Abstract
Hybridisation is an evolutionary process that generates genetic diversity in organisms. However, the relationship between reproductive features, such as spawning synchronisation and gamete compatibility, and the degree of introgression leading to hybridisation are poorly understood. The reef-building coral Acropora spp. have a complex evolutionary history, and the link between their ecology, life-history traits, and potential to hybridise is disputed. Here, we examined the relationship among the reproductive features involved in the intercrossing of three species, Acropora florida, Acropora gemmifera, and Acropora intermedia, at two sites: Akajima and the Sesoko islands in southern Japan. Although the examined species showed synchronous spawning and high rates of gamete compatibility, spawning synchronisation and gamete compatibility were less strongly associated with high rates of interbreeding among the three species. Model-based genetic clustering and site-pattern frequency-based tests with single nucleotide polymorphisms supported genetic admixture among the three species in each location. Demographic analyses using fastsimcoal implied that the admixture among the three species in each location might have occurred in the past (> 2,000 generations) and recently (< 50 generations). Furthermore, the recent admixture of these three species is potentially associated with heavy bleaching events and population declines. The principal component analysis, structure, and fastsimcoal showed that the extensive admixture of A. intermedia and A. gemmifera on Sesoko Island occurred recently. Therefore, gamete interactions that lead to hybridisation in the field must be clarified. Furthermore, the connectivity between the two locations needs to be identified; however, our results implied that population fluctuations could be associated with introgression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most widely used concept regarding biological species is that ‘a species’ comprises interbreeding populations (Mayr 1963; Stankowski and Ravinet 2021), and reproductive isolation is crucial for its maintenance. Hybridisation often threatens the long-term survival of species by suppressing assimilation and inbreeding (Frankham et al. 2002) and contributing to species extinction (Levin et al. 1996; Rhymer & Simberloff 1996). Under the species concept of Mayr (1963), hybridisation can threaten to blur the lines of what is considered a species. In contrast, Veron (1995) hypothesised that coral species are parts of the syngameon, which is defined as a species linked by hybridisation that lacks strong reproductive barriers (Grant 1957). In the coral genus Acropora, some Acropora species consist of syngameons (Miller and Benzie 1997; Diekmann et al. 2001; van Oppen et al. 2002), although van Oppen et al. (2002) indicated an important difference between the syngameons of Acropora and those of other organisms, which is the distributional pattern of hybridising species: overlapping distributions for corals vs parapatric distributions for other organisms.
The genus Acropora is species-rich (135 species, Hoeksema and Cairns 2023), with diverse morphologies, such as tabular, arborescent, and bushy-shaped colonies. Hybridisation occurs repeatedly in Acropora (Wallace 1999). In the Caribbean Sea, F1 hybrids occur in nature, and introgression among parental species has been suggested (e.g. Nylander-Asplin et al. 2021; Vollmer and Palumbi 2004). In contrast, despite the presumed importance of hybridisation in diversity (Willis et al. 2006), evidence of hybrids in nature is limited in the Indo-Pacific (Fukami et al. 2019). This lack of evidence may be due to the species richness and morphological diversity of corals. Furthermore, hybrids and parental lineages are difficult to identify because of their morphology and confusing taxonomy (Richards et al. 2013). Most Acropora species are not monophyletic, although they are divided into five or more genetically closely related groups, including several species, based on mitochondrial and nuclear gene trees (Cowman et al. 2020; Márquez et al. 2002; Nakajima et al. 2012; van Oppen et al. 2001; Vollmer and Palumbi 2004; Wolstenholme et al. 2004), further complicating the interpretation of such patterns due to the inherent difficulty of distinguishing between the processes underlying them (i.e. hybridisation vs. incomplete lineage sorting; Funk and Omland 2003; Degnan and Rosenberg 2009).
Species boundaries and hybridisation in Acropora depend on pre- and post-zygotic reproductive isolation. Pre-zygotic traits include the synchronisation of spawning and fertilisation specificity, and post-zygotic traits include the mortality of larvae. Several congeneric sympatric species spawn synchronously (Babcock et al. 1986; Hayashibara et al. 1993), and cross-fertilisation has been shown by more than 20 sympatric species with sympatric synchronous spawning under in vitro experiments (e.g. Fukami et al. 2003; Hatta et al. 1999; van Oppen et al. 2002; Vollmer and Palumbi 2004; Willis et al. 1997, 2006). Although gametes are compatible with heterospecifics, hybridisation occurs only conditionally (Kitanobo et al. 2016; Willis et al. 2006). For example, eggs can be fertilised by heterospecific sperm when the number of sperms is limited (Kitanobo et al. 2016). In other words, prezygotic barriers may play essential roles at species boundaries. In contrast, the post-zygotic barriers are weak in several species. Hybrid larvae have high survivability (Chan et al. 2018; Isomura et al. 2013) and sexual reproduction (Isomura et al. 2016). As the pre-zygotic barrier has a stronger effect on corals than the post-zygotic barrier, hybridisation is favoured when there is a reduction in the number of individuals in a population (Kitanobo et al. 2016 and 2022b); however, the hybridisation process and its degree are not completely understood. In addition, the involvement of reproductive features, such as spawning synchronisations and gamete compatibility, has not been extensively investigated.
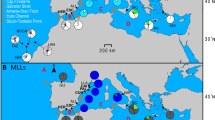
At the population level, spawning synchronicity varies between intercrossing species, and consequently, the degree of hybridisation may differ. For example, the overlap in the spawning times of Acropora spp. differs between the Akajima and Sesoko islands. The two islands are located 75 km apart in the Ryukyu Archipelago in southern Japan. Both islands have long been sites of research on coral spawning (Baird et al. 2021a; Isomura and Fukami 2018), partly because of the presence of laboratories on both islands. On Akajima Island, multi-specific synchronous spawning occurs almost every year, i.e. multiple species spawning at the same time (Hayashibara et al. 1993; Isomura and Fukami 2018). In contrast, the frequency of synchronous spawning around Sesoko Island is comparatively low (Baird et al. 2021a; Isomura and Fukami 2018). However, the relationship between the degree of spawning overlap and genetic introgression following hybridisation has not yet been verified.
Spawning synchronisations and gamete compatibility in introgressions could be associated with the intercrossing Acropora spp. Therefore, to understand the actual state or maintenance mechanisms of coral syngameons, this study aimed to compare the degree of introgression among populations of three Acropora species (A. florida, A. gemmifera, and A. intermedia) at two sites (Akajima Island and Sesoko Island) with different reproductive features. In addition, we analysed the genetic structure and whether genetic introgression occurred among the three intercrossing Acropora species.
Materials and methods
Coral colonies for genetic analyses and crossing experiments
We investigated two sites, Akajima Island (26° 12 'N, 127° 17' E) and Sesoko Island (26° 37' N, 127° 51' E), in southern Japan (Fig. 1). In the present study, we targeted three Acropora species: A. florida, A. gemmifera, and A. intermedia (Fig. 2a–c). The Acropora species were identified based on the taxonomic references (Wallace 1999). The latitudes and longitudes were recorded in 2018 on Akajima Island and in 2020 on Sesoko Island for A. florida, A. gemmifera, and A. intermedia. The latitude and longitude of each colony were obtained as follows: pairs of snorkelers were used to search for colonies of the target species. Once a colony was found, one tagged the colony, and the other stopped at the surface directly above it to acquire global positioning system (GPS) information. In addition to these three species, one colony on Akajima Island was categorised as a ‘presumed hybrid’, and based on morphology, the parental species of the presumed hybrids were inferred to be A. florida and A. intermedia (Fig. 2d, see in Fukami et al. 2019; Isomura et al. 2013). Thereafter, we collected branches (~ 10 cm) from the GPS-tagged colonies, removed a small piece (5 × 5 mm) from each branch, and preserved them in 2 mL of CHAOS for several days (modified guanidine solution: see Fukami et al., 2004). Total DNA was extracted from the CHAOS solution using the DNeasy Blood & Tissue Kit (QIAGEN, Venlo, Netherlands) and stored at − 20 °C for further genetic analyses. The sample remnants were bleached and deposited at the National Institute of Technology, Okinawa College (catalogue numbers: A. florida, ONCT_F1–ONCT_F44; A. gemmifera, ONCT_G1–ONCT_G68; A. intermedia, ONCT_I1–ONCT_I72; presumed hybrid, ONCT_FI1; real hybrids, ONCT_HYA, ONCT_HYB1 and ONCT_HYB2). The fragments were collected as follows: on Akajima Island, 8 colonies of A. florida, 24 A. gemmifera, 34 A. intermedia, and 1 presumed hybrid, and on Sesoko Island, 36 A. florida, 44 A. gemmifera, and 38 A. intermedia.
Spawning time analyses and cross experiments
To examine cross-fertilisation among the three species, fertilisation experiments were performed in 2007, 2013, 2014, 2015, and 2016 on Akajima Island and in 2012, 2013, 2014, 2017, 2018, 2019, and 2021 on Sesoko Island. Although most of the data (up to 2015) were presented by Isomura et al. (2013, 2016) and Kitanobo et al. (2016), we used them to estimate long-term fertilisation changes. The gametes were collected from the spawned colonies. To identify spawning activity, gamete bundles were checked at 20:30 (local time) and the set colonies were transferred to buckets. The spawning time was recorded, and the released gamete bundles were collected for crossing experiments. After collecting the bundles, the gametes were separated into sperm and eggs using a plankton mesh, as described previously (Nozawa et al. 2015). The eggs were washed twice with filtered seawater, and the sperm suspensions were diluted to the appropriate fertilisation concentration for Acropora.
To conduct the fertilisation experiments, eggs (approximately 100) and sperm (final concentration; 0.5–2.6*106/mL) were subsequently mixed in pairwise combinations within 2 h after spawning. Self-fertilisation experiments were conducted simultaneously. The number of fertilised and unfertilised eggs was scored at the 16-cell/morula stage 4–5 h after gamete mixing. The experiments were conducted in a room maintained at 26–27 °C.
The detailed information of colonies used in this study is listed in Table S1.
Coincidence of spawning time among the three species
After conducting experiments to determine the spawning date and time, we calculated ‘Date of Spawning Relative to the Nearest Full Moon’ (hereafter ‘DoSRtNFM’) using the time zone and date of observation for days before ( −) or after ( +) the nearest full moon (ranging from − 15 to + 14 d) to evaluate the relationship between moon age and spawning date in multiple years using the same criteria (Baird et al. 2021a).
MIG-seq analyses
We performed multiplexed inter-simple sequence repeat (ISSR) genotyping using sequence (MIG-seq) analysis, following the protocol described by Suyama and Matsuki (2015), to determine the degree of nuclear genetic admixture potentially caused by hybridisation. In brief, the MIG-seq method amplifies 100–1000 genome-wide single nucleotide polymorphisms (SNPs) around ISSR regions in two PCR steps. First, eight and four universal pairs of multiplex ISSR primers were used for the first PCR. DNA libraries with different indices were produced using a second PCR. PCR was conducted as previously described with some modifications. All PCR reactions were performed using PrimeStar HS (Takara, Shiga, Japan). All PCR products were pooled and sequenced using MiSeq (MiSeq Control Software v. 2.0.12, Illumina, San Diego, USA) and MiSeq Reagent v. 3 150-cycle Kit (Illumina). A total of 188 individuals were analysed: 44 A. florida, 68 A. gemmifera, 72 A. intermedia, one suspected hybrid, and three real hybrid individuals (Fig. 2e and f; see in Fukami et al. 2019).
HISAT2 (Kim et al. 2019) was used to map these sequences against the symbiotic algal reference genome (Cladocopium goreaui v. 1.0) to remove their sequences. Genome information of symbiotic algae was obtained from Reef Genomics (http://reefgenomics.org/) (Liew et al. 2016). After the symbiotic algal clade C1 sequence reads were removed, 32,723,390 reads remained unmapped.
Isolation of SNP with Stacks
In total, 32,855,176 raw reads, with an average of 343,706 reads per sample (SD = 172,586), were obtained from 188 individuals using MIG-seq analysis and 50,260 SNPs were isolated after filtering minor allele frequency (MAF) (< 0.05) and Hardy–Weinberg equilibrium (HWE; p < 0.00001) with PLINK 1.9. The coverage of the variant sites was approximately 10–36 times.
We used denovo_map.pl in Stacks v. 2.59 to generate the following analysis files: five nucleotide mismatches were allowed between stacks within individuals (-M 5) and paired-end reads (–samples–paired) were set. Files for each analysis, such as Structure and TreeMix, were converted according to the ‘popmap’ definition of colonies and species (“–popmap definition file -X “populations: –Plink” -X “populations: –var.phylyip” -X “populations: –vcf”). After the output files were generated, we created a bed file with a vcf file using PLINK 1.9 and filtered SNPs in terms of calling rate 50%, minor allele frequency < 0.05 and HWE statistics p < 0.00001 (–geno 0.50, –maf 0.01 –hwe 0.00001 –make-bed). Pairwise FST values were calculated using filtered bed files with Plink from heterozygosity (-het). The bed files were subsequently transformed into a vcf file for eastSFS to call the minor allele frequency for demographic analyses (– recode vcf–iid –allow-extra-chr).
Population structure
Admixture analyses were conducted to analyse the population structure of the three species on the Akajima and Sesoko islands using Admixture (Alexander et al. 2009). PLINK 1.9 was used to prepare the input file for Admixture, which was used to estimate the population structure of the target population group. Nine independent runs were performed for K = 1–9. The analysed files were compressed and applied to CLUMPAK, and StructureSelector was used to estimate the K values. We estimated delta K (Evanno et al. 2005), the most likely number of clusters, using STRUCTURE HARVESTER (Earl & vonHoldt 2012), CLUMPAK (Kopelman et al. 2015), and STRUCTURE PLOT (Ramasamy et al. 2014) to summarise and visualise the admixture results.
Phylogeny of the populations
To build the phylogenetic tree, phylip files were pretreated with raxml_asc.phy to remove invariant sites, and the files were used to select the appropriate model using ModelTest-NG. The selected models were used in RaxMl-NG to estimate the maximum likelihood (ML) with Lewis modifications (Lewis 2001) for the SNP phylip file and set–force perf_threads. ModelTest-NG and RaxMl-Ng analyses were performed using the NIG supercomputer system of the international nucleotide sequence database.
PCA with SNP data
PCA of the SNP data was conducted using PLINK1.9, filtered Plink.map, and Plink.ped files (see above). First, we calculated eigencec and eigencal files with PLINK by allowing extra chromosomes because of the lack of linkage groups as chromosomes. The eigenvectors and eigenvalues were subsequently plotted R script. The results were plotted using Rscript with output eigenval and eigenpac files (Supplementary Information 1 and 2).
Detection of hybridisation and demographic analyses among populations
We used two approaches to detect hybridisation and gene flow among the three species at the two locations. First, we performed ABBA-BABA analyses using Dsuite (Malinsky et al. 2021). In this analyses, the vcf file and phylogenetic tree built with RaxML-NG was used and A. gemmifera in the distinctive locations was set as ‘outgroup’, and A. intermedia and A. florida were excluded. For example, we set A. gemmifera as an outgroup for the analyses with three species in Sesoko Island.
Demographic analyses were conducted using fastsimcoal. We set four hypotheses: (1) different gene flows at distinct times, (2) constant gene flow, (3) ancient gene flow, and (4) recent gene flow among species in the same locations (fig.6a). For the analyses, we used the minor allele frequencies among the three species. We calculated the joint MAF files among the three species using easySFS with a Plink-filtered vcf file. Prior to running easy SFS, we performed a review of easySFS to set the projections for the joint MAF files. The highest values for each species on Akajima and Sesoko islands were selected. Thereafter, we ran fastsimcoal to estimate the parameters with 100,000 simulations and 40 expectation-conditional maximisation cycles for composite likelihoods. We calculated the Akaike’s information criterion values (AIC) from MaxEstlhoods in the output file and the K values of the analyses. The AIC values of the four models were compared to calculate the differences, and the rescaled AIC (∆AIC) was obtained for all models. The smallest rescaled AIC (∆AIC) value was selected. Thereafter, estimation of the parameters was selected for the highest maximum composite likelihood. The selected model was analysed again with parametric bootstrapping 100 times to divide the vcf files into 100 files and create the SFS files. We again calculated 100 times for each file to obtain 95% confidence intervals.
Statistical analyses
A generalised linear model (GLM) was used to examine whether fertilisation rates in crossing experiments differed within species and combinations of species, with fertilisation rate (number of eggs fertilised/number of eggs counted to confirm fertilisation) as the objective variable and the combination of species as the explanatory variable. The error structure and link function of the model had a negative binomial distribution and log, respectively. Pemanova was perfomed in PCA analyses. The statistical analyses were performed with number of principal compenents accounting for at least 70% of the cumulative variance explained. Pairwise comparison between groups using PERMANOVA, then apply Bonferroni corrections to the P-values. The above analysis was performed using R ver. 4.0.2 (R Development Core Team 2020) using the lme4 package ver. 1.1.25 (Bates et al. 2015).
Results
Date and time of spawning
The relationships between spawning day and time in each year are shown in Fig. 3. Regardless of the species, most colonies spawned from the full moon (DoSRtNFM = 0) to the day before the last quarter-moon (DoSRtNFM = 6) on Akajima Island (Fig. 3a). Colonies on Sesoko Island spawned over a wide range of moon ages, from DoSRtNFM-6 to 8 (Fig. 3b). During spawning, all except one colony (in 2021, Sesoko) of A. florida in both areas spawned 130–170 min after sunset, earlier than the other two species. Most colonies of A. intermedia and A. gemmifera had partially overlapping spawning times: A. gemmifera spawned 160–215 min, and A. intermedia spawned 150–190 min after sunset.
Relationship between moon age and spawning time per year. a Akajima Island; b Sesoko Island. The x-axis is the date of spawning relative to the nearest full moon (DoSRtNFM) using the time zone and date of observation for days before (-) or after ( +) the nearest full moon (− 15 to + 14). The y-axis is minutes after sunset in each year
On Akajima Island, the spawning times of the three species were always synchronous, except in 2016, when the gap in synchronous spawning between A. florida and the other two species was < 2 d (Fig. 3a). On Sesoko Island, two to three species spawned together, and the gap in synchronous spawning was confirmed to be < 2 d (see 2018 and 2021 in Fig. 3b). In 2013, 2014, 2017, and 2019, the two species spawned synchronously on some days; however, the overall spawning duration was prolonged (up to 12 d). In 2012, there was no overlap in spawning among the species (Fig. 3b).
Cross fertilisation rates
The results of the intra- and interspecific crosses are shown in Fig. 4. On both Akajima and Sesoko islands, fertilisation rates were higher in intraspecific crosses than those in interspecific crosses (Fig. 4a and b). In intraspecific crosses, there were no differences in fertilisation rates between A. gemmifera and A. intermedia relative to A. florida (Akajima: A. gemmifera, z = 0.08, p = 0.94; A. intermedia, z = − 1.26, p = 0.21; Table S2; Sesoko: A. gemmifera, z = 0.15, p = 0.88; A. intermedia, z = − 0.61, p = 0.54; Table S3). However, A. intermedia showed greater variation in fertilisation rate than the other two species. Comparing the fertilisation rates of intraspecific crosses among the regions, fertilisation rates were statistically different for A. intermedia in both Akajima and Sesoko islands and for A. florida on Akajima Island when A. florida was used as the intercept (Akajima Island: z = − 14.92, p = < 2e − 16, Sesoko: z = − 25.951, p = 2e − 16, Table S4).
Fertilisation rates in all the crossing experiments. a Fertilisation rates in the crossing experiments performed in 2007, 2013, 2014, 2015, and 2016 on Akajima Island; b Fertilisation rates in the crossing experiments performed in 2012, 2013, 2014, 2017, 2018, 2019, and 2021 on Sesoko Island. flo, Acropora florida; gem, A. gemmifera; int, A. intermedia. In pair, uppercase text indicates eggs and lowercase text indicates sperm. Uppercase “SELF” indicates self-crossing in each species
For interspecific crosses, the average fertilisation rate of all combinations, except A. florida eggs × A. gemmifera sperm, exceeded 20% on Akajima Island (Fig. 4a). Among all combinations, A. intermedia eggs × A. gemmifera sperm showed the highest fertilisation rate (52%). Approximately 25% interspecific fertilisation was observed for A. florida eggs × A. gemmifera sperm. On Sesoko Island, interspecific crosses of A. gemmifera eggs × A. intermedia sperm and A. intermedia eggs × A. gemmifera sperm showed fertilisation rates approximately equal to or higher than those of intra-species crosses; in particular, A. intermedia eggs × A. gemmifera sperm showed a higher fertilisation rate of 72.4% than the intra-species crosses. The fertilisation rates of the other combinations were approximately 0% (Fig. 4b; Table S3).
Most colonies showed approximately 0% self-fertilisation at both sites, whereas one colony each of A. intermedia from Akajima Island and Sesoko Island showed self-fertilisation rates of 19% and 89%, respectively.
Genetic structure and phylogenetic analysis
The heterozygosity and inbreeding coefficients among the examined populations were not significantly different among populations (Figure S2; Table S5). The inbreeding coefficient F of all populations ranged from 0.3 to 0.6, Heterozygosity (Ho and He) ranged from 0.22 to 0.28 and from 0.1 to 0.2, respectively.
Pairwise Fst also indicated that there were differences among populations, except A. florida, on Akajima and Sesoko islands (Table S6). In addition, the presumed hybrids were < 0 when comparing A. intermedia on Akajima and Sesoko islands. This may be because of the limited number of samples and potential hybrids of A. intermedia.
The population structure and phylogeny of the three species, one presumed hybrid and one artificially crossed F1 hybrid, were analysed. The ML tree from the SNP data clustering with the categorisation of species and locations showed that each of the three species formed a different cluster and that all F1 hybrids were located at an intermediate position between the parental species (Figure S3). The presumed hybrid was located near an A. intermedia branch. In contrast, individual SNP data without clustering showed that several colonies of A. intermedia and A. gemmifera were nested, with bootstrap values of < 50%.
As a result of the admixture analyses, A. florida populations in the two sites were distinct from the other species in each location (K = 5 and K = 3). In contrast, A. gemmifera differed between Akajima and Sesoko islands. The genetic structure of the artificially crossed F1 hybrids was a mixture of the parental species A. intermedia and A. florida (Fig. 5a). Although the slight distinctiveness of the three species presented by Admixture analyses and the following PCA analyses, the population structure of each species in two locations were similar (Figure S5).
Genetic structure of the examined species around the Akajima and Sesoko islands based on model-based analysis and PCA a The population structures of Sesoko and Akajima islands were examined using an admixture. The best-fit values determined using the Structure Harvester software were K = 5 or K = 3 for both mixture analyses. b PCA with a filtered bed file (Calling rate 50%, MAF = 0.05, HWE P < 0.00001)
The PCA also showed that A. gemmifera and A. intermedia were genetically closely related but significantly different (Fig. 5b, Figure S6; PEMANOVA P < 0.05). The F1 hybrids of A. intermedia and A. florida (HyA and HyB) were located between the clusters of both species (Fig. 5b).
ABBA–BABA test and demographic analyses for estimating the admixture event
According to the F4 statistics (ABBA-BABA test) for the same island based on the Z score, admixture events were to have occurred among the three species (Table 1). In this analysis, we set the out group as A. florida.
Demographic analyses suggested that introgression might have occurred at different times. We established four models: recent, ancient, constant, and different gene flows (Fig. 6a). A model comparison supported the scenario of different gene flows (Table 2). In this scenario, the recent admixture timings of Sesoko and Akajima islands were different. The former might have occurred recently (< 50 generations); however, the latter might have occurred 20 to 1500 generations ago (Table 3). According to our previous study (Isomura et al. 2016), Acropora takes 7 years to spawn (Isomura et al. 2016).
Demographic analyses of A. florida, A. intermedia, and A. gemmifera in the Akajima and Sesoko islands a The model for the analyses is (1) Ancient gene flow, (2) Different gene flow, (3) Recent gene flow, and (4) Constant gene flow. We set each pair of the species in each location and compared them according to AIC values calculated from the estimated maximum likelihood values in the fastsimcoal 2.7. b the detailed model of the different gene flow
Admixture events on Sesoko Island may have arisen within approximately 20 to 350 years. A heavy bleaching event occurred in 1998 (20 years ago) around Sesoko Island; therefore, the correlation between introgression and population declines after heavy bleaching warrants consideration.
Discussion
This study examined the relationship between reproductive features (spawning synchronisation and gamete compatibility) and the degree of introgression using SNP data. In the present study, the degree of introgression was marginally related to spawning synchronisation and gamete compatibility. However, the demographic simulation implied that the admixture event may be correlated with the bleaching event i.e. the 1998 bleaching event in Okinawa, and past climate change. Although distinct species show gamete compatibility, they rarely hybridise with each other in the colony-rich reef; however, extensive hybridisation might have occurred during the low population breeding crisis. These results match the hybridisation that potentially occurs in a lower number of populations (Wong et al. 2022; Zhou et al. 2022). However, this study did not show evidence of adaptation due to hybridisation (F1 generation) followed by introgression (backcrossing with the parental species).
This study indicated that the population structure of the examined species on Akajima Island was well separated from that on Sesoko Island. Gamete compatibility and spawning synchronisation may be related to the degree and direction of introgression (Van Open et al., 2002; Morita et al. 2019); however, hybridisation may occur conditionally with a low population number. In addition, gamete compatibility and spawning synchronisation were less strongly associated with introgression. For example, on Akajima Island, the three examined species exhibited tight spawning synchronisation and high gamete compatibility. In contrast, those on Sesoko Island spawned less synchronously, and their cross-fertilisation rates were lower than those on Akajima Island. Spawning synchronisation differs among sites (Isomura and Fukami 2018; Baird et al. 2021a, b). In addition, gametes disperse rapidly after spawning and sperm concentration markedly changes (Kitanobo et al. 2022a), implying that fertilisation conditions change after spawning and that spawning synchronisations are not strong determinants. Therefore, spawning synchronisation and gamete compatibility must be considered for their hybridisation functions.
Demographic analyses and the ABBA-BABA test indicated that introgression occurred in the past and recently, and the hybridisation era was linked to climate change and mass bleaching. We set several scenarios to examine how gene flow among the three species occurred. The most befitting scenario was different gene flow, and the recent gene flow era differed between Sesoko and Akajima islands. On Sesoko Island, the estimated current gene flow events (20–350 years ago) matched the mass bleaching events. For example, mass bleaching occurred in 1998 (> 20 years ago) around Okinawa Island, Japan. After bleaching, living coral coverage decreased to less than 1% of its previous level on Sesoko Island (Sakai 2006), and species density returned to levels similar to those before the mass bleaching event (van Woesik et al. 2011). The genus Acropora is regarded as a loser to bleaching (Loya et al. 2001), and we can predict that the rapid decline in coral coverage induced a breeding crisis owing to the low number of gametes at spawning. However, except for A. florida and A. intermedia, gene flow between heterospecifics may not have occurred recently (< 50 years ago) on Akajima Island. Although coral coverage has decreased to 32.2% of its previous level on Akajima Island (Iwao and Taniguchi 1999), the decrease in population is far less than that on Sesoko Island. Although the association between coral coverage and breeding crises has not been well studied, Akajima Island reached 106 sperm/mL after spawning in 1998 (Omori et al. 2001), and we showed that hybridisation occurred at < 104 sperm/mL (Kitanobo et al. 2016). However, as described above, sperm concentration in the ocean changes after spawning, and a field survey after spawning is a prerequisite for this conclusion.
Coral population communities can change considerably. Thus, ancient gene flow among the examined species is suspected to represent how local populations hybridise with each other. Furthermore, Akajima and Sesoko islands are < 75 km away; the Kuroshio Current might affect larval dispersal, and Akajima Island is potentially a strong source of larvae for Sesoko Island (Nishikawa et al. 2003). The Kuroshio Current facilitates the transport of larvae from Akajima Island to Sesoko Island (Tsuchiya et al. 2022). Therefore, the differences in the current gene flow do not credibly reflect introgression events in the examined populations on Sesoko Island.
The delimitation of the two examined species, A. intermedia and A. gemmifera, must be separated in several analyses, which implies less robustness. Morphological similarities do not strongly represent the consequences of hybridisation; however, the colony shape of these two species often needs to be clarified, especially in small colonies. In addition, kinship analyses showed that the two species have several colonies that are presumed to be within third degree, implying colonies of first cousin or great-grandchild of the hybrid. The precision of the analysis depends on the identification of the colonies and species; however, detailed morphological analyses of these two species are warranted (Wolstenholme, 2004; Ramirez-Portilla et al., 2022). PCA, population structures, and pairwise comparisons implied that these species on Sesoko Island were similar. With morphological analyses, a more genome-wide sequence for calling SNPs may be convenient and support a more robust analysis. However, these two approaches are necessary for a more comprehensive analysis.
In addition, complicated hybridisation of A. gemmifera with other related species was suspected. For example, our preliminary data with more SNP analyses (> 60,000) suggested that extensive introgression might have occurred among A. gemmifera and related species and that these species spawn synchronously and have high gamete compatibility (Kitanobo et al., unpublished data). In addition, because of the limited number used and the existence of several sister species (> 20) participating in sympatric synchronous spawning, it is difficult to discriminate between incomplete lineage sorting and multiple introgressive events among species (Funk and Omland 2003; Degnan and Rosenberg 2009). Therefore, additional species related to A. gemmifera and other species should be examined in future studies. In addition, adaptive introgression and phenotypic outcomes resulting from introgression remain obscure.
In southern Japan, the hybrid hot spot of the Indo-Pacific (Hobbs et al. 2021), integrative approaches have helped investigate how hybridisation occurs in Acropora. In this study, we set good models to determine whether adaptive introgression contributes to species and phenotypic diversity, resulting in fitted traits in admixture populations. However, comprehensive strategies based on the morphology, reproductive features, and heat tolerance are required.
References
Alexander DH, Novembre J, Lange K (2009) Fast model-based estimation of ancestry in unrelated individuals. Genome Res 19:1655–1664
Babcock RC, Bull GD, Harrison PL, Heyward AJ, Oliver JK, Wallace CC, Willis BL (1986) Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef. Mar Biol 90:379–394. https://doi.org/10.1007/BF00428562
Baird AH, Edwards AJ, Guest JR, Harii S, Hatta M, Lachs L, Mera H, Sinniger F, Abrego D, Zvi OB, Bronstein O, Cabaitan PC, Cumbo VR, Eyal G, Eyal-Shaham L, Feldman B, Figueiredo J, Flot JF, Grinblat M, Heyward A, Hidaka M, Hirose M, Iguchi A, Isomura N, Kinzie RA, Kitanobo S, Kuba A, Levy O, Loya Y, Mezaki T, Mohamed AR, Morita M, Nojima S, Nozawa Y, Prasetia R, Puill-Stephan E, Ramirez-Portilla C, Rapuano H, Rosenberg Y, Sakai Y, Sakai K, Shlesinger T, Terraneo TI, Yakovleva I, Yamamoto HH, Yamazato K (2021a) A coral spawning calendar for Sesoko Station, Okinawa, Japan. Galaxea, J Coral Reef Stud 24:41–49. https://doi.org/10.3755/galaxea.G2021_S10O
Baird AH, Guest JR, Edwards AJ, Bauman AG, Bouwmeester J, Mera H, Abrego D, Alvarez-Noriega M, Babcock RC, Barbosa MB, Bonito V, Burt J, Cabaitan PC, Chang CF, Chavanich S, Chen CA, Chen CJ, Chen WJ, Chung FC, Connolly SR, Cumbo VR, Dornelas M, Doropoulos C, Eyal G, Eyal-Shaham L, Fadli N, Figueiredo J, Flot JF, Gan SH, Gomez E, Graham EM, Grinblat M, Gutiérrez-Isaza N, Harii S, Harrison PL, Hatta M, Jin Ho NA, Hoarau G, Hoogenboom M, Howells EJ, Iguchi A, Isomura N, Jamodiong EA, Jandang S, Keyse J, Kitanobo S, Kongjandtre N, Kuo CY, Ligson C, Lin CH, Low J, Loya Y, Maboloc EA, Madin JS, Mezaki T, Min C, Morita M, Moya A, Neo SH, Nitschke MR, Nojima S, Nozawa Y, Piromvaragorn S, Plathong S, Puill-Stephan E, Quigley K, Ramirez-Portilla C, Ricardo G, Sakai K, Sampayo E, Shlesinger T, Sikim L, Simpson C, Sims CA, Sinniger F, Spiji DA, Tabalanza T, Tan CH, Terraneo TI, Torda G, True J, Tun K, Vicentuan K, Viyakarn V, Waheed Z, Ward S, Willis B, Woods RM, Woolsey ES, Yamamoto HH, Yusuf S (2021b) An Indo-Pacific coral spawning database. Sci Data 8:35. https://doi.org/10.1038/s41597-020-00793-8
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models Usinglme4. J Stat Softw. https://doi.org/10.18637/jss.v067.i01
Chan WY, Peplow LM, Menendez P, Hoffmann AA, Van Oppen MJ (2018) Interspecific hybridization may provide novel opportunities for coral reef restoration. Front Mar Sci 5:160
Cowman PF, Quattrini AM, Bridge TC, Watkins-Colwell GJ, Fadli N, Grinblat M, Baird AH (2020) An enhanced target-enrichment bait set for Hexacorallia provides phylogenomic resolution of the staghorn corals (Acroporidae) and close relatives. Mol Phylogenetics and Evol 153:106944. https://doi.org/10.1016/j.ympev.2020.106944
Degnan JH, Rosenberg NA (2009) Gene tree discordance, phylogenetic inference and the multispecies coalescent. Trends Ecol Evol 24:332–340. https://doi.org/10.1016/j.tree.2009.01.009
Diekmann OE, Bak RPM, Stam WT, Olsen JL (2001) Molecular genetic evidence for reticulate speciation in the coral genus Madracis on a Caribbean fringing reef slope. Mar Biol 139:221–233
Earl DA, vonHoldt BM (2012) Structure Harvester: a website and program for visualizing structure output and implementing the Evanno method. Conserv Genet Resour 4:359–361. https://doi.org/10.1007/s12686-011-9548-7
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Frankham R, Ballou JD, Briscoe DA (2002) The broader context: population viability analysis (PVA). Introduction to conservation genetics. Cambridge University Press, New York, pp 502–528
Fukami H, Omori M, Shimoike K, Hayashibara T, Hatta M (2003) Ecological and genetic aspects of reproductive isolation by different spawning times in Acropora corals. Mar Biol 142:679–684. https://doi.org/10.1007/s00227-002-1001-8
Fukami H, Budd AF, Levitan DR, Jara J, Kersanach R, Knowlton N (2004) Geographic differences in species boundaries among members of the Montastraea annularis complex based on molecular and morphological markers. Evol 58:324–337
Fukami H, Iwao K, Kumagai NH, Morita M, Isomura N (2019) Maternal inheritance of F1 hybrid morphology and colony shape of the coral genus Acropora. PeerJ 7:e6429
Funk DJ, Omland KE (2003) Species-level paraphyly and polyphyly: frequency, causes, and consequences, with insights from animal mitochondrial DNA. Annual Rev Ecol Evol Syst 34:397–423
Grant V (1957) The plant species in theory and practice. In: Mayr E (ed) The species problem. American Association for the Advancement of Science, Washington, DC, p 67
Hatta M, Fukami H, Wang W, Omori M, Shimoike K, Hayashibara T, Ina Y, Sugiyama T (1999) Reproductive and genetic evidence for a reticulate evolutionary history of mass-spawning corals. Mol Biol Evol 16:1607–1613
Hayashibara T, Shimoike K, Kimura T, Hosaka S, Heyward A, Harrison P, Kudo K, Omori M (1993) Patterns of coral spawning on Akajima Island. Marine Ecology Progress Series, Okinawa, Japan. https://doi.org/10.3354/MEPS101253
Hobbs J-PA, Richards ZT, Popovic I, Lei C, Staeudle TM, Montanari SR, DiBattista JD (2021) Hybridisation and the evolution of coral reef biodiversity. Coral Reefs 41:535–549. https://doi.org/10.1007/s00338-021-02193-9
Hoeksema BW, Cairns S (2023) World List of Scleractinia. Acropora Oken, 1815. Accessed through: World Register of Marine Species at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=205469 on 2023–03–15
Isomura N, Fukami H (2018) Coral reproduction in Japan. In: Iguchi A, Hongo C (eds) Coral Reef Studies of Japan. Coral Reefs of the World, Springer, Berlin
Isomura N, Iwao K, Fukami H (2013) Possible natural hybridization of two morphologically distinct species of Acropora (Cnidaria, Scleractinia) in the Pacific: Fertilization and larval survival rates. PLoS ONE 8:1–8. https://doi.org/10.1371/journal.pone.0056701
Isomura N, Iwao K, Morita M, Fukami H (2016) Spawning and fertility of F1 hybrids of the coral genus Acropora in the Indo-Pacific region. Coral Reefs 35:851–855. https://doi.org/10.1007/s00338-016-1461-9
Iwao K, Taniguchi H (1999) The report on recovery and death process from the bleaching of the Reef-building corals in Maenohama, Akajima Island, Okinawa. Midoriishi 10:23–28
Kim D, Paggi JM, Park C, Bennett C, Salzberg SL (2019) Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol 37:907–915. https://doi.org/10.1038/s41587-019-0201-4
Kitanobo S, Isomura N, Fukami H, Iwao K, Morita M (2016) The reef-building coral Acropora conditionally hybridize under sperm limitation. Biol Let 12:20160511. https://doi.org/10.1098/rsbl.2016.0511
Kitanobo S, Toshino S, Morita M (2022a) Genetic variation in released gametes produces genetic diversity in the offspring of the broadcast spawning coral Acropora tenuis. Sci Rep 12:5026. https://doi.org/10.1038/s41598-022-08995-3
Kitanobo S, Iwao K, Fukami H, Isomura N, Morita M (2022b) First evidence for backcrossing of F1 hybrids in Acropora corals under sperm competition. Sci Rep 12:5356. https://doi.org/10.1038/s41598-022-08989-1
Kopelman NM, Mayzel J, Jakobsson M, Rosenberg NA, Mayrose I (2015) Clumpak: a program for identifying clustering modes and packaging population structure inferences across K. Mol Ecol Resour 15:1179–1191. https://doi.org/10.1111/1755-0998.12387
Levin DA, Francisco-Ortega J, Jansen RK (1996) Hybridization and extinction of rare plant species. Conserv Biol 10:10–16
Lewis PO (2001) A likelihood approach to estimating phylogeny from discrete morphological character data. Syst Biol 50:913–925
Liew YJ, Aranda M, Voolstra CR (2016) Reefgenomics. Database, Org - a repository for marine genomics data. https://doi.org/10.1093/database/baw152
Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, Van Woesik R (2001) Coral bleaching: the winners and the losers. Ecol Lett 4:122–131. https://doi.org/10.1046/j.1461-0248.2001.00203.x
Malinsky M, Matschiner M, Svardal H (2021) Dsuite - Fast D-statistics and related admixture evidence from VCF files. Mol Ecol Resour 21:584–595
Márquez LM, Van Oppen MJH, Willis BL, Reyes A, Miller DJ (2002) The highly cross-fertile coral species, Acropora hyacinthus and Acropora cytherea, constitute statistically distinguishable lineages. Mol Ecol 11:1339–1349. https://doi.org/10.1046/j.1365-294X.2002.01526.x
Mayr E (1963) Animal species and evolution. Harvard University Press
Miller KJ, Benzie JAH (1997) No clear genetic distinction between morphological species within the coral genus Platygyra. Bull Mar Sci 61:907–917
Morita M, Kitanobo S, Nozu R, Iwao K, Fukami H, Isomura N (2019) Reproductive strategies in the intercrossing corals Acropora donei and A. tenuis to prevent hybridization. Coral Reefs 38:1211–1223. https://doi.org/10.1007/s00338-019-01839-z
Nakajima Y, Nishikawa A, Iguchi A, Sakai K (2012) The population genetic approach delineates the species boundary of reproductively isolated corymbose acroporid corals. Mol Phylogenet Evol 63:527–531. https://doi.org/10.1016/j.ympev.2012.01.006
Nishikawa A, Katoh M, Sakai K (2003) Larval settlement rates and gene flow of broadcast-spawning (Acropora tenuis) and planula-brooding (Stylophora pistillata) corals. Mar Ecol Prog Ser 256:87–97
Nozawa Y, Isomura N, Fukami H (2015) Influence of sperm dilution and gamete contact time on the fertilization rate of scleractinian corals. Coral Reefs 34:1199–1206. https://doi.org/10.1007/s00338-015-1338-3
Nylander-Asplin HF, Hill RL, Doerr JC, Greer L, Fogarty ND (2021) Population dynamics and genotypic richness of threatened Acropora species and their hybrid in the U.S. Virgin Islands Coral Reefs 40:965–971. https://doi.org/10.1007/s00338-021-02093-y
Omori M, Fukami H, Kobinata H, Hatta M (2001) Significant drop of fertilization of Acropora corals in 1999: an after-effect of heavy coral bleaching? Limnol Oceanogr 46:704–706. https://doi.org/10.4319/lo.2001.46.3.0704
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria https://www.R-project.org/.Austria. https://www.R-project.org/
Ramasamy RK, Ramasamy S, Bindroo BB, Naik VG (2014) Structure Plot: a program for drawing elegant STRUCTURE bar plots in user friendly interface. Springerplus 3:431. https://doi.org/10.1186/2193-1801-3-431
Ramírez-Portilla C, Baird AH, Cowman PF, Quattrini AM, Harii S, Sinniger F, Flot JF (2022) Solving the coral species delimitation conundrum. Syst Biol 71:461–475
Rhymer JM, Simberloff D (1996) Extinction by hybridization and introgression. Annu Rev Ecol Syst 27:83–109. https://doi.org/10.1146/annurev.ecolsys.27.1.83
Richards ZT, Miller DJ, Wallace CC (2013) Molecular phylogenetics of geographically restricted Acropora species: implications for threatened species conservation. Mol Phylogenet Evol 69:837–851. https://doi.org/10.1016/j.ympev.2013.06.020
Sakai K (2006) Changes in hard coral cover around Sesoko Island, Okinawa: looking back 25 Years. Midoriishi 17:15–19
Stankowski S, Ravinet M (2021) Quantifying the use of species concepts. Curr Biol 31:428–429. https://doi.org/10.1016/j.cub.2021.03.060
Suyama Y, Matsuki Y (2015) MIG-seq: an effective PCR-based method for genome-wide single-nucleotide polymorphism genotyping using the next-generation sequencing platform. Sci Rep 5:16963. https://doi.org/10.1038/srep16963
Tsuchiya K, Zayasu Y, Nakajima Y, Arakaki N, Suzuki G, Satoh N, Shinzato C (2022) Genomic analysis of a reef-building coral, Acropora digitifera, reveals complex population structure and a migration network in the Nansei Islands, Japan. Mol Ecol 31:5270–5284
van Oppen MJ, McDonald BJ, Willis B, Miller DJ (2001) The evolutionary history of the coral genus Acropora (Scleractinia, Cnidaria) based on mitochondrial and anuclear marker: reticulation, incomplete lineage sorting, or morphological convergence? Mol Biol Evol 18:1315–1329. https://doi.org/10.1093/oxfordjournals.molbev.a003916
van Oppen MJ, Willis BL, Van Rheede T, Miller DJ (2002) Spawning times, reproductive compatibilities and genetic structuring in the Acropora aspera group: evidence for natural hybridization and semi-permeable species boundaries in corals. Mol Ecol 11:1363–1376. https://doi.org/10.1046/j.1365-294x.2002.01527.x
van Woesik R, Sakai K, Ganase A, Loya Y (2011) Revisiting the winners and the losers a decade after coral bleaching. Mar Ecol Prog Ser 434:67–76. https://doi.org/10.3354/meps09203
Veron JEN (1995) Corals in space and time: the biogeography and evolution of the Scleractinia. Cornell University Press, New York, p 321
Vollmer SV, Palumbi SR (2004) Testing the utility of internally transcribed spacer sequences in coral phylogenetics. Mol Ecol 13:2763–2772. https://doi.org/10.1111/j.1365-294X.2004.02265.x
Wallace CC (1999) Staghorn corals of the world: a revision of the genus Acropora. CSIRO Publishing, Collibgwood, Victoria
Willis BL, Babcock RC, Harrison PL, Wallace CC (1997) Experimental hybridization and breeding incompatibilities within the mating systems of mass spawning reef corals. Coral Reefs 16:S53–S65
Willis BL, van Oppen MJ, Miller DJ, Vollmer SV, Ayre DJ (2006) The role of hybridisation during the evolution of reef corals. Annual Rev Ecol, Evol, and Syst 37:489–517. https://doi.org/10.1146/annurev.ecolsys.37.091305.110136
Wolstenholme JK, Wallace CC, Chen CA (2004) Species boundaries within the Acropora humilis species group (Cnidaria; Scleractinia): a morphological and molecular interpretation of evolution. Coral Reefs 22:155–166. https://doi.org/10.1007/s00338-003-0299-0
Wong ELY, Hiscock SJ, Filatov DA (2022) The Role of interspecific hybridisation in adaptation and speciation: insights from studies in Senecio. Front Plant Sci 13:907363. https://doi.org/10.3389/fpls.2022.907363
Zhou J, Yang Y, Lv Y, Pu Q, Li J, Zhang Y, Deng X, Wang M, Wang J, Tao D (2022) Interspecific hybridization is an important driving force for origin and diversification of Asian cultivated rice Oryza sativa L. Front Plant Sci 13:932737. https://doi.org/10.3389/fpls.2022.932737
Funding
JSPS KAKENHI, 26440225, Naoko Isomura, 17K07545, Naoko Isomura, 17K07414, Masaya Morita, 19J14375, Seiya Kitanobo.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Isomura, N., Inoha, K., Shimura, A. et al. Effect of the frequency of multi-specific synchronous spawning on genetic introgression among three Acropora species. Coral Reefs 43, 1497–1509 (2024). https://doi.org/10.1007/s00338-024-02554-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-024-02554-0