Abstract
Herbivorous fishes consume algae on coral reefs, and this ecological function is pivotal in helping reefs to resist and recover from disturbance. Although numerous studies have differentiated between those fishes that graze on low-profile algae and those that browse on larger fleshy macroalgae, little is known about the feeding behaviours of some herbivorous fishes (e.g. rabbitfishes, Siganidae), limiting our understanding of whether, and how, these species contribute to ecological functions on coral reefs. Here, we examine how the feeding ecology of four species of rabbitfishes that dominate the artisanal fishery in the Seychelles changed spatially and temporally. Siganus argenteus and S. sutor were generalist herbivores feeding on a range of substrata (e.g. turf algae, macroalgae, seagrass and epiphytic algae), whereas S. corallinus and S. stellatus were specialist herbivores feeding primarily on substrata covered in turf algae. Bite rates of S. argenteus and S. sutor were positively correlated with the cover of macroalgae, seagrass and epiphytic algae. By contrast, bite rates of S. corallinus and S. stellatus were not correlated with changes in the cover of turf algae. These findings illustrate possible differences in the ecological contributions among rabbitfish species on coral reefs, and emphasize the need for caution when assigning species to functional groups and assuming within-group functional equivalence. The results also support the classic niche theory that species within a community must use resources differently in order to coexist over evolutionary timescales. These results further provide valuable insights for the management of rabbitfishes in tropical fisheries because it implies that the conservation of different species might result in distinct shifts in the competitive dominance of coral and algae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fishes that perform crucial ecological roles on coral reefs (e.g. herbivory) and that are also important targets of fisheries should be a primary focus for research effort and management action (Bellwood et al. 2012). Coral reefs with relatively intact fish assemblages experience intense herbivory from the feeding activities of fishes that consume algal material (Green and Bellwood 2009; Fong et al. 2018). The foraging actions of herbivorous fishes help to maintain a low biomass of algae on reefs (Hughes et al. 2007; Rasher and Hay 2010) and can prevent the competitive dominance of canopy-forming macroalgae (e.g. Hoey and Bellwood 2009; Green and Bellwood 2009; Bonaldo and Hay 2014). If, however, large and/or dense beds of canopy-forming macroalgae become established, they may be difficult to remove as these algae are unpalatable to many species of herbivorous fish (Bellwood et al. 2006; Ledlie et al. 2007; Hoey and Bellwood 2011).
Herbivorous fishes are commonly placed in functional groups in an attempt to understand and examine ecological functions on coral reefs (Brandl et al. 2019). The broadest distinction in the herbivore functional groups is between fishes that graze predominantly on algal turfs and those that browse on fleshy macroalgae (Heenan and Williams 2013). ‘Grazers’ may prevent macroalgae from becoming established by feeding on diminutive macroalgae and turf algae (Paddack et al. 2006; Hughes et al. 2007). ‘Browsers’ have the potential to reverse macroalgal phase shifts as they can reduce the overgrowth and shading of coral by selectively feeding on mature macroalgae (Hoey and Bellwood 2009; Green and Bellwood 2009). However, recent findings suggest that the relationship between nominally herbivorous fishes and benthic algae is not a simple relationship, but influenced by many differences of consumer and producer ecological traits, as well as environmental factors (Brandl and Bellwood 2016). Thus, more detailed work on the relationship between herbivorous fishes and the benthic community is required in order to develop a deeper understanding of consumer–producer dynamics on tropical coral reefs (Adam et al. 2015).
The diet and foraging behaviours of many herbivorous fishes on coral reefs are characterized by high variability, both among and between habitat features on reefs, with differences often relating to changes in both the available nutritional content and chemical defenses of algal resources (e.g. Fox et al. 2009; Bruggemann et al. 1994; Hanmer et al. 2017). Coral reefs support a high diversity of algal species, which vary in their nutritional value to herbivores, and it is widely accepted that herbivores regulate their foraging efforts to optimize nutritional benefits (Zemke-White et al. 2002; Simpson et al. 2004; Dromard et al. 2015). To better appreciate the ecological roles that herbivorous fishes perform on reefs, we require empirical data to explain why they select certain food items and to describe how preferences vary in time and space (Suding et al. 2004; Miller et al. 2011). Identification of such behaviour is a necessary step towards understanding feeding preferences in herbivores, and how different species modify their feeding actions and help reefs to either resist, or recover from, disturbances that would otherwise lead to macroalgae overgrowth (Bellwood et al. 2006; Adam et al. 2015; Loffler et al. 2015).
We know surprisingly little about the feeding behaviours of many herbivorous fish species, and this limits our understanding of the ecological roles they perform on reefs (Fox and Bellwood 2013; Hoey et al. 2013; Yabsley et al. 2016). Rabbitfishes are a family comprising of 28 species, characterized by their morphology and ecology; dull coloured, fusiform species that typically occur in schools within seagrass and/or macroalgal habitats (e.g. the streamlined spinefoot, Siganus argenteus, and the shoemaker spinefoot, S. sutor) and brightly coloured, deep-bodied reef-associated species that typically occur in in pairs (e.g. the blue-spotted spinefoot, S. corallinus, and the brown-spotted spinefoot, S. stellatus) (Woodland 1990; Borsa et al. 2007). They are considered to be important browsers and grazers on tropical reefs and feed on a diverse assortment of algae within these seascapes (Cvitanovic and Bellwood 2009; Hoey et al. 2013; Brandl and Bellwood 2015). This family has recently gained attention, particularly on the Great Barrier Reef (GBR), for not only their functional role but also their ability to coexist with closely related species. Fox and Bellwood (2013) demonstrated that rabbitfishes had very different feeding microhabitats from other herbivorous fish species, such as parrotfishes and surgeonfishes. Moreover, Hoey et al. (2013) showed that 11 rabbitfish species on the GBR exhibited species-specific variations in diet composition, and Fox et al. (2009) demonstrated that two closely related rabbitfishes had clear differences in diet composition and feeding periods. Thus, it appears that feeding niche partitioning is an important component of coexistence among herbivorous species, even within families.
Rabbitfishes are vital food sources for humans in many tropical coastal regions and are subjected to heavy fishing pressures in the Indo-West Pacific coastal regions of the world (Woodland 1990; Kuiter 1993; Kaunda-Arara and Rose 2004; McClanahan and Mangi 2004). The harvesting of rabbitfishes is particularly intense in the Republic of Seychelles, where four species, including S. argenteus, S. corallinus, S. stellatus, and S. sutor, constitute over half (approx. 60%) of the annual artisanal fishery catch (Grandcourt and Cesar 2003; Robinson et al. 2011). Unfortunately, empirical data on their feeding behaviours are lacking for many of these species (e.g. S. sutor) and for most coral reefs outside of the GBR (Chong-Seng et al. 2014). This information is necessary to better understand how rabbitfishes, which dominate artisanal fisheries in the Seychelles, possibly contribute to the function of herbivory (i.e. either graze and/or browse on algal material) on coral reefs.
The majority of the reefs around the inner islands have undergone macroalgal (predominantly Sargassum sp.) regime shifts following the major bleaching event of 1998 (Graham et al. 2015), resulting in increased herbivore productivity that has sustained the local artisanal reef fishery (Robinson et al. 2019). With recent habitat restructuring, the region provides a unique opportunity to study foraging behaviours of the family Siganidae, as a contrast to the well-studied GBR herbivores where coral cover is generally higher, macroalgal habitat minimal [except on inshore reefs (Wismer et al. 2009)], and species are not crucial fishery targets (Fox and Bellwood 2008). We tested how the feeding ecology of rabbitfishes changed spatially and temporally, with variation in the relative abundance of algal food resources among reefs. This was done by focusing on the feeding rates and types of substrata targeted on each reef using direct bite rate observations in the field, an approach that has been adopted widely to quantify the functional roles of herbivorous fishes on coral reefs (e.g. Burkepile and Hay 2008; Cardoso et al. 2009; Fox and Bellwood 2013; Hanmer et al. 2017). The overall aim was to describe the ecological roles of harvested rabbitfishes on coral reefs in the Seychelles and to determine whether, and how, these species display differences in their diet and foraging behaviours. Indeed, classic niche theory suggests that species within a community must use resources differently in order to coexist over evolutionary timescales (Chesson et al. 2001); therefore, we hypothesized that the four species would show variations in their diet and foraging behaviours by targeting different microhabitats on reefs in the Seychelles.
Materials and methods
Study region and sampling design
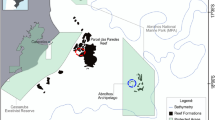
We surveyed the composition of benthic assemblages and quantified the foraging behaviour of harvested rabbitfishes, on 16 coral reefs, which provided a strong gradient in the cover and composition of algal communities across seven of the inner islands of the Seychelles (Fig. 1, Electronic Supplementary Materials, ESM Fig. S1). The Republic of Seychelles is an archipelago of 115 granitic and carbonate islands spread over an exclusive economic zone of 1.37 million km2 in the Western part of the Indian Ocean (Robinson et al. 2011). The islands’ economy is driven mainly by tourism and fisheries, whereby rabbitfish dominate the local artisanal fishery catch (Grandcourt and Cesar 2003; Robinson et al. 2011).
All data were collected from shallow (< 10 m) reef slopes using SCUBA. This is the primary operating depth for the near-shore artisanal trap fishery that targets rabbitfishes, as it supports the four focal species in high abundance (Seychelles Fishing Authority 2016).
Surveying the composition of coral and algal assemblages
To quantify variation in the composition of coral and algal assemblages among reefs, benthic surveys were performed using the point intercept transect (PIT) method (sensu Hodgson et al. 2003). At each reef, coral and algal assemblages were surveyed at 40 points along a 10-m tape. A diver swam directly above the tape and identified and recorded the benthos at 0.25-m intervals along the transect line. The survey was replicated five times at each site, yielding a total of 200 points for analysis from each reef. Transect tapes were laid in a haphazard fashion at each site, approximately 10 m apart without any overlap. Benthic life forms were identified and classified as fleshy macroalgae (i.e. Sargassum sp.) (FMA), turf algae (TA), live coral (LC), dead coral (DC), coral rubble (CR), sand (S) and seagrass (SG).
Quantifying the foraging behaviours of herbivorous rabbitfishes
Foraging behaviours of the four focal rabbitfish species were recorded using timed foraging observations (e.g. Cardoso et al. 2009; Fox and Bellwood 2013; Hanmer et al. 2017), which were conducted between January 2015 and November 2016. During each observation, an individual fish was followed for a period of 8–10 min, at a distance of 2–3 m, and data were recorded to describe the species and size (fork length) of each fish, and the number of bites taken on each type of benthic substrata (i.e. turf algae, fleshy macroalgal fronds, fleshy macroalgal thallus, seagrass, or epiphytic algae). Feeding on epiphytes, as distinct from feeding on either the macroalgal fronds or seagrass blades that supported epiphytes, was distinguished by the rapid bite rates of fishes (Fox and Bellwood 2008; Hoey and Bellwood 2009), as well as the absence or very little macroalgal and seagrass material removed. To test whether foraging behaviours varied throughout the day, divers followed individuals of each species in six distinct time periods: early morning (06:00 to 08:00 h); mid-morning (08:00 to 10:00 h); late morning (10:00 to 12:00 h); early afternoon (12:00 to 14:00 h); mid-afternoon (14:00 to 16:00 h); late afternoon (16:00 to 18:00 h). If fish behaviour appeared to be affected by the presence of divers, observations were stopped and data for that fish were excluded from analyses. In total, 480 foraging observations were completed to describe the feeding behaviours of S. sutor (n = 142), S. argenteus (n = 140), S. corallinus (n = 110) and S. stellatus (n = 88). Data from behavioural observations were summarized as the foraging rate (bites.min–1) of each rabbitfish species on different food items and times of day at each reef.
Data analysis
Foraging rates and behaviours
Bite rate data (bites.min−1) for each individual fish, on each algal resource (i.e. fleshy macroalgae, turf algae, and seagrass), were transformed using log(x + 1) to normalize the data (Anderson et al. 2008). Bray–Curtis similarity (BCS) distances (BCS = 1 − Bray–Curtis dissimilarity distance) were then calculated for transformed data (Clarke and Gorley 2006). A principal coordinate analysis (PCO) plot was then used to visualize the BCS values among the different rabbitfish species’ bite data on different feeding substrata. We then averaged the PCOs per species, per site using the first two axes of the eigenvalues, as they accounted for over 90% of the total variation observed, and plotted the centroids in order to visualize the variation among species, among sites. Standard error bars were also displayed to show the dispersion from the mean.
To understand whether foraging behaviours differed among species, sites and at various times of the day, we used a three-way permutation-based multivariate analysis of variance (PERMANOVA; Anderson et al. 2008), in which fish species, site and time period were the fixed factors, and pseudo-F was calculated using 999 restricted permutations of data. Post hoc pairwise comparisons were used to test for significant differences in bite rates between species, time periods and the reef sites. All multivariate statistical analyses were performed using Primer-E v7 software (Clarke and Gorley 2015) with the PERMANOVA+ add-on package (version 7.0.13) (Anderson et al. 2008). Bar charts with standard error bars were used to display the foraging behaviours of each species, at each time period, at each site.
To display the diurnal patterns of feeding of each species, we used simple scatter plots of the average foraging rates across all sites. The foraging rates (bites.min−1) of each species on each substratum were averaged across the sites and displayed using a stacked bar chart with the corresponding error bars. In order to determine how the rates differed between sites, simple regressions were plotted for each species at each site, with the abundance of the target resource as the independent variable, and the foraging rate of each rabbitfish as the dependent variable.
Foraging selectivity
We examined foraging selectivity using Vanderploeg and Scavia’s Relativized Electivity Index (Vanderploeg and Scavia 1979; Lechowicz 1982). This index is calculated by first finding the selectivity coefficient for foraged item i, Wi:
where ri is the proportion of bites taken in each category i and pi is the proportional cover of each category i. The index Wi ranges from 0 (total avoidance) to 1 (total preference).
The relativized index is then
where n represents the number of foraged categories available at each site. The values of Ei range from − 1 (total avoidance) to 1 (total preference).
Only the algal resources (i.e. fleshy macroalgae fronds (FMAF) and thallus (FMAT); seagrass epiphytes (SGE) and blades (SGB); and turf algae (TA)) were used in the calculations of the electivity indices.
The calculated electivity indices of each algal resource were averaged across sites for each species and are presented as bar graphs.
Results
Foraging rates and behaviours
The overall foraging behaviour of each rabbitfish species was significantly different among species, sites and the time of day (PERMANOVA Pseudo-F = 3.7, Pperm = 0.001; Table 1; ESM Figs. S2, S3, S4 and S5). When analysing the pairwise tests between sites, for each species at the different time intervals, S. argenteus and S. sutor showed significant values for the majority of the interactions (ESM Figs. S2 and S5; Tables S1 and S4), suggesting that most of their foraging rates varied among reefs and perhaps over time. Contrastingly, S. corallinus and S. stellatus demonstrated very few significant interactions between sites at the different time intervals (ESM Figs. S3 and S4; Tables S2 and S3), suggesting that these two species had similar foraging rates at different reefs, and possibly over time.
Individual species comparisons confirmed that S. corallinus displayed quite similar foraging habits among sites (~ 77% similarity) (ESM Table S5). Likewise, S. stellatus showed high foraging similarity between sites (~ 73%) and, when compared to each other, showed ~ 70% similarity (ESM Table S5). Conversely, individual comparisons of S. argenteus and S. sutor showed only ~ 43–44% similarity between sites (ESM Table S5), and when compared to each other, they were only ~ 43% similar (ESM Table S5). Correspondingly, when S. sutor and S. argenteus were compared to S. corallinus and S. stellatus, similarity ranged from ~ 44 to 47% (ESM Table S5). These patterns were confirmed through the average PCO plot, which displays the average foraging rate (bites.min−1) for each species (± SE), at the different sites (ESM Fig. S6).
All four species were observed predominantly biting on turf algae (TA) (Fig. 2). On average, S. corallinus took the most bites on TA (9.0 bites.min−1), followed by S. stellatus (7.4 bites.min−1), S. sutor (5.3 bites.min−1) and least by S. argenteus (4.3 bites.min−1) (Fig. 2). In addition to bites taken on TA, S. sutor took the most bites on fleshy macroalgae fronds (FMAF) (3.1 bites.min−1), followed by S. argenteus (2.1 bites.min−1), S. corallinus (0.9 bites.min−1) and least by S. stellatus (0.2 bites.min−1) (Fig. 2). Bites were also taken on the thallus of the macroalgae (FMAT), mostly by S. sutor (1.8 bites.min−1), followed by S. argenteus (0.8 bites.min−1) and least by S. corallinus (0.2 bites.min−1) (Fig. 2). No bites were taken by S. stellatus on FMAT (Fig. 2). Bites on seagrass blades (SGB) were mostly taken by S. sutor (1.8 bites.min−1), followed by S. argenteus (1.7 bites.min−1) and least by S. stellatus (0.4 bites.min−1) (Fig. 2). Bites on seagrass epiphytes (SGE) were done generally by S. argenteus (2.6 bites.min−1), followed by S. sutor (1.8 bites.min−1) and least by S. stellatus (0.7 bites.min−1) (Fig. 2). Siganus corallinus was not observed foraging on seagrass (Fig. 2). These observations were confirmed by the PCO plot, which displays the similarity of each rabbitfish surveyed, distinguished by species, and their distribution in 2D space in relation to the preferred foraged resource (Fig. 3).
Average proportion of bites (± standard errors) on the different resources by the four species of rabbitfish. Resources included: FMAF fleshy macroalgae fronds, FMAT fleshy macroalgae thallus, SGE seagrass epiphytes, SGB seagrass blades, TA turf algae. Also displayed are the total bites (± standard errors) for each species
For the majority of feeding substrata, foraging rates were higher when the feeding substrata abundance was high (ESM Fig. S7a, b, c and d). Specifically, foraging rates by S. argenteus on the fronds of macroalgae (R2 = 0.84), seagrass epiphytes (R2 = 0.77) and seagrass blades (R2 = 0.88) increased linearly as the abundance of these resources increased (ESM Fig. S7a). Siganus sutor demonstrated similar characteristics when foraging on the thalli of macroalgae (R2 = 0.64), seagrass epiphytes (R2 = 0.93) and seagrass blades (R2 = 0.98) (ESM Fig. S7d). Conversely, S. corallinus and S. stellatus showed no strong linear increase in foraging rates as the resource abundances increased (ESM Fig. S7b and c).
All rabbitfishes exhibited a pattern of foraging rate typical of diurnal herbivores, with bite rates increasing through the morning periods, peaking in the mid-afternoon and decreasing again in the late afternoon or early evening (Fig. 4; ESM Figs. S2, S3, S4 and S5). At T1 (06:00 to 08:00 h), overall foraging rates averaged between 5.8 and 8.7 bites.min−1 across the 16 sites, increasing to a peak of between 13.1 and 21.0 bites.min−1 at T4 (12:00 to 14:00 h) (Fig. 4). At T6 (16:00 to 18:00 h), average foraging rates had declined to between 3.6 and 7.1 bites.min–1 (Fig. 4).
Foraging selectivity
Resource selection, measured by the Vanderploeg and Scavia’s Relativized Index, showed that the average electivity of S. argenteus and S. sutor was similar. Both showed neutral or positive electivity (Ei ≥ 0; Fig. 5) for all types of algae on offer. The electivity for S. corallinus and S. stellatus was similar (Fig. 5). On average, both showed positive electivity (Ei ≥ 0) for TA and a negative electivity for FMAF and FMAT (Ei < 0; Fig. 5).
Bar charts showing rabbitfish resource electivity (Vanderploeg and Scavia’s Relativized Index, Ei) averaged across sites for each the five foraged items: FMAF fleshy macroalgal fronds, FMAT fleshy macroalgal thallus, SGE seagrass epiphytes, SGB seagrass blades, TA turf algae. Average electivity is represented on the vertical axis: values > 0 represent active selection disproportionate to abundance, values < 0 represent resource avoidance
Discussion
Foraging rates and behaviours
Rabbitfishes in the Seychelles exhibited differences and similarities in foraging behaviours and rates. S. corallinus and S. stellatus displayed rather specific foraging preference, whereby both species foraged primarily on turf algae. This finding, specifically for S. corallinus, is consistent with that found on the Great Barrier Reef (GBR), Australia (Fox and Bellwood 2013; Hoey et al. 2013). Contrastingly, our results also show S. argenteus and S. sutor to have a much more generalist foraging behaviour compared to the other two species. They were observed foraging on turf algae, brown fleshy macroalgal (i.e. Sargassum sp.) epiphytes and thalli, seagrass epiphytes and blades, indicating dietary plasticity. Dietary plasticity is not uncommon in rabbitfishes (e.g. Wu 1984; Fox and Bellwood 2011; Hoey et al. 2013), although our results for S. argenteus oppose those from other global regions. Hoey et al. (2013) on the GBR demonstrated S. argenteus had similar foraging habits to S. corallinus, made up primarily of turf algae. Furthermore, gut content analyses in their study confirmed no presence of Sargassum sp., nor seagrass (Hoey et al. 2013). It must be noted, however, that Hoey et al. (2013) only sampled S. argenteus from mid-shelf reefs where Sargassum sp. is rare/absent (Wismer et al. 2009), although other studies in the Pacific Ocean have found little evidence of S. argenteus feeding on Sargassum sp. when available (Fiji: Rasher et al. 2013; Guam: Paul et al. 1990). This signifies that S. argenteus may only feed on Sargassum sp. when it dominates the seascape (i.e. after regime shifts), as observed in the Seychelles. Our findings for S. sutor seem to be consistent with others within the Western Indian Ocean region. Almeida et al. (1999), and Lugendo et al. (2006) found seagrass in their gut contents in eastern Africa, while Chong-Seng et al. (2014), and Humphries et al. (2015) documented them foraging and assimilating macroalgae within this same region.
Turf algae was the primary feeding substrata of all four rabbitfish species in the present study. This is common with many other herbivorous fishes globally, whereby turf algae is selected over other algal sources due to their morphology and nature (Kelly et al. 2016; Tootell and Steele 2016). Turf algae are primarily composed of filamentous, palatable, and fast-growing species that can be readily digested as compared to more structurally or chemically defended macroalgae species (Kelly et al. 2016), such as Sargassum sp. However, we also discovered foraging on Sargassum sp., dominated mostly by S. argenteus and S. sutor. We distinguished between thallus and frond foraging of macroalgae, as it has been shown that bites on the fronds by fishes typically target epiphytes, rather than actual macroalgal material (Fox and Bellwood 2008; Hoey and Bellwood 2009). Our results also indicate that S. stellatus may not browse on Sargassum sp. at all, but rather graze on the epiphytes. The targeting of the epiphytes has little impact on the reduction in macroalgal biomass (Hoey and Bellwood 2009) and may actually enhance macroalgal growth and longevity (Fox and Bellwood 2008) by increasing photosynthetic capacity through cleaning of the fronds. However, incidental ingestion or dislodgement of the fronds may occur; therefore, the role of epiphyte grazing should not be underestimated (Streit et al. 2016; Puk et al. 2016). Interestingly, in a recent review by Puk et al. (2016), it was declared that rabbitfishes are only able to keep macroalgae growth in check by targeting the fronds, but are unlikely to remove whole thalli. We found that S. argenteus, S. sutor, and to a smaller extent, S. corallinus were indeed foraging on the thallus tissue of Sargassum sp., suggesting that their roles as macroalgal browsers should not be ignored. From an ecosystem perspective, foraging on different parts of macroalgae by different species can have a significant effect on its overall removal (Streit et al. 2016). This may lead to overall positive effects of browser diversity on macroalgal removal (Topor et al. 2019).
Seagrass habitats occupy a small proportion of the world’s oceans, but provide a disproportionately large range of ecological services, including nutrient recycling, sediment stabilization and carbon sequestration (Waycott et al. 2005; Fourqurean et al. 2012). They are important habitats and foraging areas for many key fish species (Orth et al. 2006; Unsworth and Cullen 2010). During our surveys, we distinguished between foraging on seagrass blades vs. foraging on the epibiota because herbivores can be effective at reducing algal loads, but some species consume seagrass directly and may have a more detrimental effect (Heck et al. 2006). Most of the blade foraging was done by S. argenteus and S. sutor and to a small extent by S. stellatus, although, when compared to grazing by macro-herbivores, such as dugongs and turtles (e.g. Fourqurean et al. 2012), their impact is considered light and can actually stimulate seagrass growth and productivity (e.g. Valentine et al. 1997; Christianen et al. 2012). Foraging on the epiphytes of seagrass has a positive effect by increasing light availability to seagrass, which in turn improves photosynthetic capacity (e.g. Whalen et al. 2013). We found that, again, S. argenteus and S. sutor foraged the most on seagrass epiphytes and, to a smaller extent, S. stellatus. We also discovered that S. corallinus did not forage on seagrass blades or seagrass epiphytes, demonstrating a functional distinction between the species.
Foraging rates varied significantly between sites, with a general trend of increased foraging on resources as they became more abundant. Specifically, foraging rates by S. argenteus on the fronds of macroalgae, seagrass epiphytes and seagrass blades increased as the abundance of these resources increased. Similarly, S. sutor foraged most on the thalli of macroalgae, seagrass epiphytes and seagrass blades, when these resources were in high abundance. This contradicts many other foraging studies (e.g. Hoey and Bellwood 2010, 2011; Bennett and Bellwood 2011; Chong-Seng et al. 2014), which all found the opposite trend towards resource increase. This may be explained by the findings of Boyer et al. (2004), who found that nutrient enrichment on coral reefs and seagrass habitats causes increased quantity and quality of plant and algal material, thereby increasing the foraging rates by herbivorous fishes in these areas. All of the contradicted studies (i.e. Hoey and Bellwood 2010, 2011; Bennett and Bellwood 2011; Chong-Seng et al. 2014) used transplanted bioassays of macroalgae, which may have had reduced nutritional quality from transportation and handling (due to algal fragility) (Lefèvre and Bellwood 2010), thus rendering conflicting results to our study. Therefore, our results indicate that S. argenteus and S. sutor may be foraging at a greater rate in areas where the quality of seagrass and macroalgae is higher. This finding, particularly for areas that have a high macroalgal coverage, insinuates that macroalgal overgrowth may have been favoured in locations with high nitrogen loads and suggests that nutrient enrichment enhanced macroalgal regime shifts (Graham et al. 2015). As a result, this may have contributed to the higher quality of algal tissue for S. argenteus and S. sutor. This could be an interesting area for future research, by understanding the links between function, foraging and food quality.
All species of rabbitfishes exhibited a pattern of foraging rate typical for diurnal herbivores, with bite rates increasing through the morning periods, peaking in the mid-afternoon and decreasing again in the late afternoon or early evening (Fox et al. 2009; Zemke-White et al. 2002; Polunin et al. 1995). Zemke-White et al. (2002) showed that the nutritional value of algal sources increases until midday and remains high throughout the afternoon, and this correlates with the diel pattern of feeding by herbivorous fishes, which may be seeking nutrient-rich sources of algal material (Zemke-White et al. 2002). Furthermore, the patterns exhibited may be attributed to predator avoidance at certain times of the day. Several studies (e.g. Catano et al. 2017; Madin et al. 2019) demonstrate highest predator presence at dusk and lowest in the mid-afternoon; corresponding well to our observations.
Foraging selectivity
Understanding the selectivity of a particular resource by a certain species may allow us to determine their precise impact on the ecosystem, especially following large increases in that particular resource. Following the 1998 bleaching event, most of the reefs surrounding the Inner Seychelles Islands underwent macroalgal regime shifts (Graham et al. 2015), and the ecological roles of many herbivorous fishes post-bleaching are poorly understood. However, increases in their biomass and heavy exploitation suggest that the current status of reefs around these islands is sustaining these fisheries (Robinson et al. 2019), further insinuating that herbivore populations have responded positively to habitat change, but species-specific responses are not well known.
We showed that the average electivity of S. argenteus and S. sutor across sites was similar by having neutral or positive electivity for all types of algae on offer, highlighting them as generalist foragers. On the other hand, S. corallinus and S. stellatus both showed a positive electivity for only turf algae on average, indicating that they may be specialists in turf algae foraging. Furthermore, they both selected against Sargassum sp. fronds and thalli, signifying that they may not be efficient browsers (generally speaking). We also demonstrate that, on average, S. stellatus selected against seagrass blades and epiphytes. These patterns may be due to chemical defences released by Sargassum sp. (e.g. Soliman et al. 2008; Rasher et al. 2013) and seagrass (e.g. Vergés et al. 2007, 2011), acting as deterrents towards them.
Many reefs globally have already phase-shifted from coral dominance to alternative states dominated predominantly by algae, and future coral reefs are likely to vary in appearance and functionality to those of the past and present. Our results emphasize the need for caution when assigning species to functional groups and assuming within-group functional equivalence (cf. Streit et al. 2016). The utilization of foraging substrata needs to be considered when characterizing species with regard to their functional impact (Adam et al. 2015).
References
Adam TC, Kelley M, Ruttenberg BI, Burkepile DE (2015) Resource partitioning along multiple niche axes drives functional diversity in parrotfishes on Caribbean coral reefs. Oecologia 179:1173–1185
Almeida AJ, Marques A, Saldanha L (1999) Some aspects of the biology of three fish species from the seagrass beds at Inhaca Island, Mozambique. Cybium 23:369–376
Anderson M, Gorley R, Clarke K (2008) PERMANOVA+ for PRIMER. Primer-E, Plymouth
Bellwood DR, Hughes TP, Hoey AS (2006) Sleeping functional group drives coral-reef recovery. Curr Biol 16:2434–2439
Bellwood DR, Renema W, Rosen BR (2012) Biodiversity hotspots, evolution and coral reef biogeography. In: Biotic evolution and environmental change in Southeast Asia, p 216
Bennett S, Bellwood DR (2011) Latitudinal variation in macroalgal consumption by fishes on the Great Barrier Reef. Mar Ecol Prog Ser 426:241–252
Bonaldo RM, Hay ME (2014) Seaweed-coral interactions: variance in seaweed allelopathy, coral susceptibility, and potential effects on coral resilience. PLoS ONE 9:85786
Borsa P, Lemer S, Aurelle D (2007) Patterns of lineage diversification in rabbitfishes. Mol Phylogenet Evol 44:427–435
Boyer KE, Fong P, Armitage AR, Cohen RA (2004) Elevated nutrient content of tropical macroalgae increases rates of herbivory in coral, seagrass, and mangrove habitats. Coral Reefs 23:530–538
Brandl SJ, Bellwood DR (2015) Coordinated vigilance provides evidence for direct reciprocity in coral reef fishes. Sci Rep 5:14556
Brandl SJ, Bellwood DR (2016) Microtopographic refuges shape consumer–producer dynamics by mediating consumer functional diversity. Oecologia 182:203–217
Brandl SJ, Hoey AS, Bellwood DR (2014) Microtopography mediates interactions between corals, algae, and herbivorous fishes on coral reefs. Coral Reefs 33:421–430
Brandl SJ, William RD, Bellwood DR (2015) Exploring the nature of ecological specialization in a coral reef fish community: morphology, diet and foraging microhabitat use. Proc R Soc B Biol Sci 282:20151147
Brandl SJ, Rasher DB, Côté IM, Casey JM, Darling ES, Lefcheck JS, Duffy JE (2019) Coral reef ecosystem functioning: eight core processes and the role of biodiversity. Front Ecol Environ 17:445–454
Bruggemann JH, van Oppen MJH, Breeman AM (1994) Foraging by the stoplight parrotfish Sparisoma viride. I. Food selection in different, socially-determined habitats. Mar Ecol Prog Ser 106:41–55
Burkepile DE, Hay ME (2008) Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proc Natl Acad Sci 42:16201–16206
Cardoso SC, Soares MC, Oxenford HA, Côté IM (2009) Interspecific differences in foraging behaviour and functional role of Caribbean parrotfish. Mar Biodivers Rec 2:148
Catano LB, Barton MB, Boswell KM, Burkepile DE (2017) Predator identity and time of day interact to shape the risk–reward trade-off for herbivorous coral reef fishes. Oecologia 183:763–773
Chesson P, Pacala S, Neuhauser C (2001) Environmental niches and ecosystem functioning. In: Kingzig A, Pacala S, Tilman D (eds) The functional consequences of biodiversity. Princeton University Press, Princeton, New Jersey, pp 213–245
Chong-Seng KM, Nash KL, Bellwood DR, Graham NAJ (2014) Macroalgal herbivory on recovering versus degrading coral reefs. Coral Reefs 33:409–419
Christianen MJA, Govers LL, Bouma TJ, Kiswara W, Roelofs JGM, Lamers LPM, van Katwijk MM (2012) Marine megaherbivore grazing may increase seagrass tolerance to high nutrient loads. J Ecol 100:546–560
Clarke KR, Gorley RN (2006) PRIMER v6: User manual/tutorial. PRIMER-E, Plymouth
Clarke KR, Gorley RN (2015) PRIMER v6. Plymouth Marine Laboratory, Plymouth
Cvitanovic C, Bellwood DR (2009) Local variation in herbivore feeding activity on an inshore reef of the Great Barrier Reef. Coral Reefs 28:127–133
Dromard CR, Bouchon-Navaro Y, Harmelin-Vivien M, Bouchon C (2015) Diversity of trophic niches among herbivorous fishes on a Caribbean reef (Guadeloupe, Lesser Antilles), evidenced by stable isotope and gut content analyses. J Sea Res 95:124–131
Fong CR, Frias M, Goody N, Bittick SJ, Clausing RJ, Fong P (2018) Empirical data demonstrates risk-tradeoffs between landscapes for herbivorous fish may promote reef resilience. Mar Environ Res 133:1–5
Fourqurean JW, Duarte CM, Kennedy H, Marbà N, Holmer M, Mateo MA, Apostolaki ET, Kendrick GA, Krause-Jensen D, McGlathery KJ, Serrano O (2012) Seagrass ecosystems as a globally significant carbon stock. Nat Geosci 5:1–5
Fox RJ, Bellwood DR (2008) Remote video bioassays reveal the potential feeding impact of the rabbitfish Siganus canaliculatus (f: Siganidae) on an inner-shelf reef of the Great Barrier Reef. Coral Reefs 27:605–615
Fox RJ, Bellwood DR (2011) Unconstrained by the clock? Plasticity of diel activity rhythm in a tropical reef fish, Siganus lineatus. Funct Ecol 25:1096–1105
Fox RJ, Bellwood DR (2013) Niche partitioning creates a unique ecosystem function for rabbitfishes (Perciformes, Siganidae) on coral reefs. Coral Reefs 32:13–23
Fox RJ, Sunderland TL, Hoey AS, Bellwood DR (2009) Estimating functional roles in coral reef ecosystems: behaviour drives contrasting roles in herbivorous fishes (f. Siganidae). Mar Ecol Prog Ser 385:261–269
Graham NAJ, Jennings S, MacNeil MA, Mouillot D, Wilson SK (2015) Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 518:94–97
Grandcourt EM, Cesar HSJ (2003) The bio-economic impact of mass coral mortality on the coastal reef fisheries of the Seychelles. Fish Res 60:539–550
Green AL, Bellwood DR (eds) (2009) Monitoring functional groups of herbivorous reef fishes as indicators of coral reef resilience: a practical guide for coral reef managers in the Asia Pacific Region (No. 7) IUCN
Hanmer J, White JW, Pawlik JR (2017) Application of diet theory reveals context- dependent foraging preferences in an herbivorous coral reef fish. Oecologia 184:127–137
Heck Jr KL, Valentine JF, Pennock JR, Chaplin G, Spitzer PM (2006) Effects of nutrient enrichment and grazing on shoalgrass Halodule wrightii and its epiphytes: results of a field experiment. Mar Ecol Prog Ser 326:145–156
Heenan A, Williams ID (2013) Monitoring herbivorous fishes as indicators of coral reef resilience in American Samoa. PLoS ONE 8:e79604
Hodgson G, Maun L, Shuman C (2003) Reef check survey manual for Coral Reefs in the Indo-Pacific, Hawaii, Red Sea, Atlantic/Caribbean, and Arabian Gulf. Reef Check-UCLA, Institute of the Environment, Los Angeles, California, U.S.A
Hoey AS, Bellwood DR (2009) Limited functional redundancy in a high diversity system: single species dominates key ecological process on coral reefs. Ecosystems 12:1316–1328
Hoey AS, Bellwood DR (2010) Cross-shelf variation in browsing intensity on the Great Barrier Reef. Coral Reefs 29:499–508
Hoey AS, Bellwood DR (2011) Suppression of herbivory by macroalgal density: a critical feedback on coral reefs? Ecol Lett 14:267–273
Hoey AS, Brandl SJ, Bellwood DR (2013) Diet and cross-shelf distribution of rabbitfishes (f. Siganidae) on the northern Great Barrier Reef: implications for ecosystem function. Coral Reefs 32:973–984
Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli D, Hoegh- Guldberg O, McCook L, Moltschaniwsky N, Pratchett MS, Steneck RS, Willis B (2007) Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol 17:360–365
Humphries AT, McQuaid CD, McClanahan TR (2015) Context-dependent diversity-effects of seaweed consumption on Coral Reefs in Kenya. PLoS ONE 10:e0144204. https://doi.org/10.1371/journal.pone.0144204
Kaunda-Arara B, Rose GA (2004) Effects of marine reef National Parks on "fishery CPUE in coastal Kenya. Biol Conserv 118:1–13
Kelly EL, Eynaud Y, Clements SM, Gleason M, Sparks RT, Williams ID, Smith JE (2016) Investigating functional redundancy versus complementarity in Hawaiian herbivorous coral reef fishes. Oecologia 182:1151–1163
Kuiter R (1993) Coastal fishes of south-eastern Australia. University of Hawaii Press, Honolulu
Lechowicz MJ (1982) The sampling characteristics of electivity indices. Oecologia 52:22–30
Ledlie MH, Graham NAJ, Bythell JC, Wilson SK, Jennings S, Polunin NVC, Hardcastle J (2007) Phase shifts and the role of herbivory in the resilience of coral reefs. Coral Reefs 26:641–653
Lefèvre CD, Bellwood DR (2010) Seasonality and dynamics in coral reef macroalgae: variation in condition and susceptibility to herbivory. Mar biol 157:955–965
Loffler Z, Bellwood DR, Hoey AS (2015) Among-habitat algal selectivity by browsing herbivores on an inshore coral reef. Coral Reefs 34:597–605
Lugendo BR, Nagelkerken I, Van Der Velde G, Mgaya YD (2006) The importance of mangroves, mud and sand flats, and seagrass beds as feeding areas for juvenile fishes in Chwaka Bay, Zanzibar: gut content and stable isotope analyses. J Fish Biol 69:1639–1661
Madin E, Precoda K, Harborne A, Atwood TB, Roelfsema CM, Luiz O (2019) Multi-trophic species interactions shape seascape-scale coral reef vegetation patterns. Front Ecol Evol 7:102
McClanahan TR, Mangi SC (2004) Gear-based management of a tropical artisanal "fishery based on species selectivity and capture size. Fish Manag Ecol 11:51–60
Miller AD, Roxburgh SH, Shea K (2011) How frequency and intensity shape diversity-disturbance relationship. Proc Natl Acad Sci USA 108:5643–5648
Orth RJ, Carruthers TJB, Dennison WC, Duarte CM, Fourqurean JW, Heck KL Jr, Randall Hughes A, Kendrick GA, Kenworthy W, Olyarnik S, Short FT, Waycott M, Williams SL (2006) A global crisis for seagrass ecosystems. Bioscience 56:987–996
Paddack MJ, Cowen RK, Sponaugle S (2006) Grazing pressure of herbivorous coral reef fishes on low coral-cover reefs. Coral Reefs 25:461–472
Paul VJ, Nelson SG, Sanger HR (1990) Feeding preferences of adult and juvenile rabbitfish Siganus argenteus in relation to chemical defenses of tropical seaweeds. Mar Ecol Prog Ser Oldendorf 60:23–34
Polunin NVC, Harmelin-Vivien M, Galzin R (1995) Contrasts in algal food processing among five herbivorous coral-reef fishes. J Fish Biol 47:455–465
Puk LD, Ferse SC, Wild C (2016) Patterns and trends in coral reef macroalgae browsing: a review of browsing herbivorous fishes of the Indo-Pacific. Rev Fish Biol Fish 1:53–70
Rasher DB, Hay ME (2010) Chemically rich seaweeds poison corals when not controlled by herbivores. Proc Natl Acad Sci USA 107:9683–9688
Rasher DB, Hoey AS, Hay ME (2013) Consumer diversity interacts with prey defenses to drive ecosystem function. Ecology 94:1347–1358
Robinson J, Samoilys MA, Grandcourt E, Julie D, Cedras M, Gerry C (2011) The importance of targeted spawning aggregation fishing to the management of Seychelles’ trap fishery. Fish Res 112:96–103
Robinson JP, Wilson SK, Robinson J, Gerry C, Lucas J, Assan C, Govinden R, Jennings S, Graham NA (2019) Productive instability of coral reef fisheries after climate-driven regime shifts. Nat Ecol Evol 3:183
Seychelles Fishing Authority (2016) Seychelles artisanal fisheries statistics. Seychelles Fishing Authority Technical Report, Government of Seychelles, Seychelles
Simpson SJ, Sibly RM, Lee KP, Behmer ST, Raubenheimer D (2004) Optimal foraging when regulating intake of multiple nutrients. Anim behav 68:1299–1311
Soliman VS, Mendoza AB, Yamaoka K (2008) Seaweed-associated fishes of Lagonoy Gulf in bicol, the Philippines with emphasis on Siganids (Teleoptei: Siganidae). Kuroshio Sci 2:67–72
Streit RP, Hoey AS, Bellwood DR (2016) Feeding characteristics reveal functional distinctions among browsing herbivorous fishes on coral reefs. Coral Reefs 34:1037–1047
Suding KN, Gross KL, Houseman GR (2004) Alternative states and positive feedbacks in restoration ecology. Trends Ecol Evol 19:46–53
Tootell JS, Steele MA (2016) Distribution, behavior, and condition of herbivorous fishes on coral reefs track algal resources. Oecologia 181:13–24
Topor ZM, Rasher DB, Duffy JE, Brandl SJ (2019) Marine protected areas enhance coral reef functioning by promoting fish biodiversity. Conserv Lett 12:e12638
Unsworth RKF, Cullen LC (2010) Recognising the necessity for Indo-Pacific seagrass conservation. Conserv Lett 3:63–73
Valentine JF, Heck KL Jr, Busby J, Webb D (1997) Experimental evidence that herbivory can increase shoot density in a subtropical turtlegrass (Thalassia testudinum) meadow. Oecologia 112:193–200
Vanderploeg HA, Scavia D (1979) Calculation and use of selectivity coefficients of feeding: zooplankton grazing. Ecol Model 7:135–149
Vergés A, Becerro MA, Alcoverro T, Romero J (2007) Experimental evidence of chemical deterrence against multiple herbivores in the seagrass Posidonia oceanica. Mar Ecol Prog Ser 343:107–114
Vergés A, Alcoverro T, Romero J (2011) Plant defences and the role of epibiosis in ediating within-plant feeding choices of seagrass consumers. Oecologia 166:381–390
Waycott M, Longstaff BJ, Mellors J (2005) Seagrass population dynamics and water quality in the Great Barrier Reef region: a review and future research directions. Mar Pollut Bull 51:343–350
Whalen MA, Duffy JE, Grace JB (2013) Temporal shifts in top-down vs. bottom-up control of epiphytic algae in a seagrass ecosystem. Ecology 94:510–520
Wismer S, Hoey AS, Bellwood DR (2009) Cross-shelf benthic community structure on the Great Barrier Reef: relationships between macroalgal cover and herbivore biomass. Mar Ecol Prog Ser 376:45–54
Woodland DJ (1990) Revision of the fish family Siganidae with description of two new species and comments on distribution and biology. Indo-Pac Fishes 19:1–136
Wu RSS (1984) The feeding habits of seven demersal fish species in a subtropical estuary. Asian Mar Biol 1:17–26
Yabsley NA, Olds AD, Connolly RM, Martin TSH, Gilby BL, Maxwell PS, Huijbers CM, Schoeman DS, Schlacher TA, Genner M (2016) Resource type influences the effects of reserves and connectivity on ecological functions. J Anim Ecol 85:437–444
Zemke-White LW, Choat J, Clements K (2002) A re-evaluation of the diel feeding hypothesis for marine herbivorous fishes. Mar Biol 141:571–579
Acknowledgements
We thank R. Melanie from the Seychelles Fishing Authority (SFA) for field assistance and Justin Prosper from the Ministry of Environment and Climate Change for map production. This research was financed by the SFA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Topic Editor Andrew Hoey
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ebrahim, A., Martin, T.S.H., Mumby, P.J. et al. Differences in diet and foraging behaviour of commercially important rabbitfish species on coral reefs in the Indian Ocean. Coral Reefs 39, 977–988 (2020). https://doi.org/10.1007/s00338-020-01918-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-020-01918-6