Abstract
Boring microorganisms (microendoliths) are ubiquitous in living corals, constituting the skeleton microbiome important for coral health and reef resilience. Numerous microborings were recognized in Upper Jurassic (ca. 160 million years ago) corals (Pomerania, Poland) providing a glimpse into the oldest scleractinian skeleton microbiome so far. Scanning electron microscope study of resin casts of microborings (ca. 4 μm) revealed that they represent mostly the ichnospecies Ichnoreticulina elegans, commonly considered as traces of Ostreobium quekettii, an alga adapted for a low-light environment. The distribution pattern of microborings (occurrence in the inner part of the skeletal elements, commonly upward orientation) implies that they were not done post-mortem, but by microendoliths inhabiting the coral skeleton during coral life. These findings imply that the most common boring microorganism inhabiting the skeleton of Jurassic corals was, like in modern corals, O. quekettii or a similar green alga. The microbiome of dead parts of modern living colonies revealed by analysis of microborings is an unexplored, but is a perspective topic for research by reef biologists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
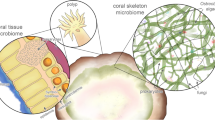
Coral is a holobiont, a complex consortium comprised of the coral host (polyp) and its tissue (e.g., Symbiodinium, commonly termed as zooxanthellae), mucus, and skeleton-associated microbiota (suite of bacteria, algae, fungi, and viruses) (Rohwer et al. 2002). Recent papers on coral microbiomes show that there is a growing interest in the endolithic microorganisms inhabiting skeletons of living corals because their contribution to coral reef bioerosion, coral health, and reef functioning is more important than previously appreciated (for review: van Oppen and Blackall 2019; Ricci et al. 2019; Pernice et al. 2020).
Boring microorganisms (algae, fungi, bacteria), termed microeuendoliths (general term: microendoliths; Golubic et al. 1981), are ubiquitous in modern, living corals. Traces produced by them have a high fossilization potential; thus, analysis of microborings can help to reveal the skeleton microbiome of fossil corals. Due to physical and chemical changes in the sediment, findings of such microborings have been rarely reported in fossil corals, in contrast with post-mortem traces. However, previous (Elias and Lee 1993) and recent studies (Kołodziej et al. 2012, 2016; Salamon and Kołodziej 2021; Salamon et al. 2019, 2021) indicate that these traces are more common than would be expected (for review: Salamon and Kołodziej 2021). Microendoliths are common in various dead biogenic substrates, but symbiotic endolithic microbes were commonly reported from corals, but very rare from other macroorganisms (see references in Salamon and Kołodziej 2021).
Material, geological background, and methods
The specimens for the present study were collected in the 1980s in a gravel pit near Ostromice, ca. 10 km east of the town of Wolin (Pomerania, NW Poland) (Roniewicz 1984) (Fig. 1a). The Upper Jurassic (Oxfordian–Kimmeridgian, ca. 152,1–163,5 Mya) colonial corals occurred as pebbles (erratics) in the Pleistocene glacial sediments.
Detailed taxonomic studies performed by Roniewicz (1984) revealed colonies of some extinct coral species. Samples of Thamnasteria concinna (family Thamnasteriidae), a species that dominates in Ostromice and other sites in Pomerania, were studied in detail in this paper. Well-preserved colonies are massive and submassive, 1 to 20 cm in diameter (Fig. 1b).
A series of cuboid blocks (25 × 40 × 5 mm) representing whole profiles of nine colonies (ca. 100 × 100 × 70 mm) were prepared in accordance to standard methodology to receive resin casts of microborings to be studied under SEM (Golubic et al. 1970; see also Salamon et al. 2019). Additionally, seven standard thin sections (40 × 27 mm), without a cover glass, etched with dilute HCl (3–3.5%), were studied under SEM. Microborings showing a similar morphology and distribution pattern were recognized in all studied samples. Samples ZPAL H. IV 150, 335, 753, and Szcz2 are documented in this paper.
The samples were analyzed with a Nikon Eclipse Ni-U multipurpose microscope (normal light and fluorescence) and a Hitachi S-4700 SEM at the Institute of Geological Sciences, Jagiellonian University in Kraków, where they are also housed. The other samples of Ostromice corals are housed at the Institute of Paleobiology, Polish Academy of Sciences, Warszawa (collection ZPAL H. IV), and in collection of H. H. Schnick in Germany (samples abbreviated as Szcz).
Results
Interseptal space (between radial elements) in corals is usually empty, rather than filled with sediment or cement. Microborings in skeletal elements remain empty and hardly visible in thin sections observed under the petrographic microscope (Fig. 2a). However, resin-filled microborings (in transverse and longitudinal sections) are evident in SEM (Figs. 2d, 3) and even in etched thin sections, under petrographic (Fig. 2b) and fluorescence microscopes (Fig. 2c). Microborings occur in all the studied samples, in the whole coral colony. Their abundance is higher in the inner part of skeletal elements (Fig. 3a, b), whereas resin casts distributed at their margins are rare (Fig. 3a).
Microborings in coral septa in longitudinal petrographic thin sections. a Hardly visible microborings (arrows) in an unetched thin section under normal transmitted light. Microborings and interseptal space (is) are filled by resin. b–d Resin casts of microborings made visible by lightly etched thin section under transmitted light (b) and under fluorescence (c) as well as in cuboid blocks under SEM (d)
SEM images of numerous resin-replicated microborings (Ichnoreticulina elegans) in transverse (a, b) and longitudinal sections (c). a, b Cross sections of etched skeletal elements show a scattered casts in the central part the columella (a) and the septum (b). c Bundles of the branched casts of I. elegans oriented upward and parallel to growth direction of the coral colony
The inner network of microborings is composed mainly by flat zig-zag or undulated casts reaching a diameter of 3.5–4 μm (Figs. 2, 3). Microborings show dichotomous (Y-shape) or lateral ramification and delicate lateral swellings or short, triangular branches (ca. 10 µm long) at the points of bending. The resin casts in the central part of skeletal elements are predominantly scattered (Fig. 3a) and form more or less tangled bundles (Figs. 2b–d, 3b, c). The casts retain an upward orientation, parallel to the growth direction of the coral in all cases (Fig. 3c), and are barely connected with surfaces of skeletal elements (Fig. 3a). Additionally, there are, but much rarer, simple, slightly undulated, curved or straight, thin (1–1.5 μm in diameter), tubular resin casts.
Discussion and conclusions
Skeletons of live corals are bored by symbiotic endoliths from the inside out (Le Campion-Alsumard et al. 1995). The described resin casts of microborings in Jurassic corals show a similar pattern. They occur in the whole colony of the all studied specimens, predominantly in the inner part of the skeletal elements, and are usually oriented upward, roughly along longitudinal axis of the colonies, in the direction of coral growth. The distribution pattern of microborings in Jurassic corals clearly indicates that most of them, if not all, were produced during the growth of the host coral, as was shown in the classic case study of microendoliths in modern corals by Le Campion-Alsumard et al. (1995; see also Salamon and Kołodziej 2021, Fig. 9). If the microborings were formed post-mortem, they would be denser on the margins of the skeletal elements (dead coral surfaces). Moreover, microborings in dead modern corals are essentially restricted to the upper (usually less than 1 mm) surface layer of the coral colony, and their abundance decreases rapidly toward the interior (Le Campion-Alsumard et al. 1995).
Recently, Salamon et al. (2021) revealed the diverse nature of microborings in Cretaceous corals. Most of those were done by microendoliths in vivo. Figure 4 shows an illustrative drawing showing distribution of two microborings types in modern and fossil corals: (i) produced by coral-associated microendoliths and (ii) those produced post-mortem (very rare in samples studied here). Microborings produced in vivo occur roughly along the longitudinal axis of the septa (Fig. 4a, c; distribution observed in studied here corals) or are orientated randomly (Fig. 4b, d) (see also Salamon and Kołodziej 2021).
General patterns of post-mortem (black and dark gray, located close to the surface) and in vivo microborings (green and light gray, in inner parts) in coral septa in longitudinal (a, b) and transverse (c, d) sections. a, c Upward-oriented network of I. elegans parallel to growth direction of the coral colony. b, d Randomly oriented I. elegans not or hardly reaching the surface of septum
The characteristics of the microborings in this study are consistent with the morphological properties described as ichnospecies Ichnoreticulina elegans (Radtke 1991; Radtke and Golubic 2005). These are commonly considered as traces produced by the chlorophyte green alga Ostreobium quekettii Bornet et Flahault 1889 (Radtke 1991; Tribollet 2008). Morphology of thinner microborings suggests their fungal or cyanobacterial origin (see Radtke 1991).
We conclude that the microborings in the studied corals were formed during the coral’s life and are convincing record of a fossil skeleton microbiome. Ostreobium quekettii (or algae referred to as Ostreobium sp.) is the most common species of the boring algal flora in coral reefs, mostly in dead carbonate substrates. It is also the main endolithic species (symbiotic microendoliths or symbiotic endolithic microbes, in accordance with commonly accepted broad definition of symbiosis) of the skeleton microbiome of modern scleractinian corals. This filamentous microalga is well adapted to the extreme, very dimly lit environment of the skeleton, which also experiences strong fluctuations of pH and O2 (see Iha et al. 2021). Fungi and bacteria also contribute to the skeleton microbiome (van Oppen and Blackall 2019; Ricci et al. 2019; Pernice et al. 2020). However, only some of them are euendoliths that leave microborings and thus have fossilization potential, while others (cryptoendoliths) live in the pores of the skeleton.
Commonly, morphology of microborings matches the outline of its producer; thus, they can be attributed to modern organisms with a fair degree of confidence and applied as paleoenvironmental proxies. Based on the microborings’ morphology, the skeletal microbiome in Jurassic corals studied here and in Cretaceous and Paleogene corals (Salamon and Kołodziej 2021; Salamon et al. 2019, 2021) was, like in modern corals, dominated by O. quekettii or similar algae. This microalga has recently been considered as a complex of genotypes separated by the host that vary along depth gradients and may represent more than a single species (Gutner-Hoch and Fine 2011). It is probable that traces of I. elegans known since the Ordovician (geologic period of the Paleozoic, 443.8–485.4 Mya; Vogel and Brett 2009) were also produced by other chlorophyte species. However, phylogenetic analyses have indicated that the Ostreobium clade originated in the Ordovician (Marcelino and Verbruggen 2016).
Much less is known about the activity of the endolithic microbial community than about tissue- and mucus-associated bacteria. Ostreobium may be vital for the survivorship of corals during bleaching (Fine and Loya 2002). Blooms of these algae have been observed in the skeleton when corals bleach. In contrast, it has been demonstrated that the coral-associated endolithic fungi are mostly parasitic (Bentis et al. 2000).
Although studies of the coral skeleton microbiome are increasing, knowledge of its role in fossil corals is in its infancy. Microborings produced by symbiotic microorganisms are rarely reported from fossil corals, but have been recognized even in Paleozoic (Ordovician) corals (Elias and Lee 1993). Previous studies revealed microborings in the Cretaceous and Paleogene corals that were mostly produced by Ostreobium, rarely by red algae, bacteria, and fungi (Kołodziej et al. 2012, Kołodziej et al. 2016; Salamon et al. 2019, Salamon et al. 2021; for review: Salamon and Kołodziej 2021). The fossil coral skeleton microbiome has yet to be fully elucidated, but analysis of traces of boring microorganisms may be the key to its full recognition. However, studies of coral samples of stratigraphically different ages are necessary. Continued research is essential to reveal the composition and changes of the skeleton microbiome in corals of different ages, taxa, environmental settings, and paleogeographic domains. SEM studies of resin-replicated microborings are necessary. Some simple methods allow faster recognition of such traces and allow selection of proper samples for further SEM studies (Salamon et al. 2019).
Recent genomic studies indicate that Ostreobium is key member of the coral holobiont and plays important roles in coral biology (Iha et al. 2021). Data presented herein imply that this green alga was a main member of the skeleton microbiome of corals as old as the Late Jurassic. Analysis of microborings produced by coral-associated microorganisms offers an additional tool in studies of modern corals: subfossil Holocene samples and dead parts of living corals. Such studies could have to analyze bands which are originally colored (green, black, pink) and related with different microbial endolithic taxa (green algae, fungi, bacteria) that are known to bloom under some conditions, including environmental stresses (see Ricci et al. 2021). Green algal banding response of coral mass bleaching events has a great fossilization potential and is expected to be preserved in fossil corals as zones with more dense network of microborings.
Interspecific differences in endolithic green algal biomass are likely influenced by how algae respond to skeletal variability, its density, coral species’ capacity for capturing, and scattering light (Fordyce et al. 2021). Microborings in dead parts of modern colonies provide information about the trace producer that allows comparison with the skeleton microbiome of living part of the colony. This research topic is unexplored, but provides biologists a fascinating insight into changes in the microbiome through time.
References
Bentis CJ, Kaufman L, Golubic S (2000) Endolithic fungi in reef-building corals (Order: Scleractinia) are common, cosmopolitan, and potentially pathogenic. Biol Bull 198:254–260
Elias RJ, Lee D-J (1993) Microborings and growth in Late Ordovician halysitids and other corals. J Paleontol 67:922–934
Fine M, Loya Y (2002) Endolithic algae—an alternative source of energy during coral bleaching. Proc Biol Sci 269:1205–1210
Fordyce AJ, Ainsworth TD, Leggat W (2021) Light capture, skeletal morphology, and the biomass of corals’ boring endoliths. mSphere 6:e00060-e121
Golubic S, Brent G, Le Campion T (1970) Scanning electron microscopy of endolithic algae and fungi using a multipurpose casting embedding technique. Lethaia 3:203–217
Golubic S, Friedmann I, Schneider J (1981) The lithobiontic ecological niche, with special reference to microorganisms. J Sediment Petrol 51:475–478
Gutner-Hoch E, Fine M (2011) Genotypic diversity and distribution of Ostreobium quekettii within scleractinian corals. Coral Reefs 30:643–650
Iha C, Dougan KE, Varela JA, Avila V, Jackson CJ, Bogaert KA, Chen Y, Judd LM, Wick R, Holt KE, Pasella MM, Ricci F, Repetti SI, Medina M, Marcelino VR, Chan CX, Verbruggen H (2021) Genomic adaptations to an endolithic lifestyle in the coral-associated alga Ostreobium. Curr Biol 31:1393–1402
Kołodziej B, Golubic S, Bucur II, Radtke G, Tribollet A (2012) Early Cretaceous record of microboring organisms in skeletons of growing corals. Lethaia 45:34–45
Kołodziej B, Idakieva V, Ivanov M, Salamon K (2016) New record of endolithic algae syn vivo associated with an Early Cretaceous coral. Carnets Geol 16:633–640
Le Campion-Alsumard T, Golubic S, Hutchings P (1995) Microbial endoliths in the skeletons of live and dead corals: Porites lobata (Moorea, French Polynesia). Mar Ecol Prog Ser 117:149–157
Marcelino VR, Verbruggen H (2016) Multi-marker metabarcoding of coral skeletons reveals a rich microbiome and diverse evolutionary origins of endolithic algae. Sci Rep 6:31508
Pernice M, Raina JB, Rädecker N, Cárdenas A, Pogoreutz C, Voolstra CR (2020) Down to the bone: the role of overlooked endolithic microbiomes in reef coral health. ISME J 14:325–334
Radtke G (1991) Die mikroendolithischen Spurenfossilien im Alt-Tertiär West-Europas und ihre palökologische Bedeutung. Cour Forsch Senck 138:1–185
Radtke G, Golubic S (2005) Microborings in mollusk shells, Bay of Safaga, Egypt: morphometry and ichnology. Facies 51:118–134
Ricci F, Marcelino VR, Blackall LL, Kühl M, Medina M, Verbruggen H (2019) Beneath the surface: community assembly and functions of the coral skeleton microbiome. Microbiome 7:159
Ricci F, Fordyce A, Leggat W, Blackall LL, Ainsworth T, Verbruggen H (2021) Multiple techniques point to oxygenic phototrophs dominating the Isopora palifera skeletal microbiome. Coral Reefs 40:275–282
Rohwer F, Seguritan V, Azam F, Knowlton N (2002) Diversity and distribution of coral-associated bacteria. Mar Ecol Prog Ser 243:1–10
Roniewicz E (1984) Aragonitic Jurassic corals from erratic boulders on the south Baltic coast. Ann Soc Geol Pol 54:65–77
Salamon K, Kołodziej B (2021) Unravelling the microbiome of fossil corals: a message from microborings. Hist Biol. https://doi.org/10.1080/08912963.2021.1971213
Salamon K, Kołodziej B, Stefanskyi VL (2019) Simple methods for detection of microborings produced by coral-associated microendoliths. Facies 65:16
Salamon K, Kołodziej B, Löser H (2021) Diverse nature of ubiquitous microborings in Cenomanian corals (Saxonian Cretaceous Basin, Germany). Cretac Res 126:104888
Tribollet A (2008) The boring microflora in modern coral reef ecosystems: a review of its roles. In: Wisshak M, Tapanila L (eds) Current developments in bioerosion. Springer, Berlin, pp 67–94
van Oppen MJH, Blackall LL (2019) Coral microbiome dynamics, functions and design in a changing world. Nat Rev Microbiol 17:557–567
Vogel K, Brett CE (2009) Record of microendoliths in different facies of the Upper Ordovician in the Cincinnati Arch region USA: the early history of light-related microendolithic zonation. Palaeogeogr Palaeoclimatol Palaeoecol 281:1–24
Acknowledgements
We are grateful to Professor Ewa Roniewicz (Polish Academy of Sciences, Warszawa) for providing coral samples for investigation. The research is a contribution to K. Salamon’s project, funded by the National Science Centre, Poland (No. 2016/23/N/ST10/01334). B.K. and K.S. were additionally supported by Jagiellonian University. We are grateful to Dr. Lauren T. Toth and two anonymous referees for corrections and helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Topic Editor Lauren T. Toth
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salamon, K., Kołodziej, B., Radtke, G. et al. Microborings in Jurassic scleractinians: a glimpse into the ancient coral skeleton microbiome. Coral Reefs 41, 863–867 (2022). https://doi.org/10.1007/s00338-022-02248-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-022-02248-5