Abstract
In this study, an organometallic complex based on aluminum ions is synthesized. And it is utilized as fluorescent material in the organic light-emitting diodes (OLEDs). The synthesized complex was characterized using XRD, UV–Vis, FT-IR as well as PL spectroscopy analyses. The energy levels of Al complex were determined by cyclic voltammetry measurements. Then, the effects of ZnO nanoparticles (NPs) of poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate), PEDOT:PSS, on the electrical and optical performance of the organic light-emitting diodes have been investigated. For this purpose, two samples containing ITO/PEDOT:PSS/PVK/Alq3/PBD/Al with two different concentration and two samples containing ITO/PEDOT:PSS:ZnO/PVK/Alq3/PBD/Al with two different concentration were prepared. Then, hole transport, electron transport and emissive layers were deposited by the spin coating method and the cathode layer (Al) was deposited by the thermal evaporation method. The OLED simulation was also done by constructing the model and choosing appropriate parameters. Then, the experimental data were collected and the results interpreted both qualitatively and quantitatively. The results of the simulations were compared with experimental data of the J–V spectra. Comparing experimental data and simulation results showed that the electrical and optical efficiency of the samples with ZnO NPs is appreciably higher than the samples without ZnO NPs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The organic light-emitting diodes (OLEDs) more often have been investigated in both mercantile and scientific fields, because of their exclusive advantages such as light weight, slender structure, high resolution and low driving voltage [1]. Recently, polymer light-emitting devices (PLEDs) attracted a great attention due to their advantages of emission in a wide visible region, solution processing, including screen printing and inkjet deposition, large area coverage, low and poor consumption in application as light sources for optical signal circuits, etc. [2]. Although important progress has been achieved on the presentation of PLEDs, they still need additional improvements such as stability, brightness and lifetime to attract commercial applications [3]. The basic structure of a PLED consists of several organic thin films sandwiched between two electrodes. Nowadays, due to their specific properties, the application of nanoparticles and nanostructures is attracting a lot of research efforts, to enhance the performance of PLEDs. Several studies showed that doped NPs enhanced the abilities of charge injection and charge transport and consequently the turn-on voltage of the device reduced. Therefore, the device characteristics in current density were obviously improved by doping nanoparticles (NPs) into the hole transport (HTL) layer of PLEDs [1, 4, 5]. It has been demonstrated that the optoelectronic efficiency of OLEDs can be practically improved by measuring doped nanoparticles or nanostructures in and/or between organic thin films [6]. By doping suitable inorganic nanoparticles, the electrical properties of PEDOT:PSS can be improved, and the ensuing of PEDOT:PSS nanocomposite layers in PLEDs makes the injection of holes easy [7, 8]. In this study, we have made an organometallic complex based on aluminum ions, then the effect of PEDOT:PSS/ZnO nanocomposite hole–injection layer on the enhancement of performance of PLEDs has been investigated. Simulation device was also done by constructing the model and choosing appropriate parameters. The experimental data were collected and the results interpreted both qualitatively and quantitatively. Also experimental results and simulated data were compared.
2 Experimental
2.1 Materials
The poly(9-vinylcarbazole) (PVK), 2-(4-biphenylyl)-5-phenyl-1,3,4-oxadiazole (PBD), poly(3,4-ethylenedioxythiophene) polystyrene sulfonate and ZnO NPs were purchased from Aldrich.
2.2 Synthesis
8-Hydroxyquinoline (Aldrich, 4.35 g) was dissolved in a mixture of 15 mL methanol (Merck); it was stirred well until orange transparent solution was obtained. Separately, Al (NO3)3·9H2O (99%, Aldrich, 3.75 g) was dissolved in methanol and stirred well till crystal transparent solution was obtained. These two mixtures were added together, and the resulted suspension was stirred for 20 min. The final product was dried in the room temperature and was used for further experiments.
2.3 Physical measurements
The crystal structures of Al complex were determined by means of X-ray diffraction (XRD, Philip X’pert X-ray diffractometer with Cu Kα radiation, λ = 1.5418 Å). Infrared spectra from 200 to 4000 cm−1 were recorded on a Shimadzu 470 FT-IR instrument, using KBr pellets. Voltammetric experiments were performed using a m-Autolab Type III electrochemical system. A conventional three-electrode cell consisting of a glassy carbon working electrode, a platinum wire counter electrode and a saturated Ag/AgCl reference electrode was used for voltammetric experiments. The current–voltage characteristics of the devices were measured by Keithley, as 2400 source meter. The EL and PL spectra were measured with USB2000 and HR4000 ocean optic, under ambient conditions. All measurements were carried out immediately after fabricating the devices.
3 Fabrication OLED
3.1 Simulation method
Simulation is an influential method to examine the underlying mechanism of the devices. More importantly, it can tell us to what extent the mechanism can affect the device performance [9]. Meanwhile, simulation can regain detailed information, such as the charge carrier distribution which is difficult to be directly measured through experiments. The first step in the simulation of PLED devices is to divide the organic layers into a set of discrete monolayers, sandwiched between an anode and a cathode [9–11]. The simulations presented in this work are based on injection, transport and recombination of charge carriers, as well as the electric field distribution. Injection of charge carrier from a metal electrode into an inorganic semiconductor can be described by the thermionic emission model [12], which assumes that the current flow across the barrier is dependent on the barrier height only [11, 13, 14]. Based on this model, the injection current can be calculated by the following equations:
The energy and position disorder impedes the transport and causes a low mobility of organic materials. Bässler [15] used the Monte Carlo method to analyze the transport behavior in disordered materials. The dependence of mobility on electric field can be described by the Poole–Frenkel equation [15, 16] as follows
The electric field of the device is calculated by the following equation [9, 10, 17–20]:
3.2 Fabrication of OLED by utilization of prepared complex
3.2.1 The first step of the fabrication process
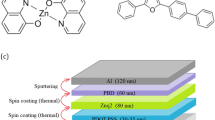
ITO substrates were patterned by the etching process using an acidic mixture of HCl and HNO3 as etching agent. Patterned ITO glasses were cleaned with acetone, dichloromethane, methanol and deionized water under an ultrasonic bath for 10 min and then dried in an oven for 20 min. The PEDOT:PSS and PEDOT:PSS:ZnO layers were spin-coated on ITO glasses. To remove residual water, the layers were baked into the oven at 120 °C for 20 min. The PVK in dichloromethane solution was spin-coated on the PEDOT:PSS and PEDOT:PSS:ZnO layers. The Alq3 and PBD were spin-coated in separated dichloromethane solution and baked in the oven, respectively, as the former stage. The devices were prepared after thermally evaporating Al that is as a cathode layer. The evaporation rate of Al was kept at 0.6–1 Å/s.
3.2.2 Fabrication of OLED
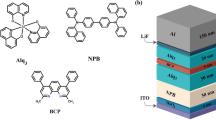
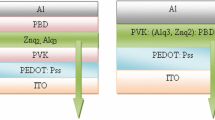
The steps of the fabrication process of a PLED device are schematically demonstrated in Fig. 1. In this study, four OLED devices were manufactured with different organic layer thickness. Active layers were used with an indium tin oxide (ITO)/PEDOT:PSS (35 nm) or PEDOT:PSS:ZnO (35 nm)/PVK (70 nm)/Alq3 (0.0032, 0.0038 M) (60 nm)/PBD(70 nm)/Al (100 nm) device structure. The size of ZnO NPs was about 31 nm. OLED devices were fabricated with the following configurations (Table 1).
ITO/PEDOT:PSS/PVK/Alq3 (0.0032 M)/PBD/Al (device 1), ITO/PEDOT:PSS/PVK/Alq3 (0.0038 M)/PBD/Al (device 2), ITO/PEDOT:PSS:ZnO/PVK/Alq3 (0.0032 M)/PBD/Al (device 3) and ITO/PEDOT:PSS:ZnO/PVK/Alq3(0.0038 M)/PBD/Al (device 4).
4 Results and discussion
4.1 Characterization synthesis
Crystal structures of Alq3 were determined by X-ray diffraction (Fig. 2). The diffraction peaks can be indexed to tris-(8-hydroxyquinoline) aluminum (Alq3) (JCPDS 26-1550). The determined α-phase of XRD pattern of the meridional isomer Alq3 is reported by Michael Cole et al. [21]. No characteristic peaks of impurity were detected; therefore, the products are pure Alq3. This result almost matches with the results observed from the FT-IR curve. The spectra are shown in the range of 200–4000 cm−1 in Fig. 3. The characteristic peaks of Alq3, which are the bands centered at 600–800 cm−1, come from the vibrations of quinoline, and the band at 400–600 cm−1 can be attributed to the stretching vibration of Al ion with ligands. As examples of them, we have C–C stretching vibrations (1502, 1469 cm−1), C–O stretching vibrations (1035, 1111, 1469 cm−1), aromatic amine (1300 cm−1), aromatic stretching C=C (1469 cm−1), Al–O–Al (758 cm−1), Al–O (557, 644 cm−1) and Al–N stretching vibrations (416, 460 cm−1). The peak at ~3416 cm−1 indicates the amount of moisture (H2O) present in the sample [22–28]. Figure 4 shows the absorption and emission spectra of Alq3 in aqueous solution. The complex Alq3 exhibits an ultraviolet absorption peak in the range of 300–450 nm, and the maximum absorption peaks are located at 292 (π–π *) and 374 (n–π *) nm, respectively [29–33]. The band at 374 nm has multiple electronic origins, that is, this band is a superposition of two or more electronic transitions, for more information see Burrows et al. [34]. As it is shown in Fig. 4, the emission peak of Alq3 in aqueous solution is 524.39 nm, and we can clearly observe the green light.
4.2 Energy levels of prepared complex
One of the most accurate methods to characterize the organic materials and estimation about energy band diagram is cyclic voltammetry (CV). The energy band diagram of the Alq3 complex was determined from the HOMO and LUMO energy levels and the band gaps was calculated from cyclic voltammetry [35, 36]. The highest occupied molecular orbital (HOMO) energy level of the complexes was evaluated by cyclic voltammetry on an electrochemical work station. The lowest unoccupied molecular orbital (LUMO) energy level was appraised by subtracting the energy gap from the energy of HOMO, and the energy gap was determined by the absorption edge of the UV absorption spectrum of the complex in the solution. Based on cyclic voltammetry results, Alq3 shows E ox, which is equal to E HOMO from the equation E HOMO = [(E ox − E 1/2(ferrocene)) + 4.8] eV. Figure 5 shows cyclic voltammetry of aluminum complex with the scan rate of 100 mV/s. The calculated E HOMO, E LUMO and E g values are listed in Table 2. The optical band gap of the complex Al (0.0032, 0.0038 M) was determined from the absorption onset (Fig. 6). The corrected base line of the absorption spectra was drawn horizontally. In order to determine the absorption edge, a straight line to the right-hand side of the maximum peak was fitted, and then, its intersection with the corrected baseline was determined [37]. The optical band gap we obtained for Alq3 corresponds well to the value reported [38].
4.3 OLED device
Figure 7 shows the XRD patterns of layers with and without ZnO NPs. As shown in Fig. 7, the intensity and width of the peak decreased when the ZnO NPs were doped with PEDOT:PSS. XRD spectrum parameters for layers with and without ZnO NPs were calculated and are reported in Table 3. According to the Scherrer equation [39]:
It can be concluded from Table 3 that the crystal size for layers with ZnO NPs increased and it is followed by the reduction of band gap. Consequently, there have been more effective electron injection/transfer. Figure 8 shows the EL spectra that are normalized at the main emissive peak at ~515 nm. The device without ZnO NPs shows the typical EL spectrum where the emission peak at ~515 nm is larger than the vibrancy peak at longer wavelengths. In comparison, the long-wavelength EL peak increases relatively, with the EL peaks slightly shifted to longer wavelengths when ZnO NPs were doped with PEDOT:PSS. This may be because of the optical interference effects. Interference effects depend on the amount of ZnO NPs or changes of the spectral characteristics of the EL emission. All electrical properties are shown in Figs. 9 and 10. Figure 9 shows the current density–voltage (J–V) as well as characteristics of prepared devices. It is clear from the Fig. 9 that the current density of device increases significantly by doped ZnO NPs compared to the device without the ZnO NPs: the turn-on voltage decreases by about 3 V and the maximum luminance increases more than ten times when doped ZnO NPs were used. The doping of ZnO can effectively enhance the conductivity in the hole–injection layer and reduce the turn-on voltage that made the progress of hole injection more smoothly and led to a higher overall device efficiency. To confirm the outcome of the simulations, the experimental J–V spectra of the devices were compared with the resulting spectra of simulation. Figure 10 shows experimental and simulated current density–voltage (J–V) spectra of four OLED structures: ITO/PEDOT:PSS/PVK/Alq3 (0.0032 M)/PBD/Al (device 1), ITO/PEDOT:PSS/PVK/Alq3 (0.0038 M)/PBD/Al (device 2), ITO/PEDOT:PSS:ZnO/PVK/Alq3 (0.0032 M)/PBD/Al (device 3) and ITO/PEDOT:PSS:ZnO/PVK/Alq3 (0.0038 M)/PBD/Al (device 4). As it is shown in the Fig. 10, good agreement was observed between simulated and experimental curves. The difference between the curves can be attributed to the carrier density.
5 Conclusion
In this study, an Al complex was synthesized and it is demonstrated that light-emitting layers of Alq3 could be formed via spin coating. The properties of Alq3 particles were studied by XRD, FT-IR, UV–Vis, CV and PL analyses. FT-IR results confirm the completion of quinoline ring formation and presence of quinoline structure in the metal complex. In the absorption spectra, the complexes show two absorption bands at 292 and 374 nm. The emission peak of Alq3 in aqueous solution is 524.39 nm, and the green light was observed clearly. The XRD and PL studies showed that the prepared yellowish green Alq3 powder in this experiment is an α-phase, that is, one of the four phases mentioned above. Examination of a device operation with an Alq3 EML confirmed that the electron transport and the green emission characteristics of Alq3 were retainable by Alq3, but the EL efficiency was very low because of the deficiency of holes that recombine radiatively with electrons. We investigated the effects of ZnO NPs doped with PEDOT:PSS on the performance of OLEDs. Then electron transport (ETL), hole transport (HTL) and emissive layers were spin-coated with spin speeds of 2000, 2000 and 1500 rpm, respectively. As a result, experiments showed that the electrical and optical efficiency in samples with ZnO NPs are appreciably higher than in samples without ZnO NPs. In summary, when ZnO NPs were used, the electroluminescence and J–V curves from PLEDs at voltages below the band gap voltage were observed. It can be concluded that the crystal size for layers with ZnO NPs increased and it is followed by the reduction of band gap. Consequently, there was more effective electron injection/transfer in device. The doping of ZnO can effectively enhance the conductivity in the hole–injection layer and reduce the turn-on voltage that made the progress of hole injection more smoothly and led to a higher overall device efficiency. In this study, the electrical properties of the highly efficient material systems for OLEDs were modeled successfully. Simulations of electron and hole-only devices verified the validity and accuracy of the used models and parameters. Also good agreement between simulated and experimental curves was observed.
References
S.A. Carter, J.C. Scott, P.J. Brock, Enhanced luminance in polymer composite light emitting devices. Appl. Phys. Lett. 71(9), 1145–1147 (1997)
Y. Ohmori, H. Kajii, T. Sawatani, H. Ueta, K. Yoshino, Enhancement of electroluminescence utilizing confined energy transfer for red light emission. Thin Solid Films 393, 407–411 (2001)
D.D. Zhang, J. Feng, Y.F. Liu, Y.Q. Zhong, Y. Bai, Y. Jin, G.H. Xie, Q. Xue, Y. Zhao, S.Y. Liu, H.-B. Sun, Enhanced hole injection in organic light-emitting devices by using Fe3O4 as an anodic buffer layer. Appl. Phys. Lett. 94, 223303–223306 (2009)
N.N. Dinh, L.H. Chi, T.T.C. Thuy, T.Q. Trung, V.V. Truong, Enhancement of current-voltage characteristics of multilayer organic light emitting diodes by using nanostructured composite films. J. Appl. Phys. 105(9), Article ID 093518 (2009)
L. Qian, Y. Zheng, K.R. Choudhury et al., Electroluminescence from light-emitting polymer/ZnO nanoparticle heterojunctions at sub-bandgap voltages. Nano Today 5(5), 384–389 (2010)
C.H. Lin, K.T. Chen, J.R. Ho, J.W. J Cheng, R.C.C. Tsiang, PEDOT: PSS/graphene nanocomposite hole-injection layer in polymer light-emitting diodes. J. Nanotechnol. Article ID 942629 (2012)
Z.B. Deng, X.M. Ding, S.T. Lee, W.A. Gambling, Enhanced brightness and efficiency in organic electroluminescent devices using SiO2 buffer layers. Appl. Phys. Lett. 74(15), 2227–2229 (1999)
I.M. Chan, F.C. Hong, Improved performance of the single-layer and double-layer organic light emitting diodes by nickel oxide coated indium tin oxide anode. Thin Solid Films 450(2), 304–311 (2004)
J. Staudigel, M. Stossel, F. Steuber, J. Simmerer, A quantitative numerical model of multilayer vapor-deposited organic light emitting diodes. J. Appl. Phys. 86, 3895 (1999)
G.G. Malliaras, J.C. Scott, Numerical simulations of the electrical characteristics and the efficiencies of single-layer organic light emitting diodes. J. Appl. Phys. 85, 7426 (1999)
B.K. Crone, I.H. Campbell, P.S. Davids, D.L. Smith, C.J. Neef, J.P. Ferraris, Device physics of single layer organic light-emitting diodes. J. Appl. Phys. 86, 5767 (1999)
W. Schottky, The influence of the structural effects, especially the Thomson graphic quality, on the electron emission of metals. Physikalische Zeitschrift 15, 872 (1914)
I.H. Campbell, P.S. Davids, D.L. Smith, N.N. Barashkov, J.P. Ferraris, The Schottky energy barrier dependence of charge injection in organic light emitting diodes. Appl. Phys. Lett. 72, 1863 (1998)
B.K. Crone, I.H. Campbell, P.S. Davids, D.L. Smith, Charge injection and transport in single-layer organic light-emitting diodes. Appl. Phys. Lett. 73, 3162 (1998)
H. Bässler, Charge transport in disordered organic photoconductors—a Monte-Carlo simulation study. Phys. Status Solidi B 175, 15 (1993)
H.C.F. Martens, P.W.M. Blom, H.F.M. Schoo, Comparative study of hole transport in poly(p-phenylenevinylene) derivatives. Phys. Rev. B 61, 7489 (2000)
P.S. Davids, I.H. Campbell, D.L. Smith, Device model for single carrier organic diodes. J. Appl. Phys. 82, 6319 (1997)
S.J. Konezny, D.L. Smith, M.E. Galvin, L.J. Rothberg, Modeling the influence of charge traps on single-layer organic light-emitting diode efficiency. J. Appl. Phys. 99, 064509 (2006)
E. Tutis, M.N. Bussac, B. Masenelli, M. Carrard, L. Zuppiroli, Numerical model for organic light-emitting diodes. J. Appl. Phys. 89, 430 (2001)
B. Ruhstaller, T. Beierlein, H. Riel, S. Karg, J.C. Scott, W. Riess, Simulating electronic and optical processes in multilayer organic light-emitting devices. IEEE J. Sel. Top. Quantum Electron. 9, 723 (2003)
M. Brinkmann, G. Gadret, M. Muccini, C. Taliani, N. Masciocchiand, A. Sironi, Correlation between molecular packing and optical properties in different crystalline polymorphs and amorphous thin films of mer-tris(8-hydroxyquinoline)aluminum(III). J. Am. Chem. Soc. 122, 5147 (2000)
M.J.J. Brigit Gilda, S. Anbarasu, Y. Samson, P.A. Devarajan, The influence of benzophenone substitution on the physico-chemical characterizations of 8-hydroxyquinoline NLO single crystals. J. Miner. Mater. Charact. Eng. 11, 769–773 (2012)
S.A. Bhagat, FTIR spectroscopy of Ca ++doped Alq3 OLED Phospor. Int. J. Sci. Res. 120, 509 (2015)
M.D. Halls, R. Aroca, Vibrational spectra and structure of tris(8-hydroxyquinoline)aluminum(III). Can. J. Chem. 76, 1730 (1998)
R.J. Curry, W.P. Gillin, J. Clarkson, D.N. Batchelder, Morphological study of aluminum tris(8-hydroxyquinoline) thin films using infrared and Raman spectroscopy. J. App. Phys. 92(4), 1902 (2002)
M.-M. Duvenhage, Investigation of the luminescent properties of metal quinolates (Mqx) for use in OLED device. Thesis (2014)
C. Cui, D.H. Park, J. Kim, J. Joo, D.J. Ahn, Oligonucleotide assisted light-emitting Alq3 microrods: energy transfer effect with fluorescent dyes. Chem. Commun. 49, 5360 (2013)
M.M. El-Nahass, A.M. Farid, A.A. Atta, Structural and optical properties of Tris(8hydroxyquinoline) aluminum (III) (Alq3) thermal evaporated thin films. J. Alloys Compd. 507, 112–119 (2010)
M. Braun, J. Gmeiner, M. Tzolov, M. Coelle, F.D. Meyer, W. Milius, H. Hillebrecht, O. Wendland, J.U. von Schűtz, W. Brűtting, A new crystalline phase of the electroluminescent material tris(8-hydroxyquinoline) aluminum exhibiting blueshifted fluorescence. J. Chem. Phys. 114(21), 9625 (2001)
Y.K. Han, S.U. Lee, Molecular orbital study on the ground and excited states of methyl substituted tris(8-hydroxyquinoline) aluminum(III) Chem. Phys. Lett. 366, 9–16 (2002)
A. Curioni, W. Andreoni, Computer simulations for organic light-emitting diodes. J. Res. Dev. IBM 45, 101 (2001)
M. Colle, J. Gmeiner, W. Milius, H. Hillebrecht, W. Brutting, Tunable photoluminescence from tris(8-hydroxyquinoline)aluminum(Alq3). Adv. Funct. Mater. 13(2), 108 (2003)
M.M. Levichkova, J.J. Assa, H. Fröb, K. Leo, Blue luminescent isolated Alq3 molecules in a solid-state matrix. Appl. Phys. Lett. 88(20), 1912 (2006)
P.E. Burrows, Z. Shen, V. Bulovic, D.M. McCarty, S.R. Forrest, Relationship between electroluminescence and current transport in organic heterojunction light-emitting devices. J. Appl. Phys. 79, 7991–8006 (1996)
M. Thelakkat, H.-W. Schmidt, Synthesis and properties of novel derivatives of 1, 3, 5-Tris (diarylamino) benzenes for electroluminescent devices. Adv. Mater. 10, 219 (1998)
F.S. Rodembusch, F.R. Brand, D.S. Corrêa, J.C. Pocos, M. Martinelli, V. Stefani, Transition metal complexes from 2-(2′-hydroxyphenyl) benzoxazole: a spectroscopic and thermogravimetric stability study. Mater. Chem. Phys. 92, 389 (2005)
R. Schlaf, P.G. Schroeder, M.W. Nelson, B.A. Parkinson, C.D. Merritt, L.A. Crisafulli, H. Murata, Z.H. Kafafi, Determination of interface dipole and band bending at the Ag/tris(8-hydroxyquinolinato) gallium organic Schottky contact by ultraviolet photoemission spectroscopy. Surf. Sci. 450, 142–152 (2000)
A. Shafiee, M.M. Salleh, M. Yahaya, Determination of HOMO and LUMO of [6,6]-phenyl C61-butyric acid 3-ethylthiophene ester and poly(3-octyl-thiophene-2,5-diyl) through voltametry characterization. Sains Malays. 40, 173–176 (2011)
P. Scherrer, Bestimmung der Gröss und der Inneren Struktur von Kolloidteilchen Mittels Röntgenstrahlen. Nachrichten von der Gesellschaft der Wissenschaften, Göttngen, Mathematisch-Physikalische Klasse 2, 98–100 (1918)
Acknowledgements
The authors would like to thank Dr. Hui Wang as Arrow Street Capital, L. P. Boston, MA for his helpful discussion and valuable cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shahedi, Z., Jafari, M.R. Synthesis Al complex and investigating effect of doped ZnO nanoparticles in the electrical and optical efficiency of OLEDS. Appl. Phys. A 123, 98 (2017). https://doi.org/10.1007/s00339-016-0715-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-016-0715-2