite green. Aquat. Toxicol. 66, 319–329 (2004)
G. Parshetti, S. Kalme, G. Saratale, S. Govindwar, Biodegradation of Malachite Green by Kocuria rosea MTCC 1532. Acta Chim. Slov. 53, 492–498 (2006)
S.W. Park, J.T. Jang, J. Cheon, H.H. Lee, D.R. Lee, Y. Lee, Shape dependent compressibility of TiO2 anatase nanoparticles. J. Phys. Chem. C 112(26), 9627–9631 (2008)
S. Hazarika, D. Mohanta, Extraction and characterization of mixed phase KNO2–KNO3 nanocrystals derived from flat-leaf green spinach. Phys. Scr. 87, 015603-1–5 (2013)
E. García-Lecina, I. García-Urrutia, J.A. Díez, J. Fornell, E. Pellicer, J. Sort, Codeposition of inorganic fullerene-like WS2 nanoparticles in an electrodeposited nickel matrix under the influence of ultrasonic agitation. Electrochim. Acta 114, 859–867 (2013)
S. Cao, T. Liu, S. Hussain, W. Zeng, X. Peng, F. Pan, Mater. Lett. 129, 205–208 (2014)
J.L. Elechiguerra, J. Reyes-Gasga, M. Jose-Yacaman, The role of twinning in shape evolution of anisotropic noble metal nanostructure. J. Mater. Chem. 16, 3906–3919 (2006)
Yu Zhang, Y. Zhang, Q. Ji, J. Ju, H. Yuan, J. Shi, T. Gao, D. Ma, M. Liu, Y. Chen, X. Song, H.Y. Hwang, Y. Cui, Z. Liu, Controlled growth of high-quality monolayer WS2 layers on sapphire and imaging its grain boundary. ACS Nano 7, 8963–8971 (2013)
C. Schuffenhauer, R. Popovitz-Biro, R. Tenne, Synthesis of NbS2 nanoparticles with (nested) fullerene-like structure (IF). J. Mater. Chem. 12, 1587–1591 (2002)
H. Zeng, G-B. Liu, J. Dai, Y. Yan, B. Zhu, R. He, L. Xie, S. Xu, X. Chen, W. Yao, X. Cui, Optical signature of symmetry variations and spin-valley coupling in atomically thin tungsten dichalcogenides. Sci. Rep. 3, 1608 (2013)
P. Atkin, T. Daeneke, Y. Wang, B.J. Carey, K.J. Berean, R.M. Clark, J.Z. Ou, A. Trinchi, I.S. Cole, K. Kalantar-zadeh, 2D WS2/carbon dot hybrids with enhanced photocatalytic activity. J. Mater. Chem. A 4, 13563–13571 (2016)
A. Berkdemir, H.R. Gutiérrez, A.R. Botello-Méndez, N. Perea-López, A.L. Elías, C.I. Chia, B. Wang, V.H. Crespi, F. López-Urías, J.C. Charlier, H. Terrones, M. Terrones, Identification of individual and few layers of WS2 using Raman spectroscopy. Sci. Rep. 3, 1755 (2013)
J. Coates, Interpretation of infrared spectra, a practical approach in encyclopedia of analytical chemistry, ed. R.A. Meyers (Wiley, Chichester, 2000), p. 10829
S.V.P. Vattikuti, C. Byon, Effect of CTAB surfactant on textural, structural, and photocatalytic properties of mesoporous WS2. Sci. Adv. Mater. 7(12), 2639–2645 (2015)
Y. Feldman, G.L. Frey, M. Homyonfer, V. Lyakhovitskaya, L. Margulis, H. Cohen, G. Hodes, J.L. Hutchison, R. Tenne, Bulk synthesis of inorganic fullerene-like MS2 (M = Mo, W) from the respective trioxides and the reaction mechanism. J. Am. Chem. Soc. 118, 5362–5367 (1996)
G.L. Frey, S. Elani, M. Homyonfer, Y. Feldman, R. Tenne, Optical-absorption spectra of inorganic fullerene like MS2 (M=Mo, W). Phys. Rev. B 57, 6666 (1998)
S.M. Notley, High yield production of photoluminescent tungsten disulphide nanoparticles. J. Coll. Interf. Sci. 396, 160–164 (2013)
S.V.P. Vattikuti, C. Byon, V. Chitturi, Selective hydrothermally synthesis of hexagonal WS2 platelets and their photocatalytic performance under visible light irradiation. Superlattices Microstruct. 94, 39–50 (2016)
H. Tributsch, Structure and bonding, vol. 49 (Springer, Berlin, 1982), p. 127
N. Paul, A. Deka, D. Mohanta, Augmented photocatalytic activity and luminescence response of Tb3+ doped nanoscale titania systems. J. Appl. Phys. 116, 144902-1–7 (2014)
S. Bingham, W.A. Daoud, Recent advances in making nano-sized TiO2 visible-light active through rare-earth metal doping. J. Mater. Chem. 21, 2041–2050 (2011)
K.M. Parida, N. Sahu, Visible light induced photocatalytic activity of rare earth titania nanocomposites. J. Mol. Catal. A 287, 151–158 (2008)
T. Peng, D. Zhao, H. Song, C. Yan, Preparation of lanthana-doped titania nanoparticles with anatase mesoporous walls and high photocatalytic activity. J. Mol. Catal. A 238, 119–126 (2005)
L. Zheng, W. Zhang, X. Xiao, Preparation of titanium dioxide/tungsten disulfide composite photocatalysts with enhanced photocatalytic activity under visible light. Korean J. Chem. Eng. 33, 107–113 (2016)
U.I. Gaya, A.H. Abdullah, Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J. Photochem. Photobiol. C 9, 1–12 (2008)
Acknowledgements
The authors acknowledge IUAC, New Delhi (Project: UFR-56322/2014). The authors thank peers and colleagues for their valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hazarika, S.J., Mohanta, D. Inorganic fullerene-type WS2 nanoparticles: processing, characterization and its photocatalytic performance on malachite green. Appl. Phys. A 123, 381 (2017). https://doi.org/10.1007/s00339-017-0965-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-017-0965-7