Abstract
Nickel titanate (NiTiO3) nanocrystallites are synthesized via a solid-state reaction from a precursor prepared by a homogeneous precipitation method. Ilmenite-structural NiTiO3 consists of alternating layers of NiO6 and TiO6 octahedra. It not only strongly absorbs ultraviolet light (wavelength <360 nm) but also selectively absorbs visible light mainly in a wavelength range of 420–540 nm and above 700 nm. The synthetic NiTiO3 is a direct-gap semiconducting material with a band gap of 3.00 eV and has obvious absorbance peaks at 448, 502, and 743 nm, corresponding to the photon energies of 2.77, 2.47, and 1.67 eV, respectively. Nevertheless, NiTiO3 does not exhibit obvious photocatalytic activity in the degradation of methylene blue in water. It is proposed that the visible light absorbance peaks of NiTiO3 mainly originate from the Ni: d → d charge-transfer transitions within its valence band. NiTiO3 has wide energy gaps from the hybridized Ni 3d and O 2p orbitals to the Ti 3d orbitals, which block both Ni2+ → Ti4+ and O2− → Ti4+ charge-transfer transitions between valence band and conduction band, and thus baffle its photocatalytic performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nickel titanate (NiTiO3) is an ilmenite-structural n-type semiconducting material with particular magnetic, electric, and optical properties [1–8]. It exhibits both semiconducting and magnetic behaviors and could be exploited for applications including microwave devices and varistors [8]. Besides applied as yellow pigments in ceramics or automobile industry [9–13], NiTiO3 is widely studied as a photocatalyst [14–25]. Although there still are arguments about its band gaps including 2.12 eV [2], 3.18 eV [3], 2.8 eV [17], 3.0–3.1 eV [4, 15, 16, 18, 20], and 3.2 eV [8], many researchers consider that NiTiO3 possesses narrower band gaps resulted from Ni2+ → Ti4+ charge-transfer transitions than TiO2 (3.3 eV for anatase, and 3.1 eV for rutile [26]) caused by O2− → Ti4+ transitions and then has visible light photocatalytic activity. Asahi et al. [27] reported that yellow nitrogen-doped TiO2 has a narrowed band gap and exhibits optical absorption and photocatalytic activity under visible light (wavelength <500 nm). They suggested that the doped nitrogen has substituted partial oxygen, and the consequential transition N 2p → Ti 3d instead of O 2p → Ti 3d makes a redshift of the absorption edge of TiO2−x N x . It is interesting to investigate the optical absorption and photocatalytic activity of NiTiO3, which are important for its applications in paints or cosmetics.
In this work, we synthesized NiTiO3 nanocrystallites by a homogeneous precipitation method and investigated their optical absorption properties. To synthesize NiTiO3 nanocrystallites, most researchers prepared precursors by wet chemical methods and then calcined the precursors to form nano-sized NiTiO3 crystallites. The precursors could be prepared by several methods, including chemical co-precipitation method [3, 10], sol–gel method [5, 8, 24], polymeric precursor method [4, 28], etc. The homogeneous precipitation method has ever been used to synthesize well-ordered crystallites including NiTiO3 [18, 29, 30]. During the process, the mixed Ti- and Ni-containing precipitate gradually forms along with the hydrolysis of urea. Hence, the well-mixed precursor more easily forms well-ordered NiTiO3 crystallites.
The investigation reveals the synthetic NiTiO3 nanocrystallites having a band gap of 3.00 eV, and obviously, selectively absorbing visible light with certain wavelength. Comparing the optical absorption properties and the band structures of TiO2, NiO, and NiTiO3, we propose that the wide energy gap from the hybridized Ni 3d and O 2p orbitals to the Ti 3d orbitals baffles the photocatalytic performance of NiTiO3.
2 Experimental
2.1 Synthesis of NiTiO3 nanocrystallites
Stoichiometric TiCl4 in a hydrochloric acid solution and Ni(NO3)2·6H2O were dissolved in deionized water under vigorous stirring. Urea was added into the solution as precipitator to gradually form a Ti- and Ni-containing precipitate. The gradually thickening suspension was filtered after being stirred for 6 h at the refluxing temperature. The green filter cake was, respectively, washed with deionized water and anhydrous ethanol and dried in air overnight at 130 °C to form the dried precursor of NiTiO3. After being ground, the precursor was annealed in air heated at 800 °C for 6 h and finally formed a yellow product.
2.2 Characterization
The dried precursor was analyzed by thermogravimetric analysis and differential scanning calorimetry (TG–DSC, STA 409 PC/PG, NETZSCH) from room temperature to 1100 °C in air flow. The synthetic powder was characterized by a power X-ray diffractometer (XRD, D/MAX 2500 V/PC, Rigaku) using Cu Kα radiation (λ = 0.15406 nm). Its mean crystal size was calculated by the Scherrer formula. The powder is a mixture of NiTiO3, rutile, and anatase phases. So their weight fractions were, respectively, calculated by following formulas [31]:
where W NT, W R, and W A are the weight fractions of NiTiO3, rutile, and anatase, respectively. I NT, I R, and I A, respectively, represent the intensities of main diffraction peaks of NiTiO3 (104), rutile (110), and anatase (101). K NT (= 2.60), K R (= 3.68), and K A (= 5.05) are the reference intensity ratios of the three phases, and are, respectively, selected from JCPDS 76-0334, 84-1284, and 84-1286. The sample’s microstructures were detected by a field-emission high-resolution transmission electron microscope (HRTEM, Tecnai G2 F20, FEI) with an energy-dispersive X-ray spectroscope (EDX). The samples’ ultraviolet–visible light diffuse reflectance spectra (UV–Vis DRS) were measured by a JASCO V-570 spectrometer in a wavelength range of 200–800 nm at room temperature. A commercial TiO2 (P-25, Degussa) was used as reference.
The samples’ photocatalytic performance was estimated by measuring the decomposition rates of methylene blue (40 mg) in an aqueous solution (400 ml) with catalyst powder (200 mg). The reactor was equipped with cooling water circulating between inner and outer quartz tubes to keep the reaction at ~30 °C. The mixed methylene blue solution and catalyst powder in the outer tubes were vigorously stirred for 30 min to equably disperse the catalyst powder before irradiation. Air was pumped into the reactor to degrade methylene blue. A 300-W high-pressure mercury arc lamp placed in the inner quartz tube was used as light source. A blank sample without a catalyst was carried out following the same procedure. After the photolysis, the remaining methylene blue concentrations in the aqueous solutions were detected by measuring the absorbance at 665 nm using a HP8453 UV–Vis spectrophotometer.
3 Result and discussion
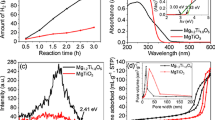
Figure 1 shows the TG–DSC curves of the precursor of NiTiO3. The precursor loses ~14.4 % weight from room temperature to 800 °C. The endothermic peak at 100 °C is attributed to the escape of surface water. The exothermic phenomenon above 800 °C is caused by the descending formation enthalpy of NiTiO3 with increasing temperature [32]. According to the results, 800 °C was selected as the reaction temperature.
Figure 2a shows XRD pattern of the synthetic powder. It is a mixture of NiTiO3, rutile, and anatase with a weight ratio of 69/22/9. The mean crystal sizes of NiTiO3, rutile, and anatase are 40, 42, and 25 nm, respectively. Most TiO2 exists in a rutile phase. It is accepted that the rutile phase is more stable than the anatase phase at high temperature. No other distinct Ni-containing compounds, such as NiO, were detected. It means that almost all Ni had been utilized to form NiTiO3 nanocrystallites. During the synthesis of NiTiO3, the raw materials contain equimolar Ti4+ and Ni2+ ions, but more Ni2+ ions lost due to the higher solubility of Ni-containing precipitate and the filtering and washing processes. The product hardly keeps the same metal ratios with that in raw materials by a homogeneous precipitation method. The similar problem exists in the conventional chemical co-precipitation method.
The lattice parameters of the synthetic NiTiO3 are \( R\bar{3} \), a = 5.0290(3) Å, c = 13.7895(2) Å, V = 302.03 Å3, Z = 6, and D x = 5.0998 g cm−3. They consist with the reported data of NiTiO3 in literature [8, 25, 28, 37] and the JCPDS 33-0960. The rhombohedral unit cell of NiTiO3 is illustrated in Fig. 2b. The alternating layers of Ni and Ti atoms are perpendicular to the c axes. The O atoms between the transition metal layers are octahedrally coordinated with the centered Ni or Ti atoms.
Figure 3a presents the nanosized particles agglomerate together. Figure 3b is an enlarged image and exhibits the particles’ crystal structures. There are different lattice spaces of 0.25 and 0.35 nm, respectively, corresponding to the (110) layers of NiTiO3 and (101) layers of rutile. An EDX spectrum indicates that the ratio of Ni/Ti/O in a selected small area is 18.9/20.2/60.9, namely Ni0.93Ti1.00O3. So the selected area almost contains NiTiO3.
Figure 4a shows the samples’ UV–Vis DRS. Degussa P-25 strongly absorbs UV light (wavelength <320 nm) and has an absorption edge of ~410 nm. It hardly selectively absorbs visible light. As comparison, the synthetic powder shows a redshift for its UV absorption edge. Its absorbance in visible light region is mainly caused by NiTiO3 because the doped TiO2 hardly absorbs visible light [32]. NiTiO3 not only strongly absorbs UV light (<360 nm) but also selectively absorbs visible light mainly within 420–540 nm and above 700 nm. It shows the absorbance peaks at 448, 502, and 743 nm, which correspond to the photon energies of 2.77, 2.47, and 1.67 eV, respectively. The high visible light absorbance centered at 448 nm (violet color) and 743 nm (red color) make NiTiO3 showing the complementary colors of yellow and green.
The band gap of NiTiO3 is estimated by the Tauc plot [15, 34, 35], namely the curve of (αhν)2/n versus hν. The α and hν, respectively, are the absorbance and photon energy. The n value indicates the transitions from the valence band (VB) to the conduction band (CB) being direct (n = 1) or indirect (n = 4). The hν value at the intersection point of the tangent and the horizontal axis is the band gap energy.
As shown in Fig. 4b, the calculation results indicate that either NiTiO3 or Degussa P-25 has a direct band gap. The band gap of Degussa P-25 is ~3.32 eV, which coincides with the band gap of anatase TiO2. NiTiO3 has a narrower band gap of 3.00 eV.
The photocatalytic performance of NiTiO3 and Degussa P-25 is evaluated by degrading methylene blue. Figure 5 shows that all methylene blue had been degraded by Degussa P-25 in 20 min. But, NiTiO3 exhibits rather low photocatalytic activity. Only 10 % methylene blue degraded in 80 min. The results approve the viewpoint of Goodenough et al. [15]. They predicted that the geometry of NiTiO3 makes the oscillator strength for Ni2+ → Ti4+ charge-transfer too weak to have an acceptable response to visible light.
The optical and photocatalytic properties of NiTiO3 are closely related with its crystal size, crystal structure, and band structure. Both experimental and theoretic results present the redshifts of UV light absorption edges for nanosized NiTiO3 [3, 7, 19, 23, 33, 36–38]. Nevertheless, NiTiO3 often has absorption peaks near 448, 502, and 745 nm, which almost do not shift with the size of NiTiO3 crystallites [3, 7, 9, 18, 19, 23, 33, 38]. Its yellow color also indicates NiTiO3 selectively absorbs visible light. However, the absorbed visible light did not obviously improve the photocatalytic performance of NiTiO3 in this work.
To understand the optical absorption properties of NiTiO3, we simply compare the energy band structures and density of states (DOSs) of TiO2, NiO, and NiTiO3 based on recent reports mainly using density functional theory (DFT) or ab initio calculations. Figure 6 shows that there is a band gap between VB and CB. According to ligand field theory, as Ti (or Ni) centers the TiO6 (or NiO6) octahedron in the crystal, the Ti 3d or (Ni 3d) orbitals split into t 2g and, e.g., orbitals. The 3d orbitals of metal ions bond with the O 2p orbitals and then form the energy bands.
As a typical photocatalyst, nanosized TiO2 (anatase or rutile) has an experimental band gap of 3.1–3.3 V [26]. TiO2 consists of TiO6 octahedra. The calculated DOSs are illustrated as Fig. 6a [39–42]. Both VB and CB consist of the hybridized O 2p and Ti 3d orbitals. Nevertheless, the VB is predominately formed from O 2p orbitals, and the CB from Ti 3d orbitals. At the bottom of CB, the high DOSs peak originates from the dominative Ti 3d (t *2g ) antibonding with less O 2p orbitals. Obviously, the electrons in Ti 3d orbitals more often occupy the t *2g orbitals than the low energy levels, e.g., and t 2g orbitals. Umebayashi et al. [39] pointed out that the location of t *2g determines the photoexcitation of TiO2, and the dopant t 2g level in the band gap plays a vital role for the visible light response of TiO2. Experimentally, Degussa P-25 hardly absorbs visible light, but exhibits nice photocatalytic activity under UV light. The most charge-transfer transitions in TiO2 should occur between O 2p and Ti 3d (t *2g ), i.e., O2− → Ti4+, under UV light.
Figure 6b shows that NiO has a wide band gap of 3.7–4.3 eV [43, 44] and generally is considered as an insulating material. NiO consists of NiO6 octahedra. Its both VB and CB are predominately from Ni 3d orbitals [43–46]. Most DOSs of Ni 3d locate within the VB. The wide energy gap between the nearby DOSs peaks of CB and VB blocks the charge-transfer transitions by either Ni 3d (t *2g ) → Ni 2d (e.g.,*) or O 2p → Ni 3d (e.g.,*). NiO (at 300 °C) shows optical absorption lines at 1.13, 1.75, 2.75, and 2.95 eV [47], which should mainly originate from the Ni: d → d transitions with low energy gaps in the VB after absorbing visible light.
Compared with TiO2 and NiO, NiTiO3 has a more complicated band structure due to its alternating layers of NiO6 and TiO6 octahedra. Its band structure is schematically shown in Fig. 6c. Ruiz-Preciado et al. [37] argued that the bulk NiTiO3 has a band gap of ~2.55 eV between the DOSs of Ni 3d and O 2p but with relatively low mobility of charges. The VB of NiTiO3 mainly derives from the hybridization of Ni 3d and O 2p orbitals; while the CB predominately consists of Ti 3d orbitals. Being similar to the case of NiO, most Ni 3d orbitals in NiTiO3 locate in the VB. The observed absorbance peaks at 1.67, 2.47, and 2.77 eV should be caused by the Ni: d → d transitions in the VB. The wide energy gap (>5.5 eV) from the hybridized Ni 3d (t *2g ) and O 2p orbital peak to the predominated Ti 3d orbital peak in CB baffles the charge-transfer transitions by both Ni 3d → Ti 3d and O 2p → Ti 3d transitions under visible light.
Ruiz-Preciado et al. [37] argued that the band gap and optical properties of NiTiO3 could be modified by the size of (NiTiO3) n nanoclusters or the surface roughness (~19 nm) of NiTiO3 thin films [25]. They suggested that the photocatalytic efficiency of NiTiO3 may be improved by a doping procedure to increase the concentration of photogenerated charge carriers and their lifetime.
In summary, NiTiO3 has a narrower band gap than TiO2 and also selectively absorbs visible light. Nevertheless, NiTiO3 has a crystal structure consisting of alternating NiO6 and TiO6 layers and induces a wide energy gap from the hybridized Ni 3d and O 2p orbitals to the predominated Ti 3d orbitals, which blocks both Ni2+→Ti4+ and O 2p → Ti 3d charge-transfer transitions. These baffle the photocatalytic performance of NiTiO3. The yellow color of NiTiO3 originates from its selective absorption of visible light and reflects the Ni: d → d charge-transfer transitions occurring in the valence band.
4 Conclusions
We synthesized NiTiO3 nanocrystallites via a solid-state reaction after the precursor being prepared by a homogeneous precipitation method. The nanosized NiTiO3 is a direct-gap semiconducting material with a band gap of 3.00 eV. It selectively absorbs visible light at 448, 502, and 743 nm. The stable wavelengths of the absorption peaks imply that they originate from the Ni: d → d charge-transfer transitions within the valence band. The NiTiO3 nanoparticles do not exhibit obvious photocatalytic activity as being used in degrading methylene blue. The mixed octahedron layer crystal structure and the wide energy gap are two suggested factors which affect the photocatalytic activity of NiTiO3.
References
Y. Ishikawa, S. Sawada, J. Phys. Soc. Jpn. 11, 496 (1956)
R.S. Singh, T.H. Ansari, R.A. Singh, B.M. Wanklyn, Mater. Chem. Phys. 40, 173 (1995)
L. Zhou, S.-Y. Zhang, J.-C. Cheng, L.-D. Zhang, Z. Zeng, Mater. Sci. Eng. B 49, 117 (1997)
Y.-J. Lin, Y.-H. Chang, W.-D. Yang, B.-S. Tsai, J. Non-Cryst. Solids 352, 789 (2006)
S.-H. Chuang, M.-L. Hsieh, D.-Y. Wang, J. Chin. Chem. Soc. 59, 628 (2012)
C. Xin, Y. Wang, Y. Sui, Y. Wang, X. Wang, K. Zhao, Z. Liu, B. Li, X. Liu, J. Alloys Compd. 613, 401 (2014)
S. Moghiminia, H. Farsi, H. Raissi, Electrochim. Acta 132, 512 (2014)
T. Acharya, R.N.P. Choudhary, J. Eelectron. Mater. 44, 271 (2015)
G.R. Rossman, R.D. Shannon, R.K. Waring, J. Solid State Chem. 39, 277 (1981)
I.V. Pishch, E.V. Radion, Glass Ceram. 60, 154 (2003)
R. Levinson, P. Berdahl, H. Akbari, Sol. Energy Mater. Sol. Cells 89, 351 (2005)
J.-L. Wang, Y.-Q. Li, Y.-J. Byon, S.-G. Mei, G.-L. Zhang, Powder Technol. 235, 303 (2013)
Y. Tong, J. Fu, Z. Chen, J. Nanomater. 2016, 5464978 (2016)
P. Salvador, C. Gutierrez, J.B. Goodenough, Appl. Phys. Lett. 40, 188 (1982)
P. Salvador, C. Gutiérrez, J.B. Goodenough, J. Appl. Phys. 53, 7003 (1982)
P.H.M. de Korte, G. Blasse, J. Solid State Chem. 44, 150 (1982)
L.G.J. de Haart, A.J. de Vries, G. Blasse, Mater. Res. Bull. 19, 817 (1984)
X. Shu, J. He, D. Chen, Ind. Eng. Chem. Res. 47, 4750 (2008)
Y.-J. Lin, Y.-H. Chang, G.-J. Chen, Y.-S. Chang, Y.-C. Chang, J. Alloys Compd. 479, 785 (2009)
M.S. Sadjadi, M. Mozaffari, M. Enhessari, K. Zare, Superlattice Microst. 47, 685 (2010)
K.S. Beenakumari, J. Exp. Nanosci. 8, 203 (2013)
P. Yuan, C. Fan, G. Ding, Y. Wang, X. Zhang, Int. J. Miner. Metall. Mater. 19, 372 (2012)
Y. Qu, W. Zhou, Z. Ren, S. Du, X. Meng, G. Tian, K. Pan, G. Wang, H. Fu, J. Mater. Chem. 22, 16471 (2012)
A. Sobhani-Nasab, S.M. Hosseinpour-Mashkani, M. Salavati-Niasari, H. Taqriri, S. Bagheri, K. Saberyan, J. Mater. Sci. Mater. Electron. 26, 5735 (2015)
M.A. Ruiz-Preciado, A. Bulou, M. Makowska-Janusik, A. Gibaud, A. Morales-Acevedo, A. Kassiba, CrystEngComm 18, 3229 (2016)
A.L. Linsebigler, G. Lu, J.T. Yates, Chem. Rev. 95, 735 (1995)
R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki, Y. Taga, Science 293, 269 (2001)
K.P. Lopes, L.S. Cavalcante, A.Z. Simões, J.A. Varela, E. Longo, E.R. Leite, J. Alloys Compd. 468, 327 (2009)
E. Matijević, Chem. Mater. 5, 412 (1993)
X.M. Gao, M.W. Li, Y.L. Hou, C.Y. Wang, Mater. Res. Innov. 19, 1 (2015)
C.R. Hubbard, E.H. Evans, D.K. Smith, J. Appl. Cryst. 9, 169 (1976)
K.T. Jacob, V.S. Saji, S.N.S. Reddy, J. Chem. Thermodyn. 39, 230 (2007)
D.H. Kim, H.S. Park, S.-J. Kim, K.S. Lee, Catal. Lett. 106, 29 (2006)
J. Tauc, R. Grigorovici, A. Vancu, Phys. Status Solidi 15, 627 (1966)
M.A. Ruiz-Preciado, A. Kassiba, A. Gibaud, A. Morales-Acevedo, Mater. Sci. Semicond. Proc. 37, 171 (2015)
R. Vijayalakshmi, V. Rajendran, E. J. Chem. 9, 282 (2012)
M.A. Ruiz Preciado, A. Kassiba, A. Morales-Acevedo, M. Makowska-Janusik, RSC Adv. 5, 17396 (2015)
N. Pal, B. Saha, S.K. Kundu, A. Bhaumik, S. Banerjee, New J. Chem. 39, 8035 (2015)
T. Umebayashi, T. Yamaki, H. Itoh, K. Asai, J. Phys. Chem. Solids 63, 1909 (2002)
F. Tian, C.B. Liu, J. Phys. Chem. B 110, 17866 (2006)
N. Martsinovich, D.R. Jones, A. Troisi, J. Phys. Chem. C 114, 22659 (2010)
H.Y. Lee, J. Robertson, J. Appl. Phys. 113, 213706 (2013)
X.-B. Feng, N.M. Harrison, Phys. Rev. B 69, 035114 (2004)
F. Tran, P. Blaha, Phys. Rev. Lett. 102, 226401 (2009)
G.A. Sawatzky, J.W. Allen, Phys. Rev. Lett. 53, 2339 (1984)
R. Gillen, J. Robertson, J. Phys. Condens. Matter 25, 165502 (2013)
R. Newman, M. Chrenko, Phys. Rev. 114, 1507 (1959)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, MW., Yuan, JP., Gao, XM. et al. Structure and optical absorption properties of NiTiO3 nanocrystallites. Appl. Phys. A 122, 725 (2016). https://doi.org/10.1007/s00339-016-0259-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-016-0259-5