Abstract
The effects of in situ-deposition temperature and post-deposition annealing on the microstructure of the sputtered SnO2 thin film are studied. These effects on the performance of the resulting gas-sensing devices have been characterized. For in situ-deposition temperature, the film was deposited on a heated substrate, while for post-deposition annealing, the film was deposited on an unheated substrate and then annealed it at various temperatures. Microstructure changes in SnO2 film were induced at various temperatures. Both films of in situ heating and post-annealing evoked only partial crystallization, especially at low temperatures, and the crystallinity was enhanced with high temperatures. SEM images confirmed that the grains and pores of the film changed with the thermal treatment. In consequence of the post-annealing, the pores grew wider; however, they grew narrow in the case of in situ heating. The film annealed at low temperature yielded high-response device to NO2 gas compared with that annealed at higher temperature. The sensing devices fabricated by in situ deposition showed highest response.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nitrogen dioxide (NO2), which is the most common oxide of nitrogen, is toxic, highly reactive oxidant, and corrosive gas. The most important sources of NO2 are internal combustion engines, thermal power stations, etc. [1], as known that NO2 causes an impairing in the lung function and respiratory system with long-term exposure [2]. Therefore, it is essential to develop a high-performance sensor for easily detecting NO2 gas. Gas sensors made of oxide semiconductors have been widely used as domestic and industrial gas sensors because of their high sensitivity, quick response, and low cost [3]. However, most of these gas sensors are operable at a high temperature of a sensing element of above 200 °C, which results in high power consumption and complexity in the integration [4–9]. Therefore, most researchers have focused their attention on the sensing material to improve the sensing properties [10–12], and some researchers have focused on the effect of electrode material and geometry on the sensing properties [13–15].
Advanced oxidation processes in the presence of semiconductor and metal oxide have received important attention in the last decade for gas sensor application. There is a considerable interest in tin oxide (SnO2) as one of the most promising n-type semiconductor material for different applications because of its physical, chemical properties and effective cost [16–18]. SnO2 film is used in many applications, such as gas sensor [19] and transparent electrode [16]. Several chemical and physical methods, such as sol–gel [20], ultrasonic spray pyrolysis [21], chemical vapor deposition [22], pulsed laser deposition [23], and sputtering [24], have been used to prepare SnO2 film. Among them, the reactive sputtering deposition has shown the advantages of controlling the crystalline orientation, growing temperatures, interfacial adhesion to the substrate, and density of the grown film. However, the thermal treatment process to stabilize or improve the operation performance of gas sensor sometimes is required for amorphous thin films deposited by sputtering technique. The thermal treatment leads to the change in the microstructure factors, such as crystallite size, pore size distribution, density of adsorption sites and the density of the film. Consequently, the control and the effect of microstructure possess the attention to control and improve the performance of the sensing material of oxide [25–27].
According to our knowledge, many experimental and theoretical studies have been performed and reviewed for SnO2 thin film [28–30]. However, the interest in the present study is the influence of in situ heating and post-annealing on the surface morphology and NO2 sensing properties of sensor fabricated by using sputtering technique. The effect of the synthesis conditions on the specific surface area, and pore structure of DC sputtering thin film which is to be used to make gas sensor are investigated. The sensing property is carried out for the SnO2 sensor to NO2 gas at various surface temperatures as well as NO2 concentrations.
2 Experimental details
2.1 Preparations of SnO2 film
A film with a thickness of 100 nm was deposited on oxidized silicon substrate by reactive DC magnetron sputtering using a circular tin target with a diameter of 100 mm. The purity of the tin target was 99.99 %. After the sputtering chamber was evacuated to a background pressure below 2 × 10−4 Pa, the chamber was maintained at mixture gas of air and O2 with a ratio of 1:1, and discharge gas pressure of 20 Pa, and then the SnO2 film was reactively deposited at a DC input power of 17 Watt with a deposition rate of 1 nm/min. For post-annealing, the film was firstly deposited and then annealed for 1 h in ambient air at temperature of 350, 500, and 650 °C. For in situ heating, the substrate was maintained at temperature of 200, 350, and 500 °C, and then SnO2 film was deposited. Before the deposition, the target was pre-sputtered for 5 min with controlling a moveable shutter placed between the target and substrate.
2.2 Characterization of SnO2 film
The crystallographic structure of the sputtered film was investigated by X-ray diffraction (XRD) using an X-ray diffractometer (Shimadzu XRD-6100) with Cu Kα source in the range of 2θ = 15°–65°. The morphology was observed by field emission scanning electron microscope (FESEM) (JEOL JSM-6700F). Pore surface area and pore size distribution were determined by using argon gas and the adsorption–desorption method described in Refs. [31, 32]. For the measurement of the physical adsorption isotherms, the film was deposited on the front and rear surfaces of 4-in Si wafer, both sides of the wafer were mirror-polished and oxidized. About 50 strips with a size of 25 × 5 mm2 were cut out of the wafer and introduced into the sample cell of the physical adsorption apparatus.
2.3 Gas-sensing properties of SnO2 film
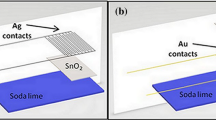
For investigation of NO2 gas-sensing properties, SnO2 film of 100 nm thick was deposited on a pair of interdigitated Pt electrodes which have a thickness of 100 nm, an area of 25 mm2, and a gap size of 20 µm, as shown in the scheme of Fig. 1. The sensors were placed in a quartz tube, which was inserted into an electric furnace. The operating temperature was varied from RT to 300 °C. Dry synthetic air, mixed with NO2 gas, was passed at a rate of 200 ml/min. It was possible with a computer-controlled measurement system to control both temperature and gas atmosphere in the measurement chamber during the operations. In order to have comparable results, the samples were measured at the same conditions so that the environment and the state of the semiconductor surface would be the same. Moreover, the measurements of gas sensing were made twice at long interval in order to monitor the reproducibility and stability of the sensor. The sensor response (S) was defined by S = (R g − R a)/R a, where R a is the resistance in the air, and R g is the resistance after introduction of gas.
3 Results and discussions
3.1 Microstructure characteristics
Typical XRD patterns recorded for the SnO2 film are shown in Fig. 2. For as-deposited film, the film is mainly amorphous with a broadening peak observed around 33°. The post-annealing temperature of 350 °C is somewhat sufficient for partial crystallization of SnO2, as shown from the peaks observed in 26.32°, 34.02°, and 61.75°. These peak intensities increased when the annealing temperature increased up to 500 °C. The same peaks for a high temperature of 650 °C film are observed with a slight increase in the intensity. These peaks were indexed on a tetragonal structure (JCPDS No. 21-1250) of SnO2 for (110), (101), (211), and (310) planes. The as-deposited and post-annealed films are partially crystallized, as known that the energy required for crystallization is related to the melting point of the formed material. The partial crystallization can be induced at low temperature if the in situ heating is applied to the substrate during the deposition. This can be seen in Fig. 3. From XRD charts of in situ heating, the change in the crystallinity of film with the temperature is hardly observed, comparing with that of post-annealing.
In the in situ-heated film, the temperature during deposition causes the surface atoms just landed not to be frozen in at their landing sites, but diffuse by the available thermal energy on the surface of substrate. Thus, the aggregations of nanocrystals are due to the thermal energy of the substrate surface, where surface adatom mobility occurs in each atomic layer during the deposition and it is dictated by the heating during the deposition [33]. As a consequence of the adatom mobility, there is in each layer an atomic environment which is sufficiently close to the crystalline SnO2. Therefore, moderate deposition temperatures are sufficient for partial crystallization. Unlike in the case of a deposition at unheated substrate, atoms arrive and settle at their landing locations with limited mobility for rearrangement for crystal formation and only short-range ordering might exist. This situation is happening in each layer and when the process of deposition is stopped, the structure remains frozen in. Therefore, high temperatures will be required to rearrange the atoms to the required crystalline formation.
The FESEM images of SnO2 film are shown in Fig. 4. Both of the in situ-heated and post-annealed films are composed of grains and pores. The grains contact with each other through the necks, and pores are observed throughout the film. At post-annealing temperature of 350 °C, the partial crystallization of the film affected the grain size, grain contacts, and pore size, as shown in Fig. 4a. The average grain size of the film annealed at low temperatures of 350 °C was ~50 nm. The grains as well as the pores grew up when the post-annealing temperature raised up to 500 °C. Large grains can be seen at a high temperature of 650 °C, and accompanied large pores can be also seen, indicating the development of pore size during the annealing. The formation of large pores is owing to the porosity and the low density of the as-deposited film due to the high discharge pressure of 20 Pa [30, 34]. Unlike in the case of in situ-heating treatment, the small grains surrounded by short pores are observed at low temperature of 200 °C. The grains grew larger and are surrounded with few large pores at a substrate temperature of 350 °C. The film became denser, and the grain boundaries can be lightly seen where the pores reduced to a very small size. However, when the density of the film was measured, it was much lower than that of a tin oxide bulk, indicating that the film is still porous.
For deeper understanding the pore size distribution of SnO2 film, the pore size distribution was measured by the physical adsorption–desorption method. Barrett–Joyner–Halenda (BJH) method was used to calculate the total pore volume and pore surface area which were measured by using argon gas. The pore size distribution of the in situ-heated and post-annealed film is shown in Fig. 5. In the post-annealed film, the pores of the film annealed at 350 °C are mostly distributed in the range of 1.5–10.0 nm and have a peak at 2.7 nm which corresponds to a specific area of ~380 cm2/g. The pores of the film annealed at 500 °C are distributed in the range of 2.0–6.5 nm and have a peak at 2.7 nm which corresponds to a specific area of ~315 cm2/g. One can also see that the film annealed at 650 °C shows a pore size distribution between small size of 1.8–3.0 nm and large size of 10–15 nm with peaks at 2 and 13 nm and specific areas of 210 and 45 cm2/g, respectively. The result of the post-annealing samples indicated that although the pores exceeded, the specific area reduced. The samples prepared on heated substrates have pores distributed in the range of 0.6–25.0, 0.6–15.0, and 0.6–7.0 nm for the film deposited at 200, 350, and 500 °C, respectively. In the later films, the pore distributed in the range of 0.6–5 nm is dominated and has large specific area. The range of pore distribution of in situ-deposition film is wider than that of post-annealing film. The pore size distribution, specific area of the peaks, and total pore volume are shown in Table 1. The total pore volume of the corresponding film is also presented in Fig. 6. The in situ- and post-deposition treatment caused a reduction in the total pore volume. The total pore volume of the post-annealed film is dramatically reduced at high temperatures, whereas it was slightly reduced for the film deposited on heated substrate. According to the context of physisorption reports, the pores observed in the present film are classified as mesopores [32].
3.2 NO2 gas-sensing characteristics
The signal of SnO2 sensors upon exposure to different concentrations of NO2 gas at operating temperature of 150 °C is shown in Fig. 7. The resistance increased to a steady value when the sensor was exposed to NO2 gas and then decreased when the gas was switched off. The resistance further increased as the sensors are being exposed to more concentration of NO2. This indicates that NO2 undergoes a reversible oxidizing interaction with the tin oxide surface. The change in resistance with concentration is remarkably observed in the resistance of a post-annealed sensor than in the in situ-heated sensors. Generally, the adsorbed layer of gas left the surface of oxide soon after the end of gas exposure, bringing the sensor signal back approximately to its baseline, indicating a good reversibility of these sensors. The very similar response to sequential gas pulse of the same concentrations showed a high reproducibility of the sensor response. Remarkably, very little baseline drift has occurred after the final concentration of NO2 had been reached. It was reported that this small drift occurs due to the poison reaction on the surface of tin oxide [28], when the sensor takes more time to return to its baseline.
The response of sensors upon exposure to 2 ppm concentration at various operating temperatures is shown in Fig. 8. For post-annealed sensor, the sensor is slightly sensitive for NO2 at low operating temperatures, and its response increased with operating temperature. The sensor became highly sensitive at operating temperature of 100 °C, and it became again less sensitive at higher temperatures. The peak response observed in the post-annealed sensor is 338, 213, and 24 for annealing temperatures of 350, 500, and 650 °C, respectively. With increasing the annealing temperature up to 500 °C, the response slightly decreased, while drastically decreased at 650 °C, as shown in Fig. 8a. The similar behavior of operating temperature dependence of the response curve for post-annealed sensors was observed for in situ-heated sensors, as shown in Fig. 8b. Interestingly, the response peak was shifted to lower operating temperature of 100 °C for the sensor deposited at a substrate temperature of 500 °C instead of the operating temperature of 150 °C for the sensors deposited at 200 and 350 °C. This might be attributed to the change in grain size, which can lead to a change in both the magnitude and operating temperature of the response peak [35, 36]. The highest responses obtained to 2 ppm NO2 were 516, 480, and 200 for the sensors deposited at 200, 350, and 500 °C, respectively. Thus, if we consider the operation of present sensors at low temperatures, such as RT or 50 °C, the in situ-heated sensor is highly sensitive for NO2 gas and thus consumes a low energy because of its low operating temperature. If we consider the sensor to be operated with the highest response, the in situ-heated sensor has a good performance and response to energy consumption slightly higher than that of post-annealed sensor which has lower response and operating temperature.
It is well known that the oxygen vacancies and oxygen species play a role in determining the electrical transport properties of SnO2 [28, 37]. For SnO2 film sputtered in an ambiance of oxygen, there should be many oxygen species existed inside the film in the form of chemisorption. The chemisorbed oxygen species trap the electrons from the bulk and effectively compensate the oxygen vacancies. With the re-crystallization of SnO2 film upon thermal treatment, these oxygen species are desorbed. The film also undergoes an oxidation process, and consequently the oxygen vacancy density is reduced at high temperatures. Since the oxidation process is not probable at low temperatures, disruption of oxygen species dominated in case of low temperatures and caused an increase in the oxygen vacancy concentration. With further increase in the temperature, oxygen molecules could get sufficient energy to react with the uncoordinated tin atoms and thus the oxygen vacancies could be reduced [38]. In addition to the change in concentration of oxygen vacancies due to the thermal treatment, the pore volume of the film was also affected. From Figs. 6 and 8, the results are in a harmony and reflect the strong correlation between the total pore volume and the response of sensor. Highly sensitive sensor possesses a high total pore volume, whereas the less sensitive sensor possesses a less value. Figure 9 shows the sensor response to various NO2 concentrations of 1–10 ppm. The response of sensors increased when the sensors were exposed to more concentration of NO2. The in situ-heated sensors are highly sensitive at lower concentrations than the post-annealed sensors. At high concentrations, both the in situ-heated and post-annealed sensors are highly sensitive, and the highest response of 1770 was observed in the post-annealed sensor of 350 °C, indicating the ability of this sensor for receiving a lot of gas concentration because of its high total pore volume. The in situ-heated sensors are superior in detecting and monitoring a low concentration of NO2 gas, whereas the post-annealed sensors are less sensitive at these concentrations although they are highly sensitive at higher concentrations. Generally, the result reflects the good ability and reliability of sensors in detecting various NO2 concentrations.
4 Conclusions
In case of post-deposition temperature, the as-deposited SnO2 film is amorphous and in order to induce structural stability, a thermal treatment is essentially required. The moderate deposition temperature of in situ heating is sufficient to induce partial crystallization in the film. XRD charts of SnO2 film show the same peaks in case of in situ heating or post-annealing. Both of the in situ-heated and post-annealed films are composed of grains and pores, and the grains contact with each other through necks. The grains and the pores grow up when the post-annealing temperature raises, contrasting in the case of in situ heating. The film became dense and the grain boundaries can be lightly seen by SEM where the pores reduced to very small sizes at high temperatures. For deeper understanding the pore size distribution of SnO2 film, the pore size distribution was measured by the physical adsorption–desorption method. The response of sensors upon exposure to 2 ppm concentration of NO2 gas at various operating temperatures is investigated. Both post-annealing and in situ-heating sensors possess similar behavior of operating temperature dependence of sensor response. Interestingly, the response peak was shifted to lower operating temperature of 100 °C for the sensor deposited at a substrate temperature of 500 °C instead of 150 °C for the sensors deposited at 200 and 350 °C. The response of sensors of in situ heating is higher than that of the sensor of post-annealing. The response is fairly good for NO2 gas at various temperatures and concentrations. The results are in a harmony and reflect the strong correlation between the total pore volume and the response of sensor.
References
B. Son, W. Yang, P. Breysse, T. Chung, Y. Lee, Estimation of occupational and nonoccupational nitrogen dioxide exposure for Korean taxi drivers using a microenvironmental model. Environ. Res. 94, 291–296 (2004)
Bonn, Germany January 2003, Report on a WHO working group, health aspects of air pollution with particulate matter, ozone and nitrogen dioxide, (2003), pp. 1–92
S.M. Kanan, O.M. El-Kadri, A.I. Abu-Yousef, M.C. Kanan, Semiconducting metal oxide based sensors for selective gas pollutant detection. Sensors 9(10), 8158–8196 (2009)
G. Sberveglieri, S. Groppelli, P. Nelli, Highly sensitive and selective NOx and NO2 sensor based on Cd-doped SnO2 thin films. Sens. Actuators B 4, 457–461 (1991)
G. Williams, G.S.V. Coles, NOx response of tin dioxide based gas sensors. Sens. Actuators B 16, 349–353 (1993)
G. Sberveglieri, G. Faglia, S. Groppelli, P. Nelli, Methods for the preparation of NO, NO2 and H2 sensors based on tin oxide thin films, grown by means of the r.f. magnetron sputtering technique. Sens. Actuators B 8, 79–88 (1992)
G. Faglia, P. Nelli, G. Sberveglieri, Frequency effect on highly sensitive NO2 sensors based on RGTO SnO2(Al) thin films. Sens. Actuators B 19, 497–499 (1994)
G. Leo, R. Rella, P. Siciliano, S. Capone, J.C. Alonso, V. Pankov, A. Ortiz, Sprayed SnO2 thin films for NO2 sensors. Sens. Actuators B 58, 370–374 (1999)
I. Hwang, S. Kim, J. Choi, J. Choi, H. Ji, G. Kim, G. Cao, J. Lee, Synthesis and gas sensing characteristics of highly crystalline ZnO–SnO2 core–shell nanowires. Sens. Actuators B 148, 595–600 (2010)
N. Yamazoe, New approaches for improving semiconductor gas sensors. Sens. Actuators B 5, 7–19 (1991)
A. Kolmakov, M. Moskovits, Chemical sensing and catalysis by one-dimensional metal-oxide nanostructures. Annu. Rev. Mater. Res. 34, 151–180 (2004)
A. Sharma, M. Tomar, V. Gupta, Low temperature operating SnO2 thin film sensor loaded with WO3 micro-discs with enhanced response for NO2 gas. Sens. Actuators B 161, 1114–1118 (2012)
J. Tamaki, J. Niimi, S. Ogura, S. Konishi, Effect of micro-gap electrode on sensing properties of dilute chlorine gas of indium oxide thin film microsensors. Sens. Actuators B 117, 353–358 (2006)
N.M. Shaalan, T. Yamazaki, T. Kikuta, Effect of micro-electrode geometry on NO2 gas-sensing characteristics of one-dimensional tin dioxide nanostructure microsensors. Sens. Actuators B 156, 784–790 (2011)
A. Ylinampa, V. Lantto, S. Leppavuori, Some differences between Au and Pt electrodes in SnO2 thick-film gas sensors. Sens. Actuators B 14, 602–604 (1993)
T. Minami, Transparent conducting oxide semiconductors for transparent electrodes. Semicond. Sci. Technol. 20, S35–S44 (2005)
S.S. Lekshmya, G.P. Daniel, K. Joy, Microstructure and physical properties of sol gel derived SnO2: Sb thin films for optoelectronic applications. Appl. Surf. Sci. 274, 95–100 (2013)
Z. Jie, H. Li-Hua, G. Shan, Z. Hui, Z. Jing-Gui, Alcohols and acetone sensing properties of SnO2 thin films deposited by dip-coating. Sens. Actuators B 115, 460–464 (2006)
B. Wei, M. Hsu, P. Su, H. Lin, R. Wu, H. Lai, A novel SnO2 gas sensor doped with carbon nanotubes operating at room temperature. Sens. Actuators B 101, 81–89 (2004)
L. Francioso, A. Forleo, S. Capone, M. Epifani, A.M. Taurino, P. Siciliano, Nanostructured In2O3–SnO2 sol–gel thin film as material for NO2 detection. Sens. Actuators B 114, 646–655 (2006)
V. Bilgin, S. Kose, F. Atay, I. Akyuz, The effect of Zn concentration on some physical properties of tin oxide films obtained by ultrasonic spray pyrolysis. Mater. Lett. 58, 3686–3693 (2004)
R.Y. Korotkov, P. Ricou, A.J.E. Farran, Preferred orientations in polycrystalline SnO2 films grown by atmospheric pressure chemical vapor deposition. Thin Solid Films 502, 79–87 (2006)
A.M. Serventi, R. Dolbec, M.A. El Khakani, R.G. Saint-Jacques, D.G. Rickerby, High-resolution transmission electron microscopy investigation of the nanostructure of undoped and Pt-doped nanocrystalline pulsed laser deposited SnO2 thin films. J. Phys. Chem. Solids 64, 2097–2103 (2003)
A.S. Reddy, N.M. Figueiredo, A. Cavaleiro, Nanocrystalline Au:Ag:SnO2 films prepared by pulsed magnetron sputtering. J. Phys. Chem. Solids 74, 825–829 (2013)
G. Korotcenkov, The role of morphology and crystallographic structure of metal oxides in response of conductometric-type, gas sensors. Mater. Sci. Eng. R 61, 1–39 (2008)
M. Hayashi, T. Hyodo, Y. Shimizu, M. Egashira, Effect of microstructure of mesoporous SnO2 powders on their H2 sensing properties. Sens. Actuators B 141, 465–470 (2009)
H. Ahn, J.H. Noh, S. Kim, R.A. Overfelt, Y.S. Yoon, D. Kim, Effect of annealing and argon-to-oxygen ration on sputtered SnO2 thin film sensor for ethylene gas detection. Mater. Chem. Phys. 124, 563–568 (2010)
B. Ruhland, T. Becker, G. Muller, Gas-kinetic interactions of nitrous oxides with SnO2 surfaces. Sens. Actuators B 50, 85–94 (1998)
A. Sharma, M. Tomar, V. Gupta, SnO2 thin film sensor with enhanced response for NO2 gas at lower temperatures. Sens. Actuators B 156, 743–752 (2011)
Y. Shen, T. Yamazaki, Z. Liu, C. Jin, T. Kikuta, N. Nakatani, Porous SnO2 sputtered films with high H2 sensitivity at low operation temperature. Thin Solid Films 516, 5111–5117 (2008)
T. Yamazaki, T. Furuta, Y. Shen, C. Jin, T. Kikuta, N. Nakatani, Effective surface area of SnO2 sputtered films evaluated by measurement of physical adsorption isotherms. Jpn. J. Appl. Phys. 45, 9180–9184 (2006)
K.S.W. Sing, D.H. Everett, R.A.W. Haul, L. Moscou, R.A. Pierotti, J. Rouquerol, T. Siemieniewska, “Reporting physisorption data for gas/solid systems” with special reference to the determination of surface area and porosity. Pure Appl. Chem. 57, 603–619 (1985)
J. Pelleg, G. Sade, M. Sinder, D. Mogilyanski, Compositional and structural changes in TiB2 films induced by bias, in situ and post-deposition annealing, respectively. Phys. B 381, 118–127 (2006)
Z. Liu, T. Yamazaki, Y. Shen, T. Kikuta, N. Nakatani, Influence of annealing on microstructure and NO2-sensing properties of sputtered WO3 thin Films. Sens. Actuators B 128, 173–178 (2007)
G. Korotcenkov, V. Brinzari, J. Schwank, A. Cerneavschi, Possibilities of aerosol technology for deposition of SnO2-based films with improved gas sensing characteristics. Mater. Sci. Eng. C 19, 73–77 (2002)
N.M. Shaalan, T. Yamazaki, T. Kikuta, Influence of morphology and structure geometry on NO2 gas-sensing characteristics of SnO2 nanostructures synthesized via a thermal evaporation method. Sens. Actuators B 153, 11–16 (2011)
C. Ke, W. Zhu, J.S. Pan, Z. Yang, Annealing temperature dependent oxygen vacancy behavior in SnO2 thin films fabricated by pulsed laser deposition. Curr. Appl. Phys. 11, S306–S309 (2011)
A.F. Khan, M. Mehmood, A.M. Bhatti, Effect of annealing on electrical resistivity of rf-magnetron sputtered nanostructured SnO2 thin film. Appl. Surf. Sci. 255, 8562–8565 (2009)
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shaalan, N.M., Rashad, M. & Yamazaki, T. Sensing performance of SnO2 film fabricated by sputtering deposition. Appl. Phys. A 120, 1555–1563 (2015). https://doi.org/10.1007/s00339-015-9355-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-015-9355-1