Abstract
Inshore coral reefs support unique ecosystems that are subject to a variety of threats and disturbances. Marine protected areas are powerful conservation tools and often include zones which vary in the level of fishing restriction, including fully protected (no-take) zones, partially protected zones (limited fishing), and less protected “open” fishing zones. Here, we compare outcomes from fully protected, partially protected, and “open” fishing zones on inshore fringing reefs of the Great Barrier Reef with a history of cyclone-induced habitat disturbance. Biomass of target and non-target fish groups, along with the coral cover and structural complexity of benthic habitats, was compared among zones. There was a strong positive effect of full protection from fishing on targeted fish biomass, and a comparatively modest effect of partial protection, even in areas where spearfishing was prohibited. Fully protected zones supported more than three times the biomass of highly targeted coral trout (Plectropomus spp.) compared to open fishing zones, and more than twice the biomass observed in partially protected zones. Similar outcomes occurred for primary targets collectively; conversely, there were no zoning-related differences for non-target fishes. Structural complexity was a consistent positive driver of fish biomass for all fish groups and among all zones. Notably high target fish biomass occurred at NTMR sites with the highest structural complexity, highlighting the importance of both habitat and protection in supporting population hotspots for targeted reef fishes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inshore coral reefs are unique habitats that provide a range of essential ecosystem services and hold significant conservation value. Their coastal location makes them important economically, socially, and culturally and also increases their exposure to a range of anthropogenic stressors (Wenger et al. 2015; Lam et al. 2018; Ceccarelli et al. 2020). Inshore reefs are often subject to significant recreational fishing pressure and have high exposure to coastal runoff and associated increased sediment, pesticide and nutrient loads (Brodie et al. 2012; Hall et al. 2021). They support distinct fish and coral assemblages, with strong connectivity to a range of other habitats in the seascape mosaic, such as mangroves, seagrass meadows, and macroalgae beds (Williams and Hatcher 1983; Sievers et al. 2020; Hall and Kingsford 2021). Such coastal mosaics may be considered key priority areas for conservation and management.
Physical disturbances such as storms and cyclones can be major drivers of the composition of coral reef habitats (Lam et al. 2018). Tropical cyclones present an acute physical disturbance that can abruptly reduce the cover of corals and subsequently diminish the structural complexity of reef habitats (Woolsey et al. 2012; Ceccarelli et al. 2016; McClure et al. 2019). Structural complexity can have a strong influence on the characteristics of fish assemblages; complex habitats positively influence both abundance and species richness (Friedlander et al. 2003; Nash et al. 2013; Hall and Kingsford 2021). For smaller and/ or juvenile fishes, the prevalence of complex coral forms such as branching corals is critical to provide microhabitats such as the interstitial space between branches (Beukers and Jones 1998; Wilson et al. 2008; Pratchett et al. 2012). For larger-bodied fishes, complex macro-habitat features such as caves, overhangs, and large tabulate corals form critical habitats (Connell and Kingsford 1998; Kerry and Bellwood 2012; Nash et al. 2013) and may hold particular importance from a management perspective, since these fishes constitute the majority of commercial and recreational fish catch (Boaden and Kingsford 2015; Webley et al. 2015).
Coral reefs provide for rich fisheries resources and may be subject to significant fishing pressure (Cinner 2014). Implementation of marine park zoning has been effective in reducing the impacts of fishing on coral reefs in a range of regions globally (e.g. Mumby et al. 2006; McClanahan et al. 2007; McCook et al. 2010; Russ and Alcala 2010). In the Great Barrier Reef Marine Park (GBRMP), implementation of no-take marine reserves (NTMRs) has resulted in dramatic increases in the biomass of fisheries targets (Russ et al. 2008; McCook et al. 2010; Boaden and Kingsford 2015). Effectively enforced NTMRs may provide a range of benefits: they provide a sanctuary for targeted species, protect key ecological processes such as predation and herbivory, and can directly benefit fisheries through larval export or spillover (Gell and Roberts 2003; Russ et al. 2004; Harrison et al. 2012; Edgar et al. 2014; Boaden and Kingsford 2015). Less certain, is the extent to which marine reserves provide resilience against impacts such as coral bleaching, floods, and cyclones, and correspondingly how habitat declines from these impacts may influence zoning outcomes (Jones et al. 2004; Graham et al. 2011; Mellin et al. 2016). Some studies have demonstrated enhanced recovery of coral communities within NTMRS after disturbance (Mumby and Harborne 2010; Mellin et al. 2016), whereas others have documented disturbance-related habitat declines for fish and corals in both fished and unfished zones (Jones et al. 2004; Wenger et al. 2015). From this, it is clear that a detailed understanding of habitat characteristics in the context of region-specific disturbance histories is needed to fully appreciate zoning outcomes.
Most research on marine protected areas (MPAs) is focussed on NTMRs; however, most MPAs consist of a range of zones that vary in their level of protection (Denny and Babcock 2004; Zupan et al. 2018). Partially protected areas (PPAs) are prevalent in MPAs and generally allow for limited fishing to occur, with the aim of providing some protection through restrictions on fishing effort (Hall et al. 2021). These zones are often situated close to the mainland shore, to allow access by small fishing vessels, and are prominent on inshore reefs. Studies on the benefits of PPAs have had dichotomous outcomes, and there is ongoing debate regarding their conservation value (Lester and Halpern 2008; Sciberras et al. 2015; Hall et al. 2021; Turnbull et al. 2021). A number of empirical studies have demonstrated some benefits of PPAs in protecting fisheries targets on tropical (Frisch et al. 2012; Bobiles and Nakamura 2019; Hall et al. 2021) and sub-tropical (Harasti et al. 2018) coral reefs. Conversely, others have found no benefit of PPAs above less regulated open fishing zones (Lester and Halpern 2008; Turnbull et al. 2021). On the central GBR, some prior studies have demonstrated conservation benefits in PPAs for targeted fishes (Boaden and Kingsford 2015; Hall et al. 2021). There is conflicting evidence, however, as to whether Special Management Area (SMA) PPAs that prohibit spearfishing can provide better protection than regular Conservation Park Zone (CPZs) PPAs that do not (Frisch et al. 2008; Hall et al. 2021). A clearer understanding of the region-specific efficacy of PPAs is needed and will be of particular importance in high-use regions such as inshore reefs.
This study focussed on evaluating the effects of long-term implementation of marine park zoning in an economically and ecologically important area of the central GBR: the Whitsundays and Mackay region. The area has been subject to a history of physical and environmental disturbances such as cyclones and floods, resulting in significant impacts to coral reef habitats among sites within the region (Williamson et al. 2019; Ceccarelli et al. 2020). This history of disturbance allowed us to examine the ecological effects of different management zones on coral reef fishes, and how they may be influenced by variation in the composition and structure of benthic habitats.
The aims of the study were to:
-
1.
Assess the effects of zoning on the biomass of target and non-target fishes;
-
2.
Evaluate the relative efficacy of PPAs in mitigating fishing impacts, through comparison with adjacent NTMRs and Habitat Protection Zones (HPZs);
-
3.
Compare outcomes of two kinds of PPAs, that either allow (regular CPZs) or prohibit (Special Management Area CPZs) recreational spearfishing;
-
4.
Examine the effect of habitat composition and structural complexity on fish biomass, and how this may influence zoning outcomes.
Methods
Great Barrier Reef Marine Park
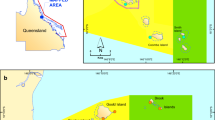
The Great Barrier Reef Marine Park (GBRMP) is a multiple use marine park with different zone types which allow varying levels and kinds of fishing activities. Approximately one-third of the GBRMP is zoned as no-take marine reserves (NTMRs; “green zones”), where fishing is prohibited (Day et al. 2019). Habitat protection zones (HPZs; “blue zones”) are less restrictive open fishing zones and allow for a range of fishing activities (excluding trawling). Partially protected areas (PPAs), or “yellow zones” provide intermediate protection from fishing through limits on fishing gear and effort; for example, line fishing is allowed with one hook/line per fisher, but large mesh netting trawling, and dive-based harvest and collection are prohibited (Day et al. 2019; Hall et al. 2021). In this study, we include two kinds of PPAs: regular Conservation Park Zones (CPZs) and Special Management Area (SMA) CPZs. Both have the aforementioned fishing limitations, but regular CPZs allow for snorkel-based recreational spearfishing, which is prohibited inside SMA CPZs (Hall et al. 2021). The two yellow coloured zones are differentiated from each other on zoning maps by a pink hashed line around the SMA region (Fig. 1).
Study area and sampling design
The study area comprised two regions of the inner-shelf of the central GBR: the Whitsundays and Mackay region (Fig. 1). Each region includes a number of inshore continental islands with fringing coral reef habitats; these islands are known to have a varying cover of soft and hard corals, along with turfing algae and macroalgae (Williamson et al. 2019). The area was impacted by Cyclone Debbie (Category 4) in 2017, which caused physical significant damage to reefs, resulting in declines in live coral cover, especially on exposed sides of islands (Williamson et al. 2019; Ceccarelli et al. 2020). As such, variation in coral cover and habitat complexity among sites is likely to be strongly influenced by previous exposure to cyclone impacts (Williamson et al. 2019; Ceccarelli et al. 2020). Both regions are popular fishing areas, especially for recreational fishing, which is mostly reef based. Recreational and (to a lesser extent) commercial fishing are prevalent in the area and predominantly focussed on reef habitats. The Whitsundays is a popular area for tourism (e.g. diving, snorkelling, sailing), with regular tourist visitation of the islands and sites included in the study.
To examine the effects of zoning on fish and benthic habitats, we surveyed sites across three zones within each of the two study regions. Both regions have fully protected NTMRS, open fishing HPZs, and either regular CPZs (spearfishing allowed; Mackay region) or SMA CPZs (spearfishing prohibited; Whitsundays region). For the purposes of statistical analysis (detailed below), both CPZ types were considered a single level of the factor “zone”. A balanced design was utilized to compare the three zone types within each region, with six sites surveyed within each zone, resulting in 18 survey sites per region, and 36 sites total (Fig. 1). Sites were selected to encompass fringing reef habitats around islands and were distributed across a number of islands within each region (Fig. 1). Surveys were conducted in September 2020.
Fish and benthic surveys
At each site, fishes were surveyed along four 50 × 5 m belt transects placed parallel to the reef crest along a depth range of 6–16 m mean tide level (MTL); average depth was 9.7 m. The number and size (to the nearest 5 cm) of fishes were estimated using underwater visual census (UVC) along each transect by a single experienced fish observer on SCUBA. The size (total length) of each individual was then converted to biomass using published length/weight relationships (Kingsford 2009; Froese and Pauly 2021) to obtain biomass estimates per species for each transect. The fish observer also estimated a complexity score for the reef habitat along each transect, which captured both rugosity and vertical relief. Complexity scores were estimated as a continuous range of values, scaled from one to four, with increasing values representing greater complexity. For example, scores close to one represented an essentially flat surface with no real habitat attributes, and a score of four represented the presence of highly complex structures, with diverse habitat features such as overhangs, caves, and bommies. Complexity scores were estimated by a trained and experienced observer and were similar in approach to that used by numerous previous studies (e.g. Williamson et al. 2014; Hall and Kingsford 2021). Benthic habitats were surveyed along a 2-m wide belt along the centre of each transect by a second diver who recorded imagery from the benthos using a GoPro camera. Footage from each transect was then analysed by randomly selecting 15 “quadrants” (i.e. points in time) from the video, and recording benthic substratum at 12 points within each quadrat. The resulting 180 points per transect were used to estimate percentage cover and gave a 95% chance of detecting benthic taxa with > 2% cover (Green 1979; Moynihan et al. 2022). Benthic substratum was categorized into broad categories of hard coral, soft coral, algae, and sand/rubble. The cover of live hard and live soft coral, along with the structural complexity score, was selected as key habitat attributes for analyses.
Fish groups
To differentiate the effects of zoning from other influences, both target and non-target species were surveyed. Primary targets were defined as those species most likely to be captured and retained by recreational and commercial fishers in the region; all primary target species had a maximum size greater than the minimum legal size limit for fisheries (Supp. Table S1). Coral trout and stripey snapper are key fisheries species that are abundant in the region, and likely to be caught and retained. As such, they were analysed separately, to infer zoning effects specific to these species, and were also included in the primary targets group for all analyses. Three species of coral trout were observed: (Plectropomus leopardus, P. maculatus, and P. laevis) and were grouped collectively as “coral trout” (Plectropomus spp.), since they are all very similar species that are key fisheries targets (Emslie et al. 2015; Hall et al. 2021). Primary targets were all medium to large-bodied piscivores or benthic foragers, so a similar suite of non-target fishes was also surveyed to enable comparisons of non-target and targeted fishes among zones (Supp. Table S1). Non-target species were defined as such if their size maximum did not exceed the minimum legal limit for fisheries, and/or if they were considered undesirable as a fisheries target (based on the available literature and discussions with relevant experts Webley et al. 2015; Hall et al. 2021). The resulting species list for target and non-target fishes included all species of Serranidae, Lutjanidae, Lethrinidae and Haemulidae, as well as large mobile wrasses from the genera Choerodon, Cheilinius and Hemigymnus (Supp. Table S1).
Analysis
Mixed effects models were conducted to analyse differences in fish biomass between regions and among zones. Separate models were run for each fish species/ group as follows: coral trout (Plectropomus spp.), stripey snapper (Lutjanus carponotatus), pooled primary targets, and pooled non-targets. All analyses were conducted using R (v.4.1.0, R Development Core Team, 2020). Models were fit in the frequentist method for model and variable selection and then run in the Bayesian framework for interpretation and analysis of results. Region and zone were included as fixed factors, with Site as a random factor. All models were fitted using a hurdle gamma distribution with a log link function, to account for the large number of zero values. Habitat covariates (complexity, live hard coral, and live soft coral) were also considered in the model selection process. Every combination of region, zone, hard coral, soft coral, and complexity was explored in both additive and interactive models. The final model output was identified as the most parsimonious model and was selected by comparing Akaike information criteria values (AICc), examination of DHARMa residual diagnostic tests (v. 0.4.3) for goodness of fit, and use of the vif function from the car package to confirm no collinearity of factors.
The Bayesian hierarchical model was fit using the ‘brms’ package (v. 2.15.0). Model selection informed by the frequentist approach was further evaluated in the Bayesian format, where region, zone, and complexity (the most informative habitat variable) were explored as additive and interactive models. Bayesian models were fit with flat, uninformative priors using the following parameters: Chains = 3, Iterations = 5000, warmup = 2000, thin = 5. Model convergence and diagnostics were assessed to confirm even mixing of chains, no autocorrelation, and appropriate effective sampling size and efficiency. Model outputs reported median posterior estimates and the 95% and 50% highest posterior density (HPD) credible intervals. Strong evidence for an effect (i.e. inferred significance) is determined when 95% credible intervals do not cross zero. The package emmeans (v. 1.6.2.1) was used to for pairwise comparisons, and to estimate biomass ratios between regions and among zones. Ratios were calculated as the first model estimate divided by the second (e.g. NTMR: HPZ = NTMR estimate/HPZ estimate).
The probability of biomass differences between zones was obtained from the modelled dataset; where ratio differences were summarized for > 1.0 (one zone having greater biomass than the other zone), > 1.5 (one zone having a 1.5 times or more the biomass of another zone), and > 2.0 (one zone having 2 times or more the biomass of another zone). The gather_emmeans_draws() function was used to compile the comparisons of predicted biomass estimate values per contrast (i.e. among zones) for all draws (samples of the chains). For each model, 1800 estimates were calculated (3 chains, 5000 iterations, 2000 warmup, 5 thin. 3*((5000–2000)/5 = 1800 estimates). From these, a proportion value was calculated by identifying the number of contrast comparisons that had selected differences in biomass estimate values. For example, calculating the number of draws where NTMRs had biomass estimate values greater than 1.5 times that of HPZs provided a probability (percentage) value for the ratio comparison of > 1.5. In this study, strong evidence of an effect was defined as probability values greater than 95% (Hannan et al. 2021). Meaning more than 95% of the 1800 estimates had the relevant ratio value (e.g. > 95% certainty that zone A has at least 1.5 times the fish biomass of zone B). Moderate evidence of an effect was defined for probabilities between 80 and 95% (e.g. 80–95% certainty that zone A has at least 1.5 times the fish biomass of zone B). Probability values less than 80% were considered to have no evidence of an effect.
Results
Fish assemblages
A total of 34 fish species within the survey groups were observed across the two regions: nine species of primary targets and 25 non-target species (Supp. Table S1). Primary targets were a mix of species from families Labridae, Lethrinidae, Lutjanidae, and Serranidae. The most abundant and commonly encountered primary target species was L. carponotatus (stripey snapper), which occurred on 73% of transects (Fig. 2 and Supp. Table S1). Two species of Plectropomus were commonly observed: P. maculatus (barcheek coral trout), and P. leopardus (common coral trout), which occurred on 62% and 63% of transects, respectively (Fig. 2 and Supp. Table S1). Blue spotted coral trout (P. leavis) also occurred but were rare, with only three individuals observed. Non-target fishes consisted of smaller species of Labridae, Lethrinidae, Lutjanidae, and Serranidae, as well as four Haemulidae species (Supp. Table S1). The two most abundant non-target species were Lutjanus vitta (brownstripe snapper), and Diagramma pictum (painted sweetlips) which both occurred in groups and were patchily distributed, occurring on only 20% of transects. Choerodon fasciatus (harlequin tuskfish) was the most commonly encountered non-target species, occurring on 76% of transects (Supp. Table S1).
Box plots of raw data for fish biomass (kilograms per 250-m2) between Mackay and Whitsunday regions and among different management zones for a Coral trout (Plectropomus spp.), b Stripey Snapper (Lutjanus carponotatus), c Primary targets and d non-targets. The pink border on the CPZ zone in the Whitsundays indicates that spearfishing is prohibited. Filled points are outliers in the boxplot, and open circles are the raw data points of individual transects
Benthic habitats
Benthic habitats varied among sites but showed no consistent trends among zones (Fig. 3). Live hard coral cover and structural complexity were mostly consistent among zones and between regions. A notable exception was for HPZs in the Whitsundays region (Fig. 3), which had the lowest median complexity and live hard coral cover, driven by particularly low values in cyclone-exposed sites. There was, however, considerable overlap in the range of values in Whitsundays HPZs with other zones and with the Mackay region. For both regions, all three habitat attributes were highly variable within zones; live hard coral cover ranged from 3 to 52% (site means), and live soft coral cover ranged from 0.7 to 26% (site means; Fig. 3). Both regions had some sites with very low coral cover; however, the Mackay region had greater maximum values for hard coral compared to the Whitsundays region (Fig. 3). Complexity varied greatly by site, with a similar range of values for both regions (Fig. 3).
Box plots of raw data for structural complexity estimates of the benthos, hard coral cover, and soft coral cover among management zones in Mackay and Whitsunday regions. The pink border on the CPZ in the Whitsundays indicates that spearfishing is prohibited. Filled points are outliers from the boxplot, and open circles are the raw data points
Fish biomass models
For all four models (coral trout, stripey snapper, primary targets, and non-targets), initial model exploration found no evidence of an interaction between fixed factors (region, zone), and an additive model was selected as most parsimonious after model comparison (Supp. Table S2). Structural complexity was consistently identified as the most informative habitat variable in model selection. Complexity was explored as both additive and interactive with region and zone. The best-fit models for all species groups included complexity as an additive variable. When complexity was included as an interactive variable, the model did not meet the requirements of the diagnostic checks. Inclusion of additional habitat covariates (live hard and soft coral) did not improve the models; therefore, complexity was the only habitat covariate used in the final models. All four models were additive, where region, zone, and complexity were fixed factors, and site was included as a random intercept factor (Supp. Table S2).
Zoning and regional effects
There was clear evidence of a positive effect of zones on fish biomass for unfished NTMRs, which supported greater biomass of coral trout and primary targets compared to both other zones (Table 1, Fig. 2). For both fish groups, there was a trend for increasing biomass with increasing protection from fishing (i.e. HPZ < CPZ < NTMR; Fig. 2), and the greatest magnitude of effect occurred when comparing fully protected NTMRs with HPZs (with the lowest level of protection from fishing; Table 1). The magnitude of effect was also greater for comparisons of NTMRs with partially protected CPZs than it was when comparing CPZs with HPZs, indicating that full protection in NTMRs provided the most powerful benefits for fished species (Table 1). There were large regional differences for coral trout and non-targets, which both had greater biomass in the Whitsundays compared to Mackay (Table 1). Coral trout and non-targets, respectively, had an estimated ≈2.28 times and ≈2.82 times the biomass in the Whitsundays compared to Mackay. No such trend occurred for stripey snapper and primary targets (Table 1).
Coral trout
Of all the species groups, coral trout (Plectropomus spp.) exhibited the greatest zoning-related response. There was a clear positive effect of protection from fishing in NTMRs; where biomass was greater in NTMRs compared to both HPZs and CPZs (Table 1 and Fig. 4). Estimated coral trout biomass in NTMRs was 3.65 times that of HPZs, and 2.13 times that of CPZs (Table 1). There was greater than 95% probability (strong evidence) of higher biomass in NTMRs compared to HPZs across all ratio contrasts, and an 86% probability (moderate evidence) that coral trout in NTMRs would have > 1.5 times the biomass of CPZs (Table 2). There was some evidence to suggest a positive effect of partial protection from fishing in CPZs, although the magnitude of effect was comparatively modest. While there was no evidence of a difference in coral trout biomass from pairwise tests between CPZs and HPZs (Table 1, Fig. 4), there was still a 94% probability (moderate evidence) that CPZs would have greater coral trout biomass (ratio ≥ 1.0) compared to HPZs (Table 2). There was, however, no evidence for CPZs to have greater than 1.5 times the biomass of coral trout compared to HPZs, indicating that the magnitude of the effect is likely to be fairly low. The spread of biomass values for coral trout was greatest in Whitsundays, which had notable outliers with extremely high biomass (up to 70 kg/250m2; Fig. 2a and Fig. 4). These occurred where coral trout were congregating on individual coral bommies in locations with high structural complexity (e.g. Black Island Reef; Fig. 1).
Effect sizes for pairwise comparisons among management zones between Mackay (square) and Whitsunday (circle) regions for each fish species group. The pink border on the CPZ in the Whitsundays indicates that spearfishing is prohibited. Comparisons are median differences in fish biomass (kilograms per 250-m2) between CPZ and NTMR zones compared to HPZ zones (reference HPZ line). Circles and squares are median posterior density estimates from Bayesian linear mixed effects models with 50% and 95% credible intervals (CI). Positive values indicate higher biomass in a zone compared to HPZs. Negative values indicate lower biomass compared to HPZs. Evidence of significance can be inferred when 95% (CI) do not cross the vertical dotted line (HPZ zone)
Stripey snapper
There was no evidence of a zoning or regional effect for biomass of stripey snapper (L. carponotatus; Table 1, Fig. 4). Estimated biomass ratios were similar between regions (ratio = 1.25) and among zones (ratios = 1.00–1.12), and biomass was quite consistent among sites overall (Fig. 2). There was no evidence for a difference in biomass among any of the zones; probability values for zone comparisons were all < 70% (Table 2). Lutjanus carponotatus had fairly uniform biomass (mean 3.4 kg/250m2 ± 0.4 SE), with the exception of two notable outliers (in CPZ and HPZ sites) where they were observed in large groups of up to 50 individuals (biomass 28–29 kg/250m2; Fig. 2b).
Primary targets
Zoning had strong evidence of an effect for primary targets, with the magnitude of difference among zones less than that observed for coral trout (Table 1, Fig. 4). The largest differences were evident between NTMRs and HPZs (Fig. 4), where NTMRs had strong evidence of greater (2.08 times) estimated fish biomass compared to HPZs (Table 1). This difference in biomass was driven strongly by coral trout species, however, smaller species such as tuskfishes (Choerodon cyanodus and C. schoenleinii) and lethrinids (e.g. Lethrinus laticaudis) also contributed to the trend (Supp. Table S1). Lutjanus carponotatus biomass had little influence on zoning trends for primary targets since their biomass was similar among zones (Figs. 2 and 4). Although median biomass values in NTMRs were greater than CPZs (ratio = 1.66), there was substantial overlap in the range of values (Figs. 2, 4 and Table 1). Biomass did not differ between CPZs and HPZs, and estimated modelled ratios were similar (ratio = 1.25; Table 1). When comparing NTMRs with other zones (HPZs and CPZs) based on probabilities, strong evidence of an effect was only found for biomass ratios > 1.0 (e.g. that NTMRs had more biomass than the other zones), but not for ratios > 1.5 or > 2.0. (Table 2). There was moderate evidence for a biomass ratio of > 1.0 between CPZs and HPZs, but no evidence of a greater difference, indicating a modest effect of zone (Table 2). The Whitsundays had an estimated 1.50 times the biomass of primary targets compared to Mackay (Table 1).
Non-targets
Non-target fish species showed no evidence of an effect of zone (Fig. 4, Table 2), but did have evidence of a strong effect of region (Table 1). Regional differences were strongest for non-targets; the Whitsundays had on average 2.82 times the biomass of non-target fish species compared to Mackay (Table 1). There was no evidence of an effect of zone when comparing probability outcomes from the model; probability values were below 30% for all zone comparisons and ratios (Table 2).
Comparison of PPA types
There was no clear evidence of a difference in biomass between Whitsundays SMA CPZs (no spearfishing) and Mackay regular CPZs (spearfishing allowed) for targeted fishes. Although pairwise model comparisons showed that both coral trout and non-targets had greater biomass in Whitsundays CPZs compared to Mackay CPZs, both groups also had strong regional effects with greater biomass in the Whitsundays region overall (Fig. 5 and Supp. Table S3). Average biomass values for coral trout were lower in Mackay CPZs (1.7 kg /250m2 ± 0.39SE) compared to Whitsundays (2.6 kg /250m2 ± 1.5SE); however, this was mostly due to one outlier site (Langford Reef) with high biomass; this reef has split CPZ/NTMR zoning (Figs. 1 and 2). Further, there were no strong differences between CPZs and HPZs for coral trout biomass in either region, indicating a similar magnitude of effect of CPZs in both regions (Supp. Table S3). Stripey snapper and primary targets had similar (overlapping) biomass in the two CPZ types (Fig. 5). This collective evidence suggests that a regional effect was likely the cause of differences in biomass between the two PPA types.
Effect sizes for pairwise comparisons of fish biomass in conservation park zones (CPZs) between regions. Whitsundays CPZs (yellow circles, pink lines) prohibit spearfishing activities, compared to Mackay CPZs (reference yellow dotted line) which allows spearfishing. Comparisons are median posterior density estimates of fish biomass (kilograms per 250-m2) from Bayesian linear mixed effects models with 50% and 95% credible intervals (CI). Positive values indicate higher biomass in the Whitsundays CPZ compared to Mackay CPZ. Evidence of significance (at the 0.05 level) can be inferred when 95% (CI) do not overlap and/or do not cross the vertical dotted line
Effects of structural complexity
Structural complexity had a positive effect on fish biomass for all species groups (Fig. 6, Supp. Table S2), and the slope of complexity ranged from ≈1.3–1.8 (Supp. Table S2). Biomass values for all groups increased with increasing complexity of habitats in both the Whitsundays and Mackay region (Fig. 6). For all fish species/groups, low complexity (score 1) sites had less than a third of the biomass compared to high complexity (score 4) sites (Table 1). The strongest relationship with complexity was observed for stripey snapper, with a 1.8 kg/250m2 increase in biomass for every 1.0 unit increase in complexity (Supp. Table S2). Although interactions were not modelled in these data, the relationship between complexity and fish biomass did vary by zone for coral trout and primary target species (Fig. 6). In both instances, the greatest fish biomass was observed in NTMRs in locations with the highest structural complexity, though there was high variability among sites (Fig. 6). This suggests that the magnitude of the effect of complexity was often greatest in NTMRs. For example, coral trout (Plectropomus spp.) biomass for sites in NTMRs with high or very high structural complexity (scores 3–4) was on average ≈3.8 times greater than the biomass of NTMR sites with low complexity (score 1). Comparatively, in fished zones, biomass in high/very high-complexity sites was ≈1.5 (CPZs), and ≈1.0 (HPZs) times greater than low complexity sites. This trend was not evident for stripey snapper, which had a similar magnitude of response for all zones, or for non-targets, where the strongest response tended to occur in HPZs (Fig. 6).
Modelled changes in fish biomass (kilograms per 250-m2) with structural complexity in Mackay and Whitsunday regions, compared among management zones. Structural complexity was modelled at the minimum (1.0), mean (3.0), and maximum (4.0) values observed in the raw data. The pink line on the CPZ zone in the Whitsundays indicates that spearfishing is prohibited. Circles are the average median biomass from modelled estimates, and error bars are 95% credibility intervals from Bayesian generalized linear mixed effects models
Discussion
Our results demonstrate the importance of marine parks in providing conservation benefits for coral reef fishes and in particular highlight the pivotal role that no-take zones play in mitigating fishing impacts. No-take marine reserves (NTMRs) supported a much greater biomass of targeted fishes compared to both other fished zones in our study regions, and there was strong evidence to conclude that variation among zones was the result of fishing effects. Differences between fished and unfished zones only occurred for targeted species, and the strongest effects occurred for the most highly targeted taxa (coral trout), indicating that the effect size of zoning differences was strongly related to fishing pressure. Although some previous studies (e.g. Hall et al. 2021; Hall et al. 2022) have demonstrated benefits from partial protection from fishing on coral reefs, it was clear in this case that the strongest outcomes were achieved for target species only when full protection was provided by management zones. We surveyed multiple zones in the region, along a spectrum of protection: fully protected (NTMRs), partially protected (CPZs) and less regulated open fishing zones (HPZs). For all taxa, the difference between full and partial protection was of greater magnitude than the difference between partial protection and open fishing zones. Thus, the provision of full protection in NTMRs was clearly irreplaceable in these inshore reef habitats.
Structural complexity was a consistent positive driver of fish biomass for all fish species/groups across all three zones. All fish groups had strong evidence of a positive relationship with complexity, regardless of their zoning relationships. For example, stripey snapper (L. carponotatus) had the strongest positive relationship with complexity, but no evidence of zoning relationships, whereas coral trout (Plectropomus spp.) were strongly influenced by zoning, but also had strong evidence of a positive relationship with complexity. There was high variation in the magnitude of this effect (Fig. 6), i.e. not all high-complexity sites supported particularly large numbers of fishes. This variability may be due to the influence of other factors (e.g. benthic composition, recruitment) at these sites. Our results align with a number of previous studies demonstrating the importance of highly complex habitats for large-bodied piscivorous and carnivorous fishes, which may be more strongly influenced by habitat complexity than by coral cover (Connell and Kingsford 1998; Emslie et al. 2008; Kerry and Bellwood 2012; Hall and Kingsford 2021). Although the cover of live hard and soft corals is undoubtedly important for a range of coral reef fishes (Wilson et al. 2008; Coker et al. 2014; Darling et al. 2017), macro-scale complexity features such as caves and overhangs can provide critical habitat features for larger-bodied fishes (Kerry and Bellwood 2012; Nash et al. 2013).
Although the presence of no-take zones may not always directly influence benthic habitats, the occurrence of optimal habitats within NTMRs may have positive synergistic effects. Notably, we found a trend for complexity effects to be of a greater magnitude in NTMRs compared to other zones. Areas of particularly high complexity attracted large groups of fishes, especially when these hotspots occurred inside NTMRs. For example, a single high-complexity bommie at Black Island Reef in the Whitsundays supported 13 coral trout, most of which were large (> 500 mm). Similar aggregations of coral trout have been observed at high complexity “hotspots” elsewhere on the GBR (e.g. One Tree Reef; Connell and Kingsford 1998). The Whitsundays region has a documented history of severe decline in coral cover, particularly due to recent cyclone impacts (Williamson et al. 2019; Ceccarelli et al. 2020). Given this, any remaining high-complexity habitats that have been minimally impacted during such disturbance events may form important post-disturbance refuges for populations of targeted fishes (Williamson et al. 2014). The ability to recover from impacts may be also helped by the life history characteristics of many of the target species. For example, coral trout have a relatively short life span (maximum 15 years) compared to many other serranids and may reach reproductive maturity as young as two years of age (Kingsford 2009). Our outcomes indicate that a strong history of protection, coupled with the presence of complex habitats, can be pivotal for supporting healthy and productive numbers of ecologically and commercially important coral reef species.
The value of partially protected marine areas as a conservation tool is widely debated (Lester and Halpern 2008; Zupan et al. 2018; Hall et al. 2021; Turnbull et al. 2021). Here, we found some evidence that limiting fishing inside PPAs provided a benefit for targeted fishes, but acknowledge that the magnitude of this effect is likely to be modest. There was a gradient effect in coral trout biomass, which was intermediate in CPZs compared to NTMRs and HPZs. However, biomass in partially protected CPZs was more similar to open fishing HPZs than it was to fully protected NTMRs. Thus, there appears to be a modest benefit to increasing protection levels from minimal (in HPZs) to moderate (in CPZs), and a much greater benefit to further increasing from partial to full protection (in NTMRs). This outcome differs to previous studies on coral reefs of GBR (Frisch et al. 2012; Boaden and Kingsford 2015) and in sub-tropical regions (Harasti et al. 2018), where fisheries target abundance and/or biomass was significantly greater in PPAs compared to open fishing areas. In the present study, it is possible that the history of habitat disturbance to the region affected the ability for more subtle zoning effects (i.e. partial protection) to be detected. However, our outcomes do concur with studies of PPAs in temperate (Turnbull et al. 2021) and sub-tropical (Malcolm et al. 2018) habitats where PPAs were found to be of minimal benefit from a social and ecological perspective.
We hypothesized that prohibiting spearfishing inside CPZs may infer additional benefits for target species, but found no compelling evidence to support this premise. A comparison of the two CPZs did show greater coral trout biomass in Whitsundays (no spearfishing) CPZs compared to Mackay (spearfishing allowed) CPZs; however, this trend was strongly influenced by a single outlier site in Whitsundays (Langford Reef), located on a reef with split CPZ/NTMR zoning. Further, a similar trend also occurred for non-target fishes, and both groups had a strong trend for greater biomass in the Whitsundays region overall. As such, the difference between CPZs most likely occurred to the regional effect rather than an effect of spearfishing. Spearfishing can be highly selective and therefore has the potential for strong impacts on key fisheries targets (Frisch et al. 2008). Studies of tropical and temperate reef fishes have shown the benefits of prohibiting spearing for fisheries targets (Frisch et al. 2012; Curley et al. 2013). On the GBR, two studies examining the impacts of spearing on inshore reefs have had differing outcomes, with one (Frisch et al. 2012) showing a significantly greater biomass of coral trout in SMA CPZs compared to regular CPZs, and another (Hall et al. 2021) showing no difference between the two zones. We propose that, in our study area, the lack of an effect of SMA (no spearfishing) CPZs, and small effect size of CPZs overall may be due to high visitation by tourists and other recreational users. Recreational fishing is thought to be popular in the area, particularly in the Whitsundays region, which receives heavy tourist visitation (Webley et al. 2015). Indeed, during our surveys, we observed large amounts of derelict fishing gear in many CPZ sites, suggesting substantial line fishing activity (Williamson et al. 2015). As such, the extent of recreational line fishing effort may be high compared to commercial fishing, resulting in minimal differences in fishing effort between CPZs and HPZs. Our outcomes suggest PPAs may be of limited utility in such high-use areas, emphasizing the importance of full protection inside NTMRs in the region.
Management implications
Our results hold important management implications that relate to both marine park zoning, and the preservation of coral reef habitats. The dual benefits of protection from fishing, alongside the presence of optimal habitats, may be critical in creating biomass hotspots, particularly for commercially and ecologically important species such as coral trout (Boaden and Kingsford 2015). Such hotspots may be an important source of larval export, and therefore benefit populations in the broader region (Harrison et al. 2012). Coral reefs face an ever-increasing range of threats, including coral bleaching, disturbance from cyclones and severe storms, and runoff/pollution (Hughes 2008; Brodie et al. 2012; Hughes et al. 2017). Management of coral reef ecosystems must therefore take into account the ongoing presence of such threats, and where possible, minimize their impact and facilitate recovery. For managers focussed on optimizing conservation outcomes in MPA systems, there are two clear and relevant implications from this study. Firstly, the placement of NTMRs within MPA systems may be of greatest benefit when located in areas that have important habitat attributes such as high structural complexity. And correspondingly, protection or restoration of coral reef habitats may be most beneficial (at least for target species) when undertaken in zones protected from fishing.
Although this study compared the three zone types only at one point in time, our results do concur with temporal studies in the Whitsundays region showing persistent benefits of NTMRs over HPZs in supporting coral trout biomass over a period of 23 years (Williamson et al. 2019). Further, our data expand upon this existing knowledge by incorporating a broader study area (including the Mackay region), and through the inclusion of PPAs in zoning comparisons. Our data highlight the ability for reserve effects to persist for over three years after significant habitat disturbance, emphasizing the importance of a history of long-term protection inside NTMRs. The ongoing recovery trajectories of these reefs, however, are unknown and will be an important component of ongoing research and monitoring. Although our study was limited to a single region of the GBR, we provide clear evidence of the critical nature of protection inside marine reserves on coral reefs, an outcome which is relevant to coral reefs, and indeed many marine ecosystems, globally. The preservation of species, ecosystem processes, and ultimately coral reef ecosystems inside protected reserves is and will continue to be, an essential component of marine conservation and management.
Change history
19 January 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00338-023-02344-0
References
Beukers JS, Jones GP (1998) Habitat complexity modifies the impact of piscivores on a coral reef fish population. Oecologia 114:50–59
Boaden AE, Kingsford MJ (2015) Predators drive community structure in coral reef fish assemblages. Ecosphere 6:art46
Bobiles RU, Nakamura Y (2019) Partially protected marine areas as a conservation tool for commercially important fishes in the Philippines: Do age, size, and design matter? Reg Stud Mar Sci 25
Brodie JE, Kroon FJ, Schaffelke B, Wolanski EC, Lewis SE, Devlin MJ, Bohnet IC, Bainbridge ZT, Waterhouse J, Davis AM (2012) Terrestrial pollutant runoff to the Great Barrier Reef: An update of issues, priorities and management responses. Mar Poll Bull 65:81–100
Ceccarelli D, Emslie M, Richards ZT (2016) Post-disturbance stability of fish assemblages measured at coarse taxonomic resolution masks change at finer scales. PLoS ONE 11:e0156232
Ceccarelli DM, Evans RD, Logan M, Mantel P, Puotinen M, Petus C, Russ GR, Williamson DH (2020) Long-term dynamics and drivers of coral and macroalgal cover on inshore reefs of the Great Barrier Reef Marine Park. Ecol App 30:e02008
Cinner J (2014) Coral reef livelihoods. Current Opinion in Environmental Sustainability 7:65–71
Coker DJ, Wilson SK, Pratchett MS (2014) Importance of live coral habitat for reef fishes. Rev Fish Biol Fish 24:89–126
Connell SD, Kingsford MJ (1998) Spatial, temporal and habitat related variation in the abundance of large predatory fish at One Tree Reef, Australia. Coral Reefs 17:49–57
Curley BG, Glasby TM, Curley AJ, Creese RG, Kingsford MJ (2013) Enhanced numbers of two temperate reef fishes in a small, partial-take marine protected area related to spearfisher exclusion. Biol Conserv 167:435–445
Darling ES, Graham NAJ, Januchowski-Hartley FA, Nash KL, Pratchett MS, Wilson SK (2017) Relationships between structural complexity, coral traits, and reef fish assemblages. Coral Reefs 36:561–575
Day JC, Kenchington RA, Tanzer JM, Cameron DS (2019) Marine zoning revisited: How decades of zoning the Great Barrier Reef has evolved as an effective spatial planning approach for marine ecosystem-based management. Aquat Cons: Mar Fresh Eco 29:9–32
Denny CM, Babcock RC (2004) Do partial marine reserves protect reef fish assemblages? Biol Conserv 116:119–129
Edgar GJ, Stuart-Smith RD, Willis TJ, Kininmonth S, Baker SC, Banks S, Barrett NS, Becerro MA, Bernard AT, Berkhout J, Buxton CD, Thomson RJ (2014) Global conservation outcomes depend on marine protected areas with five key features. Nature 506:216-+
Emslie M, Cheal A, Sweatman H, Delean S (2008) Recovery from disturbance of coral and reef fish communities on the Great Barrier Reef, Australia. Mar Ecol Prog Ser 371:177–190
Emslie MJ, Logan M, Williamson DH, Ayling AM, MacNeil MA, Ceccarelli D, Cheal AJ, Evans RD, Johns KA, Jonker MJ, Miller IR, Sweatman HPA (2015) Expectations and outcomes of reserve network performance following re-zoning of the Great Barrier Reef Marine Park. Curr Biol 25:983–992
Friedlander AM, Brown EK, Jokiel PL, Smith WR, Rodgers KS (2003) Effects of habitat, wave exposure, and marine protected area status on coral reef fish assemblages in the Hawaiian archipelago. Coral Reefs 22:291–305
Frisch AJ, Baker R, Hobbs JPA, Nankervis L (2008) A quantitative comparison of recreational spearfishing and linefishing on the Great Barrier Reef: implications for management of multi-sector coral reef fisheries. Coral Reefs 27:85–95
Frisch AJ, Cole AJ, Hobbs J-PA, Rizzari JR, Munkres KP (2012) Effects of Spearfishing on Reef Fish Populations in a Multi-Use Conservation Area. PLoS ONE 7:e51938
Froese R, Pauly D (2021) Fishbase
Gell FR, Roberts CM (2003) Benefits beyond boundaries: the fishery effects of marine reserves. Trends Ecol Evol 18:448–455
Graham NAJ, Ainsworth TD, Baird AH, Ban NC, Bay LK, Cinner JE, De Freitas DM, Diaz-Pulido G, Dornelas M, Dunn SR, Fidelman PI, Williamson DH (2011) From microbes to people: Tractable benefits of no-take areas for coral reefs. In: Gibson RN, Atkinson RJA, Gordon JDM (eds) Oceanography and Marine Biology: An Annual Review, Vol 49, pp105–135
Green R (1979) Sampling design and statistical methods for environmental biologists. Wiley-Interscience, New York
Hall AE, Kingsford MJ (2021) Habitat type and complexity drive fish assemblages in a tropical seascape. J Fish Biol 99:1364–1379
Hall AE, Cameron DS, Kingsford MJ (2021) Partially protected areas as a management tool on inshore reefs. Rev Fish Biol Fish 31:631–651
Hall A, Cameron D, Kingsford M (2022) Prohibiting spearfishing boosts conservation outcomes for partially protected areas. Biol Conserv 272:109662
Hannan KD, McMahon SJ, Munday PL, Rummer JL (2021) Contrasting effects of constant and fluctuating pCO2 conditions on the exercise physiology of coral reef fishes. Mar Environ Res 163:105224
Harasti D, Williams J, Mitchell E, Lindfield S, Jordan A (2018) Increase in relative abundance and size of snapper Chrysophrys auratus within partially-protected and no-take areas in a temperate marine protected area. Front Mar Sci 5
Harrison HB, Williamson DH, Evans RD, Almany GR, Thorrold SR, Russ GR, Feldheim KA, Van Herwerden L, Planes S, Srinivasan M, Berumen ML, Jones GP (2012) Larval export from marine reserves and the recruitment benefit for fish and fisheries. Curr Biol 22:1023–1028
Hughes TP (2008) Human impact on coral reefs. In: Hutchings PA, Kingsford MJ, Hoegh-Guldberg O (eds) The Great Barrier Reef: biology, environment and management. CSIRO Publishing, Collingwood, pp 85–95
Hughes TP, Kerry JT, Alvarez-Noriega M, Alvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R, Bridge TC, Wilson SK (2017) Global warming and recurrent mass bleaching of corals. Nature 543:373
Jones GP, McCormick ML, Srinivasan M, Eagle JV (2004) Coral decline threatens fish biodiversity in marine reserves. Proc Nat Acad Sci 101:8251–8253
Kerry JT, Bellwood DR (2012) The effect of coral morphology on shelter selection by coral reef fishes. Coral Reefs 31:415–424
Kingsford MJ (2009) Contrasting patterns of reef utilization and recruitment of coral trout (Plectropomus leopardus) and snapper (Lutjanus carponotatus) at One Tree Island, southern Great Barrier Reef. Coral Reefs 28:251–264
Lam VYY, Chaloupka M, Thompson A, Doropoulos C, Mumby PJ (2018) Acute drivers influence recent inshore Great Barrier Reef dynamics. Proc Royal Soc B: Bio Sci 285:20182063
Lester SE, Halpern BS (2008) Biological responses in marine no-take reserves versus partially protected areas. Mar Ecol Prog Ser 367:49–56
Malcolm HA, Williams J, Schultz AL, Neilson J, Johnstone N, Knott NA, Harasti D, Coleman MA, Jordan A (2018) Targeted fishes are larger and more abundant in “no-take” areas in a subtropical marine park. Estuar Coast Shelf Sci 212:118–127
McClanahan TR, Graham NAJ, Calnan JM, MacNeil MA (2007) Toward pristine biomass: Reef fish recovery in coral reef marine protected areas in Kenya. Ecol App 17:1055–1067
McClure EC, Richardson LE, Graba-Landry A, Loffler Z, Russ GR, Hoey AS (2019) Cross-Shelf Differences in the Response of Herbivorous Fish Assemblages to Severe Environmental Disturbances. Diversity 11:13
McCook LJ, Ayling T, Cappo M, Choat JH, Evans RD, De Freitas DM, Heupel M, Hughes TP, Jones GP, Mapstone B, Marsh H, Williamson DH (2010) Adaptive management of the Great Barrier Reef: A globally significant demonstration of the benefits of networks of marine reserves. Proc Nat Acad Sci 107:18278–18285
Mellin C, Aaron MacNeil M, Cheal AJ, Emslie MJ, Julian Caley M (2016) Marine protected areas increase resilience among coral reef communities. Ecol Lett 19:629–637
Moynihan JL, Hall AE, Kingsford MJ (2022) Interrelationships between soft corals and reef-associated fishes on inshore-reefs of the Great Barrier Reef. Mar Ecol Prog Ser 698:15–28
Mumby PJ, Harborne AR (2010) Marine reserves enhance the recovery of corals on Caribbean reefs. PLoS ONE 5:1–7
Mumby PJ, Dahlgren CP, Harborne AR, Kappel CV, Micheli F, Brumbaugh DR, Holmes KE, Mendes JM, Broad K, Sanchirico JN, Buch K, Gill AB (2006) Fishing, trophic cascades, and the process of grazing on coral reefs. Science 311:98–101
Nash KL, Graham NAJ, Wilson SK, Bellwood DR (2013) Cross-scale habitat structure drives fish body size distributions on coral reefs. Ecosystems 16:478–490
Pratchett MS, Coker DJ, Jones GP, Munday PL (2012) Specialization in habitat use by coral reef damselfishes and their susceptibility to habitat loss. Ecol Evol 2:2168–2180
Russ GR, Alcala AC (2010) Decadal-scale rebuilding of predator biomass in Philippine marine reserves. Oecologia 163:1103–1106
Russ GR, Alcala AC, Maypa AP, Calumpong HP, White AT (2004) Marine reserve benefits local fisheries. Ecol App 14:597–606
Russ GR, Cheal AJ, Dolman AM, Emslie MJ, Evans RD, Miller I, Sweatman H, Williamson DH (2008) Rapid increase in fish numbers follows creation of world’s largest marine reserve network. Curr Biol 18:R514–R515
Sciberras M, Jenkins SR, Mant R, Kaiser MJ, Hawkins SJ, Pullin AS (2015) Evaluating the relative conservation value of fully and partially protected marine areas. Fish Fish 16:58–77
Sievers KT, McClure EC, Abesamis RA, Russ GR (2020) Non-reef habitats in a tropical seascape affect density and biomass of fishes on coral reefs. Ecol Evol 10:13673–13686
Turnbull JW, Johnston EL, Clark GF (2021) Evaluating the social and ecological effectiveness of partially protected marine areas. Conserv Biol 35:921–932
Webley J, McInnes K, Tiexeira D, Lawson A, Quinn R (2015) Statewide Recreational Fishing Survey 2013–14. Department of Agriculture and Fisheries
Wenger AS, Williamson DH, da Silva ET, Ceccarelli DM, Browne NK, Petus C, Devlin MJ (2015) Effects of reduced water quality on coral reefs in and out of no-take marine reserves. Conserv Biol 30:142–153
Williams DM, Hatcher A (1983) Structure of fish communities on outer slopes of inshore, mid-shelf and outer-shelf reefs of the Great Barrier Reef. Mar Ecol Prog Ser 10:239–250
Williamson D, Ceccarelli D, Evans R, Jones G, Russ GR (2014) Habitat dynamics, marine reserve status, and the decline and recovery of coral reef fish communities. Ecol evol 4:337–354
Williamson DH, Ceccarelli DM, Jones GP, Russ GR (2019) Assessing the ecological effects of management zoning on inshore reefs of the Great Barrier Reef Marine Park. Great Barrier Reef Marine Park Authority, Townsville, Australia
Williamson DH, Ceccarelli DM, Evans RD, Hill JK, Russ GR (2015) Derelict Fishing Line Provides a Useful Proxy for Estimating Levels of Non-Compliance with No-Take Marine Reserves. PLoS ONE 9:e114395
Wilson SK, Burgess SC, Cheal AJ, Emslie M, Fisher R, Miller I, Polunin NV, Sweatman HPA (2008) Habitat utilization by coral reef fish: implications for specialists vs generalists in a changing environment. J Anim Ecol 77:220–228
Woolsey E, Bainbridge SJ, Kingsford MJ, Byrne M (2012) Impacts of cyclone Hamish at One Tree Reef: integrating environmental and benthic habitat data. Mar Biol 159:793–803
Zupan M, Fragkopoulou E, Claudet J, Erzini K, Costa BHE, Goncalves EJ (2018) Marine partially protected areas: drivers of ecological effectiveness. Front Ecol Enviro 16:381–387
Acknowledgements
The authors would like to thank Stephanie Garra, Jaimee Moynihan, and Daniella Martinez for assistance with field work, and Murray Logan for advice on statistical analyses. The project was funded by a Environmental Enhancement Fund from Evolution Mining awarded to A. E. Hall.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This research was carried out under an approved animal ethics permit # A2438. The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hall, A.E., Sievers, K.T. & Kingsford, M.J. Conservation benefits of no-take marine reserves outweigh modest benefits of partially protected areas for targeted coral reef fishes. Coral Reefs 42, 319–333 (2023). https://doi.org/10.1007/s00338-022-02340-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-022-02340-w