Abstract
There is increasing evidence that non-reef habitats in the seascape surrounding coral reefs are widely used by reef-associated fishes. However, our understanding of seascape use in the Indo-Pacific region is incomplete due to its large geographical range and as a consequence, considerable environmental variation (e.g. tidal regimes). We used remote video cameras to survey reef-associated fishes within five habitat types (coral reef slope, coral reef flat, macroalgal beds, mangroves and seagrass meadows) around the Tigak Islands, Kavieng, Papua New Guinea. Of the 282 shallow-water reef-associated species observed across 360 videos, 35% (99 species) were recorded in non-reef habitats, the majority (78 species) on multiple occasions. We found that macroalgal beds dominated by low-complexity algal genera (e.g. Halimeda, Caulerpa) were used extensively by reef-associated fishes, complementing previous research that has documented the use of canopy-forming macroalgae (e.g. Sargassum). Mean species richness and relative abundances (MaxN) of reef-associated fishes were twofold higher in macroalgal beds than mangroves or seagrass. Interestingly, mangroves contained the most distinct fish assemblage of the three non-reef habitats, including several reef-associated species that were not recorded from any other habitat type. This suggests that mangroves possess attributes not shared by other shallow non-reef, or even reef, habitats. Importantly, many of the fish families commonly found in non-reef habitats (i.e. lethrinids, lutjanids) are targeted by local fishers and are thus critical to sustaining local livelihoods. Our study demonstrates that non-reef habitat use is common for many reef-associated fishes and highlights the need to incorporate a range of habitats into study designs to better understand habitat use patterns in the Indo-Pacific. Given the widespread degradation of coral reefs and other shallow-water habitats, we emphasize the need to recognize that reefs are embedded within a mosaic of habitat types that influence patterns and processes and that management strategies should be scaled appropriately.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The influence of the wider landscape or seascape on patterns and processes observed within a habitat patch remains a long-standing and important question in ecology (Miller-Rushing et al. 2019). It has been proposed that the close proximity of particular kinds of habitat may have a range of effects on community composition, species diversity and ecosystem function (Fahrig et al. 2011). The effects of habitat adjacency on species diversity may occur directly, for example if a species requires resources that are found in both habitat types (e.g. different breeding and foraging habitats); or indirectly, via exchanges of nutrients and other subsidies between adjacent habitats (Alsterberg et al. 2017). Habitat adjacency and complementarity have long been recognized as important drivers of species abundance and distribution patterns in terrestrial systems (Fahrig et al. 2011), and a similar effect is increasingly evident from shallow-water coastal ecosystems (e.g. Dorenbosch et al. 2006; Pittman et al. 2007; Olds et al. 2012; Berkström et al. 2013; Aller et al. 2014).
Many coral reef fish taxa use multiple habitat types throughout their life cycle (Nagelkerken et al. 2000; Adams et al. 2006; Harborne et al. 2008). An understanding of the use of these habitats and the connections between them is critical to predict and manage the likely impacts of local and global disturbances (Berkström et al. 2012; Pittman and Olds 2015). Although numerous studies have documented the effects of coral loss on coral reef fish communities (e.g. Jones et al. 2004; Pratchett et al. 2011; Richardson et al. 2018), far fewer have considered how disturbances in the wider seascape can influence fish communities, despite many reef systems occurring within a mosaic of highly productive (Birkeland 1985) and extensive (Parrish 1989) non-reef habitat types such as mangroves and seagrass meadows. Much like coral reefs, these shallow-water ecosystems are undergoing rapid systemic change with widespread areal losses and degradation in recent decades (Valiela et al. 2001; Waycott et al. 2009; Hamilton and Casey 2016).
The number of coral reef-associated fish species that have been observed in non-reef habitats is substantial. Globally, at least 670 species of coral reef-associated fishes have been recorded in non-reef habitats in addition to reefs, representing approximately 20% of all coral reef fish species (Sambrook et al. 2019). Reef fishes use the wider seascape for a range of reasons including foraging (Beets et al. 2003; Hitt et al. 2011), spawning (Pittman and McAlpine 2003) and as juvenile habitat before migrating to reefs as subadults or adults (Dahlgren and Eggleston 2000; Nagelkerken et al. 2000, 2001; Adams et al. 2006; Jaxion-Harm et al. 2011). As a result, short- and long-term movements by reef fishes between different components of the seascape can contribute to ecosystem functioning on reefs through nutrient transfer (Meyer and Schultz 1985; Shantz et al. 2015), trophic subsidies and cascades (Heck et al. 2008; Harborne et al. 2016) and population replenishment (Nakamura et al. 2008; McMahon et al. 2012).
Much of our knowledge about seascape use by reef-associated fishes comes from the Caribbean (Nagelkerken et al. 2000; Aguilar-Perera and Appeldoorn 2008; Dorenbosch et al. 2009). Less is known about how, why and which reef fishes use non-reef habitats in the Indo-Pacific (Sambrook et al. 2019). Our limited understanding in the Indo-Pacific is, in part, complicated because of its large spatial extent and biophysical variability. For instance, unlike the Caribbean where shallow-water habitats are permanently accessible to fishes, tidal regimes in the Indo-Pacific range from micro- to macro-tidal (Krumme 2009), affecting the accessibility of non-reef habitats to reef fishes (Igulu et al. 2014). As a consequence, understanding fish–habitat relationships in the Indo-Pacific requires exploration across a wider range of locations and tidal regimes.
Understanding habitat use patterns of coral reef fishes is also highly relevant when addressing concerns around long-term food security in the Indo-Pacific (Foale et al. 2013; Blasiak et al. 2017). This is because many of the coral reef fishes that are known to use non-reef habitats are common fisheries targets (Sambrook et al. 2019) and the Indo-Pacific is home to a multitude of small island communities (Brodie et al. 2013) that rely on coral reef fisheries to satisfy daily nutritional requirements (Béné et al. 2007) and as a primary source of income (Bell et al. 2009). Many of these coastal communities are experiencing rapid population growth (Burke et al. 2011) which places increasing pressure on already stretched natural resources (Bell et al. 2009). By expanding our understanding of broader seascape use by coral reef fishes, we can identify essential fish habitats, combinations of habitats and/or target species that require better management or protection, which could contribute towards longer-term sustainable fisheries goals.
The objective of this study was to describe and compare reef-associated fish communities across five habitat types that are common in coastal tropical marine seascapes (i.e. coral reef flats, coral reef slopes, mangroves, seagrass meadows and macroalgae beds) in the Indo-Pacific. Specifically, we compared reef fish assemblages associated with the five habitat types, quantified overlap in habitat use and identified the frequency of use of non-reef habitats in Kavieng, Papua New Guinea.
Methods
Study site
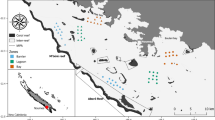
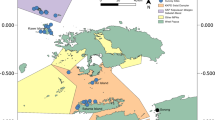
The study was conducted around the Tigak Island Group within the Kavieng lagoon, New Ireland, Papua New Guinea (2° 34′ S, 150° 48′ E; Fig. 1). The Kavieng lagoon is ~ 380 km2 and contains a range of habitats. Extensive reef formations around islands are interspersed with seagrass meadows (predominantly Enhalus and Thalassia spp.), macroalgae beds (predominantly Halimeda, Caulerpa spp.) and mangrove forests (Rhizophora spp.). The annual water temperature ranges between 28.7 °C and 31.6 °C (NOAA 2019). Tides are mixed microtidal (Krumme 2009), with a maximum tidal range of 1.09 m. As a consequence, nearshore habitats (e.g. mangroves) are generally submerged, although inundation depths can be shallow (~ 30 cm).
Data collection
To quantify habitat use by coral reef-associated fishes, we collected data from five common habitat types, specifically: (1) shallow coral reef slopes (3–6 m depth), (2) reef flats containing hard structure (e.g. coral or rock), (3) macroalgal beds, (4) non-estuarine mangroves and (5) seagrass meadows. Data on the use of these habitats by reef fishes were collected between 09:00 and 16:00 during April 2018, using unbaited underwater video cameras. This sampling method was chosen as it reduced any bias due to diver presence (Gotanda et al. 2009; Feary et al. 2011) and also due to the presence of saltwater crocodiles (Crocodylus porosus) in the area. The lack of baits on the cameras ensured that observations of habitat use were not influenced by attraction of fishes to bait (Bassett and Montgomery 2011).
Single video cameras (GoPros) mounted on steel frames were lowered to the substratum, and GPS points were recorded for each camera drop. The use of single, as opposed to stereo, video systems precluded the collection of accurate body size data for fishes and prevented the separation of individual fishes into life stages based on body size. For mangrove and reef slope habitats, care was taken to ensure that cameras faced towards the habitat as opposed to adjacent open waters. Replicate camera drops were separated by a minimum of 50 m, both among and between the five habitat types. Each camera was deployed for a minimum of 20 min to enable high replication across broad spatial scales within a relatively short time frame (e.g. Burge et al. 2012; Bradley et al. 2017; Pereira et al. 2017). The depth of the camera drops ranged from 0.3 to 5.5 m. In total, 86 reef slope, 75 reef flat, 41 macroalgae, 58 mangrove and 100 seagrass videos were analysed. This variation was due to differences in the availability of each habitat type and the exclusion of replicates with low video quality (camera fogging and limited underwater visibility).
Video analysis
For each video, a 15-min segment was analysed by a single observer (KS). Each segment began at least 1 min after the camera had stabilized on the bottom and any sediment disturbed during placement had settled. From each video, we recorded species presence and the maximum number of individuals of a species recorded in a single frame (MaxN, sensu Cappo et al. 2004). MaxN is a common metric used as a conservative measure of relative abundance (Campbell et al. 2015). Cryptic (e.g. Blenniidae, Gobiidae) and surface-dwelling (e.g. Hemiramphidae) taxa were excluded because they were not able to be consistently counted using video. Individuals were identified to genus or species where possible. We used FishBase (Froese and Pauly 2019) to provide an objective assessment of which species were considered reef-associated, hereafter termed “reef fishes”, or not reef-associated (following Sambrook et al. 2019).
Data analysis
Differences in the composition of fish assemblages between the five habitats were compared with a one-way permutational multivariate analysis of variance (PERMANOVA) using Type III sum of squares and 9999 permutations (Anderson et al. 2008). We used a zero-adjusted Bray–Curtis similarity matrix to account for the high number of zeros present in the MaxN data and applied a fourth-root transformation (Clarke et al. 2006). We used pair-wise tests to examine differences between habitats and visualized the data using non-metric multidimensional scaling (nMDS). The similarity percentages routine (SIMPER) was used to identify characteristic species for each habitat type.
Differences in the mean MaxN (i.e. relative abundance) and species richness between habitats were compared with one-way ANOVAs, followed by Tukey’s HSD post hoc tests using R. In addition, one-way ANOVAs were used to compare differences in mean MaxN for common multi-habitat users to explore whether group sizes might differ between habitat types.
Results
Across the five habitat types (reef flat, reef slope, macroalgae, mangrove and seagrass), we recorded 15,492 individuals from a total of 319 taxa, of which 288 were identified to species. Across the five habitats surveyed, 282 out of the 288 species (98%) recorded were classed as reef fishes, with only six species observed on the videos considered non-reef associated. These six non-reef-associated fish species were excluded from all further analyses.
In total, 35% of the reef fishes (99 of 282 species) observed in this study were recorded in non-reef habitats, with 20 species recorded in all three non-reef habitats (Fig. 2a; Online Resource 1). Although less speciose compared to the coral reef slope and flat habitats, the total number of reef fish species observed in each non-reef habitat was considerable (Table 1). A total of 53 reef fish species were observed in both seagrass meadows and mangroves and 60 species were observed in macroalgae beds (Fig. 2; Table 1). While the total number of species observed was similar across the three non-reef habitat types, the mean species richness and relative abundance (i.e. mean MaxN) of reef fish per video was approximately twofold higher in macroalgae beds compared to mangroves or seagrass meadows (Table 1; ANOVA F2,196 = 15.002, p < 0.001). The type and/or combination of non-reef habitats used by each of the 99 reef fish species varied widely. Over half of the species were observed in a single non-reef habitat (Fig. 2), most commonly in macroalgae (22 spp.), followed by mangroves (18 spp.) and seagrass (12 spp.). However, there was also considerable overlap in habitat use with almost half (47 of 99 species) recorded from two or more non-reef habitats (Fig. 2).
Fish community differences between habitats
Reef fish assemblages differed between the five habitats (PERMANOVA pseudo-F4,355 = 38.926, p = 0.0001, Fig. 3), with each habitat type containing a distinct assemblage of reef fishes. Macroalgae beds were broadly characterized by several species of Halichoeres, the tuskfish Choerodon anchorago, the emperor Lethrinus harak and the damselfish Dischistodus chrysopoecilus. Lethrinus harak, C. anchorago and Halichoeres spp., together with the rabbitfish Siganus canaliculatus, were characteristic of seagrass habitats. Mangroves were characterized by a different suite of species including Gerres oyena, Lutjanus ehrenbergii, Lutjanus fulviflamma, the rabbitfish Siganus lineatus and the cardinalfish Sphaeramia orbicularis.
a Non-metric multidimensional scaling plot showing the variation in the community structure of reef fishes recorded from remote underwater video in five habitat types (reef flat, reef slope, macroalgae, mangrove and seagrass). Each point represents a single video based on MaxN data. Species where adults: b ≥ 25 cm max length and c < 25 cm max length recorded on > 10% of videos across all habitats or > 25% of videos in a non-reef habitat type. Abbreviations of genera are D. = Dischistodus, H. = Halichoeres, R. = Rhinecanthus, S. = Stethojulis
Habitat use patterns by reef fish family
Two-thirds of the 41 families recorded during the surveys contained species that used non-reef habitats (27 families). Ten of these reef fish families contained a high proportion (≥ 50%) of species that were recorded in non-reef habitats including jacks (Carangidae), rabbitfishes (Siganidae), snappers (Lutjanidae), emperors (Lethrinidae) and sweetlips (Haemulidae) (Fig. 4a). Patterns of habitat use (i.e. type and number of habitats) varied both among and within families. For example, species of snapper (Lutjanidae) ranged from being only recorded in coral reef habitats to being observed in both coral reef habitats and all three non-reef habitat types. In addition, several families contained species that were not recorded from either of the coral reef habitats (Fig. 4b–d).
a Habitat use patterns of common reef fish families showing proportion of taxa within each family only recorded from coral reefs and those recorded from one, two or three non-reef habitats. [x] denotes the number of species observed in the study, b reef fish taxa observed in macroalgal beds, c mangroves, and, d seagrass. Black bars represent species recorded in a non-reef habitat type and in coral reef habitats. White bars show the number of reef fish species that were not observed in coral reef habitats
Species-level habitat use patterns
The data revealed a wide variety of habitat usage patterns by the 99 reef fish species that were observed in non-reef habitats. Over three-quarters of the species were recorded on multiple occasions away from coral reef habitats. In addition, over half were observed more frequently in at least one non-reef habitat compared to either of the coral reef habitat types (e.g. Fig. 5a, d, f–h; Online Resource 2), while others occurred in similar frequencies across a range of reef and non-reef habitats (e.g. Fig. 5b–c, e). Eighteen species were identified as widespread multi-habitat users and occurred in all five habitat types (Online Resource 2). These ranged from larger-bodied species such as the emperor Lethrinus harak, the snapper Lutjanus fulviflamma and the rabbitfish Siganus canaliculatus to smaller-bodied species such as the butterflyfish Chaetodon vagabundus, and the wrasses Halichoeres scapularis and Stethojulis strigiventer.
Comparing the relative abundance (i.e. MaxN) for some of the most frequently observed and abundant multi-habitat users highlighted potential among habitat differences in group size (Fig. 5). For instance, the mean relative abundance for Lethrinus harak was significantly lower in mangroves (1.24 ± 0.10 SE) compared to reef flat, macroalgae and seagrass habitats (ANOVA F4,158=4.68, p = 0.01). The mean relative abundance for the rabbitfish Siganus lineatus was threefold higher on reef slopes compared to mangroves (ANOVA F1,64= 6.39, p = 0.01), despite occurring on a similar number of occasions in each habitat, indicating that S. lineatus may occur in larger groups on reef slopes. Similarly, the snapper Lutjanus fulviflamma had a higher mean relative abundance on reef slopes (7.55 ± 2.26 SE) compared to all other habitat types, almost double the mean relative abundance in mangroves (4.48 ± 0.79 SE).
Discussion
There is increasing evidence of the widespread use of non-reef habitats by reef-associated fishes, with a recent meta-analysis suggesting that ~ 20% of reef fish species use non-reef habitats (Sambrook et al. 2019). By comparing fish assemblages across five habitat types in Kavieng, Papua New Guinea, we found that percentage to be even higher, with over a third (35%) of reef-associated fish species recorded in non-reef habitats, many of which occurred in multiple non-reef habitats. In addition, many of the species identified using multiple habitats are ecologically (e.g. the macroalgae browser Siganus canaliculatus) or economically important. Our findings thus provide additional support for claims of widespread use of multiple habitat types by reef fishes and for the importance of better understanding habitat complementarity in coral reef ecosystems. Our study also demonstrates the value of examining species distributions across a wider range of habitats at each study location in the Indo-Pacific, as has previously been noted for the Caribbean (Nagelkerken et al. 2000; Harborne et al. 2008). Observed species-specific patterns of habitat use would have been incomplete had we sampled a more limited range of habitats.
Macroalgal beds contained, on average, more species and higher relative abundances of reef fish compared to mangroves or seagrass meadows, albeit considerably lower than the two reef habitats. This supports the growing number of studies that have documented high abundances and diversity of coral reef fishes in macroalgae beds (e.g. Rossier and Kulbicki 2000; Wilson et al. 2010; Chaves et al. 2013; Evans et al. 2014; Eggertsen et al. 2017; Tano et al. 2017). The majority of these studies have focused on beds of canopy-forming macroalgae, such as Sargassum (but see Rossier and Kulbicki 2000), finding that the structural complexity and canopy height of algae are important factors influencing its use by coral reef fishes, particularly juvenile life stages (Wilson et al. 2014; Fulton et al. 2019, 2020; Tang et al. 2020). In contrast, our macroalgal study sites were predominantly a mixture of Halimeda and Caulerpa, which are smaller and less structurally complex than Sargassum. Despite these differences, we observed a similar suite of families (e.g. Labridae, Lethrinidae, Siganidae) to that reported from Sargassum-based studies. This suggests that factors other than structural complexity of the algae, such as the availability of food resources, visibility of predators and proximity to other habitat types (e.g. van Lier et al. 2018), may also influence the suitability of macroalgae beds for coral reef fishes. Although fish assemblages in macroalgal beds were typically more speciose than seagrass meadows or mangroves, there was considerable overlap in species using macroalgae beds and seagrass meadows, indicating that these two habitat types could act as complementary habitats for some fishes (Dunning et al. 1992). Seagrass meadows and macroalgal beds can be structurally similar (Gratwicke and Speight 2005) and contain comparable resources and refugia opportunities (Macreadie et al. 2017) which could drive similarities in habitat use.
In contrast, mangroves contained a distinct assemblage of reef fishes including some species (e.g. Lutjanus argentimaculatus) that were exclusive to this habitat. Although we were unable to separate our data into life stages based on body size, we did observe several individuals, particularly snappers (i.e. lutjanids), with juvenile markings within mangrove habitats. These findings are interesting, but require additional research, given the debate about whether mangrove habitats in the Indo-Pacific are as important for coral reef fishes, particularly juveniles, as they are in the Caribbean (Blaber & Milton 1990; Thollot 1992; Dorenbosch et al. 2005; Nakamura et al. 2008; Unsworth et al. 2009; Barnes et al. 2012; Kimirei et al. 2013; Dubuc et al. 2019). Mangrove systems in the Indo-Pacific vary considerably depending on tidal regime (Krumme 2009; Igulu et al. 2014), geomorphological and spatial context (Blaber 2007; Unsworth et al. 2008; Olds et al. 2013; Bradley et al. 2019), as well as the size, composition and structural complexity of mangrove forests (Laegdsgaard and Johnson 2001; Nanjo et al. 2014), all of which can influence habitat use patterns (Sheaves 2017). Here, we examined non-estuarine mangroves in a microtidal location and suggest that under these conditions, mangroves appear to possess certain attributes (e.g. refuge, food availability) that are not provided by the other shallow non-reef, or even reef, habitats surveyed. Therefore, the impact of mangrove loss or degradation could be greater for coral reef fishes that appear to selectively use mangroves compared to species that appear to use multiple habitats interchangeably.
Importantly, a substantial number (> 50%) of the species using multiple habitat types belong to families (e.g. Carangidae, Lethrinidae, Lutjanidae and Siganidae) caught by small-scale fisheries in the region (Papua New Guinea National Fisheries Authority 2005, 2007). Reef fishes have historically been, and continue to be, an important source of animal protein for Pacific Island communities (Dalzell et al. 1996; Pinca et al. 2012). However, increasing human populations have placed pressure on reefs, and many island nation reef fisheries are considered to be operating at unsustainable levels (Newton et al. 2007). As reefs become more degraded, it has been suggested that the availability of non-reef habitats could play an important role in maintaining the productivity of reef fisheries (Rogers and Mumby 2019). Fishing around the Tigak Islands is largely restricted to inshore waters surrounding the islands, particularly during the monsoon season (Lawless and Frijlink 2016), with the only commercial fishing targeting tuna and other pelagic fishes in offshore waters. Our findings suggest that this dependence on inshore waters for several months each year, combined with growing populations, requires careful management of the entire seascape, not just reefs, by local communities to protect food security into the future.
“Coral reef fishes” is a widely used term to describe fish assemblages that occupy waters in the vicinity of coral reefs, yet over one-third of the fishes recorded from shallow-water habitats in the Kavieng Lagoon were present in one or more non-reef habitat. Many of these species were frequently encountered away from coral reef habitat and could be considered as “seascape users” or “habitat generalists” as opposed to “coral reef fish”. Terminology aside, being flexible in habitat use could be advantageous given the widespread degradation of many shallow-water coastal habitats. As has been demonstrated from terrestrial landscapes (e.g. in birds, Salido et al. 2011), populations of such habitat generalists might be less vulnerable to the degradation of one habitat type. In contrast, species that obtain complementary resources from different habitats (e.g. food vs. shelter, Ries et al. 2004) may be negatively impacted by habitat disturbance or loss. However, the drivers of multiple habitat use are not well understood for many of the species identified here as multi-habitat users.
It is now widely recognized that coral reefs are moving into uncertain territory. However, efforts to predict how reefs might function in the future rarely consider that many reefs are embedded within, and consequently influenced by, a mosaic of other habitat types. Such connections may become increasingly important in the future both for supporting key ecological functions on reefs and providing food security for nations with strong dependencies on coral reef fisheries.
References
Adams AJ, Dahlgren CP, Kellison GT, Kendall MS, Layman CA, Ley JA, Nagelkerken I, Serafy JE (2006) Nursery function of tropical back-reef systems. Mar Ecol Prog Ser 318:287–301
Aguilar-Perera A, Appeldoorn RS (2008) Spatial distribution of marine fishes along a cross-shelf gradient containing a continuum of mangrove-seagrass-coral reefs off southwestern Puerto Rico. Estuar Coast Shelf Sci 76:378–394
Aller EA, Gullström M, Maarse FKJE, Gren M, Nordlund LM, Jiddawi N, Eklöf JS (2014) Single and joint effects of regional- and local-scale variables on tropical seagrass fish assemblages. Mar Biol 161:2395–2405
Alsterberg C, Roger F, Sundbäck K, Juhanson J, Hulth S, Hallin S, Gamfeldt L (2017) Habitat diversity and ecosystem multifunctionality – the importance of direct and indirect effects. Sci Adv 3:e1601475
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: guide to software and statistics methods. PRIMER-E, Plymouth
Barnes L, Bellwood DR, Sheaves M, Tanner JK (2012) The use of clear-water non-estuarine mangroves by reef fishes on the Great Barrier Reef. Mar Biol 159:211–220
Bassett DK, Montgomery JC (2011) Investigating nocturnal fish populations in situ using baited underwater video: with special reference to their olfactory capabilities. J Exp Mar Biol Ecol 409:194–199
Beets J, Muehlstein L, Haught K, Schmitges H (2003) Habitat connectivity in coastal environments: patterns and movements of Caribbean coral reef fishes with emphasis on bluestriped grunt, Haemulon sciurus. Gulf Caribb Res 14:29–42
Bell JD, Kronen M, Vunisea A, Nash WJ, Keeble G, Demmke A, Pontifex S, Andréfouët S (2009) Planning the use of fish for food security in the Pacific. Mar Pol 33:64–76
Béné C, Macfadyen G, Allison EH (2007) Increasing the contribution of small-scale fisheries to poverty alleviation and food security. FAO Fisheries Technical Paper 481
Berkström C, Gullström M, Lindborg R, Mwandya AW, Yahya SAS, Kautsky N, Nyström M (2012) Exploring ‘knowns’ and ‘unknowns’ in tropical seascape connectivity with insights from East African coral reefs. Estuar Coast Shelf Sci 107:1–21
Berkström C, Lindborg R, Thyresson M, Gullström M (2013) Assessing connectivity in a tropical embayment: fish migrations and seascape ecology. Biol Conser 166:43–53
Birkeland C (1985) Ecological interactions between tropical coastal ecosystems. UNEP Regional Seas Reports and Studies 73:1–26
Blaber SJM (2007) Mangroves and fishes: issues of diversity, dependence, and dogma. Bull Mar Sci 80:457–472
Blaber SJM, Milton DA (1990) Species composition, community structure and zoogeography of fishes of mangrove estuaries in the Solomon Islands. Mar Biol 105:259–267
Blasiak R, Spijkers J, Tokunaga K, Pittman J, Yagi N, Österblom (2017) Climate change and marine fisheries: least developed countries top global index of vulnerability. PLoS One 12:e0179632
Bradley M, Baker R, Nagelkerken I, Sheaves M (2019) Context is more important than habitat type in determining use by juvenile fish. Landsc Ecol 34:427–442
Bradley M, Baker R, Sheaves M (2017) Hidden components in tropical seascapes: deep-estuary habitats support unique fish assemblages. Estuaries Coast 40:1195–1206
Brodie G, Pikacha P, Tuiwawa M (2013) Biodiversity and conservation in the Pacific Islands: why are we not succeeding? In: Sodhi NS, Gibson L, Raven PH (eds) Conservation Biology: Voices from the Tropics, First Edition, pp 181–187
Burge EJ, Atack JD, Andrews C, Binder BM, Hart ZD, Wood AC, Bohrer LE, Jagannathan K (2012) Underwater video monitoring of groupers and the associated hard-bottom reef fish assemblage of North Carolina. Bull Mar Sci 88:15–38
Burke L, Reytar K, Spalding M, Perry A (2011) Reefs at risk revisited. World Resources Institute, 130 pp
Campbell MD, Pollack AG, Gledhill CT, Switzer TS, DeVries DA (2015) Comparison of relative abundance indices calculated from two methods of generating video count data. Fish Res 170:125–133
Cappo M, Speare P, De’ath G (2004) Comparison of baited remote underwater video stations (BRUVS) and prawn (shrimp) trawls for assessments of fish biodiversity in inter-reefal areas of the Great Barrier Reef Marine Park. J Exp Mar Biol Ecol 302:123–152
Chaves LTC, Pereira PHC, Feitosa JLL (2013) Coral reef fish association with macroalgal beds on a tropical reef system in North-eastern Brazil. Mar Freshw Res 64:1101–1111
Clarke KR, Somerfield PJ, Chapman MG (2006) On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray-Curtis coefficient for denuded assemblages. J Exp Mar Biol Ecol 330:55–80
Dahlgren CP, Eggleston DB (2000) Ecological processes underlying ontogenetic habitat shifts in a coral reef fish. Ecology 81:2227–2240
Dalzell P, Adams TJH, Polunin NVC (1996) Coastal fisheries in the Pacific Islands. Ann Rev Mar Sci 34:395–531
Dorenbosch M, Grol MGG, Christianen MJA, Nagelkerken I, van der Velde G (2005) Indo-Pacific seagrass beds and mangroves contribute to fish density and diversity on adjacent coral reefs. Mar Ecol Prog Ser 302:63–76
Dorenbosch M, Grol MGG, Nagelkerken I, van der Velde G (2006) Different surrounding landscapes may result in a different fish assemblages in East African seagrass beds. Hydrobiologia 563:45–60
Dorenbosch M, Grol MGG, de Groene A, van der Velde G, Nagelkerken I (2009) Piscivore assemblages and predation pressure affect relative safety of some back-reef habitats for juvenile fish in a Caribbean bay. Mar Ecol Prog Ser 379:181–196
Dubuc A, Walthan NJ, Baker R, Marchand C, Sheaves M (2019) Patterns of fish utilization in a tropical Indo-Pacific mangrove-coral seascape. New Caledonia. PLoS One 14:e0207168
Dunning JB, Dnielson BJ, Pulliam HR (1992) Ecological processes that affect populations in complex landscapes. Oikos 65:169–175
Eggertsen L, Ferreira CEL, Fontoura L, Kautsky N, Gullström M, Berström C (2017) Seaweed beds support more juvenile reef fish than seagrass beds in a south-western Atlantic tropical seascape. Estuar Coast Shelf Sci 196:97–108
Evans RD, Wilson SK, Field SN, Moore JAY (2014) Importance of macroalgal fields as coral reef fish nursery habitat in north-west Australia. Mar Biol 161:599–607
Fahrig L, Baudry J, Brotons L, Burel FG, Crist TO, Fuller RJ, Sirami C, Siriwardena GM, Martin J (2011) Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol Lett 14:101–112
Feary DA, Cinner JE, Graham NAJ, Januchowski-Hartley FA (2011) Effects of customary marine closures on fish behavior, spear-fishing success, and underwater visual surveys. Conserv Biol 25:341–349
Foale S, Adhuri D, Aliño P, Allison EH, Andrew N, Cohen P, Evans L, Fabinyi M, Fidelman P, Gregory C, Stacey N, Tanzer J, Weeratunge N (2013) Food security and the Coral Triangle Initiative. Mar Policy 38:174–183
Froese R, Pauly D (eds) (2019) FishBase. http://www.fishbase.org
Fulton CJ, Abesamis RA, Berkström C, Depczynski M, Graham NAJ, Holmes TH, Kulbicki M, Noble MM, Radford BT, Tano S, Tinkler P, Wernberg T, Wilson SK (2019) Form and function of tropical macroalgal reefs in the Anthropocene. Funct Ecol 33:989–999
Fulton CJ, Berkström C, Wilson SK, Abesamis RA, Bradley M, Åkerlund C, Barrett LT, Bucol AA, Chacin DH, Chong-Seng KM, Coker DJ, Depczynski M, Eggertsen L, Eggertsen M, Ellis D, Evans RD, Graham NAJ, Hoey AS, Holmes TH, Kulbicki M, Leung PTY, Lam PKS, van Lier J, Matis PA, Noble MM, Pérez-Matus A, Piggott C, Radford BT, Tano S, Tinkler P (2020) Macroalgal meadow habitats support fish and fisheries in diverse tropical seascapes. Fish Fish. https://doi.org/10.1111/faf.12455
Gotanda KM, Turgeon K, Kramer DL (2009) Body size and reserve protection affect flight initiation distances in parrotfishes. Behav Ecol Sociobiol 63:1563–1572
Gratwicke B, Speight MR (2005) The relationship between species richness, abundance and habitat complexity in a range of shallow tropical marine habitats. J Fish Biol 66:650–667
Hamilton SE, Casey D (2016) Creation of a high spatio-temporal resolution global database of continuous mangrove forest cover for the 21st century (CGMFC-21). Global Ecol Biogeogr 25:729–738
Harborne AR, Mumby PJ, Kappel CV, Dahlgren CP, Micheli F, Holmes KE, Brumbaugh DR (2008) Tropical coastal habitats as surrogates of fish community structure, grazing, and fisheries value. Ecol Appl 18:1689–1701
Harborne AR, Nagelkerken I, Wolff NH, Bozec Y-M, Dorenbosch M, Grol MGG, Mumby PJ (2016) Direct and indirect effects of nursery habitats on coral-reef fish assemblages, grazing pressure and benthic dynamics. Oikos 125:957–967
Heck KL Jr, Carruthers TJB, Duarte CM, Hughes AR, Kendrick G, Orth RJ, Williams SW (2008) Trophic transfers from seagrass meadows subsidize diverse marine and terrestrial consumers. Ecosystems 11:1198–1210
Hitt S, Pittman SJ, Nemeth RS (2011) Diel movements of fishes linked to benthic seascape structure in a Caribbean coral reef ecosystem. Mar Ecol Prog Ser 427:275–291
Igulu MM, Nagelkerken I, Dorenbosch M, Grol MGG, Harborne AR, Kimirei IA, Mumby PJ, Olds AD, Mgaya YD (2014) Mangrove habitat use by juvenile reef fish: meta-analysis reveals that tidal regime matters more than biogeographic region. PLoS One 9:e114715
Jaxion-Harm J, Saunders J, Speight MR (2011) Distribution of fish in seagrass, mangroves and coral reefs: life-stage dependent habitat use in Honduras. Rev Biol Trop 60:683–698
Jones GP, McCormick MI, Srinivasan M, Eagle JV (2004) Coral decline threatens fish biodiversity in marine reserves. Proc Natl Acad Sci USA 101:8251–8253
Kimirei IA, Nagelkerken I, Mgaya YD, Huijbers CM (2013) The mangrove nursery paradigm revisited: otolith stable isotopes support nursery-to-reef movements by Indo-Pacific fishes. PLoS One 8:e66320
Krumme U (2009) Diel and tidal movements by fish and decapods linking tropical coastal ecosystems. In: Nagelkerken I (ed) Ecological connectivity among tropical coastal ecosystems Dordrecht. Springer, The Netherlands, pp 271–324
Laegdsgaard P, Johnson C (2001) Why do juvenile fish utilise mangrove habitats? J Exp Mar Biol Ecol 257:229–253
Lawless S, Frijlink S (2016) Socioeconomic assessment of villages in the Tigak and Tsoi islands, northern New Ireland Province. Wildlife Conservation Society, Papua New Guinea, Papua New Guinea, p 37
Macreadie PI, Jarvis J, Trevathan-Tackett SM, Bellgrove A (2017) Seagrasses and macroalgae: importance, vulnerability and impacts. In: Phillips BF, Pérez-Ramírez M (eds) Climate change impacts on fisheries and aquaculture: a global analysis Volume II, pp 729–770
McMahon KW, Berumen ML, Thorrold SR (2012) Linking habitat mosaics and connectivity in a coral reef seascape. Proc Natl Acad Sci USA 109:15372–15376
Meyer JL, Schultz ET (1985) Migrating haemulid fishes as a source of nutrients and organic matter on coral reefs. Limnol Oceanogr 30:146–156
Miller-Rushing AJ, Primack RB, Devictor V, Corlett RT, Cumming GS, Loyola R, Maas B, Pejchar L (2019) How does habitat fragmentation affect biodiversity? A controversial question at the core of conservation biology. Biol Conserv 232:271–273
Nagelkerken I, Kleijnen S, Klop T, van den Brand RACJ, Cocheret de la Morinière E, van der Velde G (2001) Dependence of Caribbean reef fishes on mangroves and seagrass beds as nursery habitats: a comparison of fish faunas between bays with and without mangroves/seagrass beds. Mar Ecol Prog Ser 214:225–235
Nagelkerken I, van der Velde G, Gorissen MW, Meijer GJ, van’t Hof T, den Hartog C (2000) Importance of mangroves, seagrass beds and the shallow coral reef as a nursery for important coral reef fishes, using a visual census technique. Estuar Coast Shelf Sci 51:31–44
Nakamura Y, Horinouchi M, Shibuno T, Tanaka Y, Miyajima T, Koike I, Kurokura H, Sano M (2008) Evidence of ontogenetic migration from mangroves to coral reefs by black-tail snapper Lutjanus fulvus: stable isotope approach. Mar Ecol Prog Ser 355:257–266
Nanjo K, Kohno H, Nakamura Y, Horinouchi M, Sano M (2014) Effects of mangrove structure on fish distribution patterns and predation risks. J Exp Mar Biol Ecol 461:216–225
Newton K, Côté IM, Pilling GM, Jennings S, Dulvy NK (2007) Current and future sustainability of island coral reef fisheries. Curr Biol 17:655–658
Olds AD, Albert S, Maxwell PS, Pitt KA, Connolly RM (2013) Mangrove-reef connectivity promotes the effectiveness of marine reserves across the western Pacific. Global Ecol Biogeogr 22:1040–1049
Olds AD, Connolly RM, Pitt KA, Maxwell PS (2012) Primacy of seascape connectivity effects in structuring coral reef fish assemblages. Mar Ecol Prog Ser 462:191–203
Papua New Guinea National Fisheries Authority (2005) Small-scale fisheries in New Ireland Province: landing, market and buyer surveys in Kavieng. National Fisheries Authority and Coastal Fisheries Management and Development Project, 77 pp
Papua New Guinea National Fisheries Authority (2007) A review of fisheries and marine resources in New Ireland Province. Papua New Guinea, National Fisheries Authority and Coastal Fisheries Management and Development Project, p 40
Parrish JD (1989) Fish communities of interacting shallow-water habitats in tropical oceanic regions. Mar Ecol Prog Ser 58:143–160
Pereira PHC, dos Santos MVB, Lippi DL, de Paula Silva PH, Barros B (2017) Difference in the trophic structure of fish communities between artificial and natural habitats in a tropical estuary. Mar Freshw Res 68:473–483
Pinca S, Kronen M, Magron F, McArdle B, Vigliola L, Kulbicki M, Andréfouët S (2012) Relative importance of habitat and fishing in influencing reef fish communities across seventeen Pacific Island Countries and Territories. Fish Fish 13:361–379
Pittman SJ, Caldow C, Hile SD, Monaco ME (2007) Using seascape types to explain the spatial patterns of fish in the mangroves of SW Puerto Rico. Mar Ecol Prog Ser 348:273–284
Pittman SJ, McAlpine CA (2003) Movement of marine fish and decapod crustaceans: process, theory and application. Adv Mar Biol 44:205–294
Pittman SJ, Olds AD (2015) Seascape ecology of fishes on coral reefs. In: Mora C (ed) ecology of fishes on coral reefs. Cambridge University Press, Cambridge, pp 274–282
Pratchett MS, Hoey AS, Wilson SK, Messmer V, Graham NAJ (2011) Changes in biodiversity and functioning of reef fish assemblages following coral bleaching and coral loss. Diversity 3:424–452
Richardson LE, Graham NAJ, Pratchett MS, Eurich JG, Hoey AS (2018) Mass coral bleaching causes biotic homogenization of reef fish assemblages. Glob Chang Biol 24:3117–3129
Ries L, Fletcher RJ, Battin J, Sisk TD (2004) Ecological responses to habitat edges: mechanisms, models, and variability explained. Annu Rev Ecol Evol Syst 35:491–522
Rogers A, Mumby PJ (2019) Mangroves reduce the vulnerability of coral reef fisheries to habitat degradation. PLoS Biol 17:e3000510
Rossier O, Kulbicki M (2000) A comparison of fish assemblages from two types of algal beds and coral reefs in the south-west lagoon of New Caledonia. Cybium 24:3–26
Salido L, Purse BV, Marrs R, Chamberlain DE, Shultz S (2011) Flexibility in phenology and habitat use act as buffers to long-term population declines in UK passerines. Ecography 35:604–613
Sambrook K, Hoey AS, Andréfouët S, Cumming GS, Duce S, Bonin MC (2019) Beyond the reef: the widespread use of non-reef habitats by coral reef fishes. Fish Fish 20:903–920
Shantz AA, Ladd MC, Schrack E, Burkepile DE (2015) Fish-derived nutrient hotspots shape coral reef benthic communities. Ecol Appl 25:2142–2152
Sheaves M (2017) How many fish use mangroves? The 75% rule an ill-defined and poorly validated concept. Fish Fish 18:778–789
Tang S, Graba-Landry A, Hoey AS (2020) Density and height of Sargassum influence rabbitfish (f. Siganidae) settlement on inshore reef flats of the Great Barrier Reef. Coral Reefs 39:467–473
Tano SA, Eggertsen M, Wikström SA, Berström C, Buriyo AS, Halling C (2017) Tropical seaweed beds as important habitats for juvenile fish. Mar Freshw Res 68:1921–1934
Thollot P (1992) Importance of mangroves for Pacific reef fish species, myth or reality? Proc 7th Int Coral Reef Symp 2:934–941
Unsworth RKF, Garrard SL, De León PS, Cullen LC, Smith DJ, Sloman KA, Bell JJ (2009) Structuring of Indo-Pacific fish assemblages along the mangrove-seagrass continuum. Aquat Biol 5:85–95
Unsworth RKF, Salinas De León P, Garrard SL, Jompa J, Smith DJ, Bell JJ (2008) High connectivity of Indo-Pacific seagrass fish assemblages with mangrove and coral reef habitats. Mar Ecol Prog Ser 353:213–224
Valiela I, Bowen JL, York JK (2001) Mangrove forests: One of the world’s threatened major tropical environments. BioScience 51:807–815
Van Lier JR, Wilson SK, Depczynski M, Wenger LN, Fulton CJ (2018) Habitat connectivity and complexity underpin fish community structure across a seascape of tropical macroalgae meadows. Landsc Ecol 33:1287–1300
Waycott M, Duarte CM, Carruthers TJ, Orth RJ, Dennison WC, Olyarnik S, Calladine A, Fourqurean JW, Heck KL Jr, Hughes AR, Kendrick GA, Kenworthy WJ, Short FT, Williams SL (2009) Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc Natl Acad Sci USA 106:12377–12381
Wilson SK, Depczynski M, Fisher R, Holmes TH, O’Leary RA, Tinkler P (2010) Habitat associations of juvenile fish at Ningaloo Reef, Western Australia: The importance of coral and algae. PLoS One 5:e15185
Wilson SK, Fulton CJ, Depczynski M, Holmes TH, Noble MM, Radford B, Tinkler P (2014) Seasonal changes in habitat structure underpins shifts in macroalgae-associated tropical fish communities. Mar Biol 161:2597–2607
Acknowledgements
We thank the staff at the Nago Island Mariculture and Research Facility, Kavieng, Papua New Guinea (PNG), for their assistance, particularly our skipper Peksi Essau for his extensive knowledge of the region, the local communities for allowing us to conduct surveys, and the PNG National Fisheries Authority for support. Additional thanks are due to E. Aston for field assistance, T. Hill and C. Herrera Acosta for useful discussions on the manuscript, and G. Cresswell, V. Huertas, GP Jones and MI McCormick for assistance with fish identification. This project was supported by the ARC Centre of Excellence for Coral Reef Studies, James Cook University, Australia under Animal Ethics permit A2526 and the Digital Globe Foundation (satellite imagery).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Topic Editor Alastair Harborne
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sambrook, K., Bonin, M.C., Bradley, M. et al. Broadening our horizons: seascape use by coral reef-associated fishes in Kavieng, Papua New Guinea, is common and diverse. Coral Reefs 39, 1187–1197 (2020). https://doi.org/10.1007/s00338-020-01954-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-020-01954-2