Abstract
Bioerosion by reef-dwelling organisms influences net carbonate budgets on reefs worldwide. External bioeroders, such as parrotfish and sea urchins, and internal bioeroders, including sponges and lithophagid bivalves, are major contributors to bioerosion on reefs. Despite their importance, few studies have examined how environmental (e.g., nutrients) or biological drivers (e.g., the actions of other bioeroders) may influence bioeroder dynamics on reefs. For example, internal bioeroders could promote external bioerosion by weakening the coral skeletal matrix. Our study investigated: (1) whether nutrient supply influences the dynamics between internal and external bioeroders and (2) how the presence of a boring bivalve, Lithophaga spp., influences parrotfish bioerosion on massive Porites corals. We hypothesized that nutrient supply would be positively correlated with Lithophaga densities on massive Porites colonies, and that as bivalve density increased, the frequency and intensity of parrotfish bioerosion would increase. To test these hypotheses, we analyzed six time points over a 10-yr period from a time series of benthic images and nitrogen content of a dominant macroalga from the fringing reefs around Moorea, French Polynesia. We found Lithophaga densities were positively correlated with nitrogen availability. Further, massive Porites that are more infested with Lithophaga had both a higher probability of being bitten by parrotfish and a higher density of bite scars from parrotfishes. Our findings indicate that increasing nutrient availability may strengthen the relationship between internal and external bioeroders, suggesting that colonies at more eutrophic sites may experience higher bioerosion rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bioerosion, the removal of calcium carbonate structure by living organisms, is an integral process on coral reefs that contributes to the persistence of these biodiverse, structurally complex ecosystems (Glynn and Manzello 2015; Perry and Harborne 2016). Bioeroding organisms are represented in four of the six kingdoms (Hutchings 1986; Glynn and Manzello 2015; Perry and Harborne 2016). Bioerosive action is generally considered in terms of external and internal bioeroders. External bioeroders, such as parrotfishes, pufferfishes, and sea urchins, remove external reef structure mechanically. Internal bioerosion occurs when carbonate structure is removed from the inside of coral skeletons mechanically and/or chemically by worms, bivalves, sponges, and other microborers (Hutchings 1986, 2008; Tribollet and Golubic 2011; Weinstein et al. 2019). The abundance of these internal bioeroding taxa, and thus, bioerosion rates, vary considerably over space and time (e.g., Tribollet and Golubic 2005; Tribollet et al. 2006; Silbiger et al. 2016, 2017). Given that bioerosion influences the net growth and overall carbonate budget of coral reefs (Perry et al. 2013; Glynn and Manzello 2015), there is a critical need to better understand the environmental and biological drivers that shape bioerosion patterns.
The impacts of bioeroders on reef framework can be magnified by environmental conditions that hinder reef accretion, increase bioeroder populations, or favor bioerosive processes (Glynn 1997; Glynn and Manzello 2015; Perry and Harborne 2016; Silbiger et al. 2017). For example, increased nutrient supply rates often increase internal bioeroder densities (Rose and Risk 1985; Edinger et al. 2000; Holmes et al. 2000; Le Grand and Fabricius 2011), thereby stimulating bioerosion (DeCarlo et al. 2015; Prouty et al. 2017; Lubarsky et al. 2018). These relationships likely occur because eutrophication increases coral skeleton porosity (Dunn et al. 2012) and bolsters populations of filter-feeding (Le Grand and Fabricius 2011) and photosynthesizing internal bioeroders (Carreiro-Silva et al. 2005). Increased coral skeletal porosity weakens the skeletal matrix, making corals more susceptible to mechanical damage by external bioeroders (Caroselli et al. 2011; Dunn et al. 2012; Mwachireya et al. 2016). For example, Littler et al. (1989) observed that parrotfishes selectively bioerode Porites corals with weaker skeletons. Filter-feeding bioeroders, such as macroborers, become more abundant on eutrophic reefs because food resources (i.e., plankton) are available in greater quantities due to nutrient rich conditions (Scott and Cope 1990; Perry and Harborne 2016). In fact, inshore reefs, which tend to be more eutrophic due to land-based fertilizer and runoff (Fabricius 2005), often harbor higher macroborer densities than offshore reefs (Sammarco and Risk 1990; Scott and Cope 1990; Le Grand and Fabricius 2011). Yet inshore reefs tend to have lower external bioerosion rates by parrotfishes relative to offshore reefs (Hoey and Bellwood 2008; Bonaldo and Bellwood 2011). Thus, the influence of nutrient loading on bioerosion may differ for internal and external bioeroders.
Bioeroders can also be influenced by biotic interactions. The weakening of coral framework or exposure of new calcium carbonate surfaces in a colony due to the actions of one bioeroder may facilitate the action of another bioeroding group (Glynn 1997). For example, higher densities of macroborers were found in eroded versus intact portions of individual Porites astreoides colonies, suggesting that Caribbean parrotfishes target coral colony surfaces that contain macroborers (Rotjan and Lewis 2005). Thus, coral colonies with internal bioeroders, such as sponges, barnacles, polychaetes, and bivalves, might influence feeding preferences (e.g., colony targeted, or area on a given colony targeted) of external bioeroders (Rotjan and Lewis 2005). Bivalves, such as Lithophaga spp., are common internal borers that infest live corals and occur in high abundances on massive Porites corals (> 1800 ind. m−2 live tissue) on inshore, eutrophic reefs (Glynn and Manzello 2015). We predicted that high nutrient availability increases the density of internal borers in coral colonies and that their presence intensifies parrotfish bioerosion of these same colonies. Thus, we hypothesized that high nutrient availability and the bioerosive activities of internal borers facilitate external bioerosion of coral colonies by scraping and excavating parrotfishes.
Here, we tested whether internal borers, specifically lithophagid bivalves, were correlated with external bioerosion by parrotfishes (Labridae: Scarini) across a range of nitrogen availability. Specifically, we aimed to: (1) correlate the availability of nitrogen with internal bioeroders and (2) investigate the relationship between Lithophaga boreholes and parrotfish bite scars. We hypothesized that nitrogen availability would be positively correlated with the abundance of Lithophaga bivalves and that a higher density of these boring bivalves would increase the frequency and intensity of parrotfish bioerosion.
Materials and methods
Study site
This study was conducted in Moorea, French Polynesia in conjunction with the Moorea Coral Reef Long Term Ecological Research project (MCR LTER). Since 2005, the MCR LTER has taken annual photoquadrats in the fringing reefs across six LTER sites ranging in depth between 4 and 6 m (Edmunds 2018). There is a 40-m transect at each site partitioned into five permanent sections. Each section has uniquely marked stainless steel posts placed at the beginning and end, and every 1-m mark there is a permanent 50 × 50 cm plot (n = 40 plots per site). The photoquadrats (50 × 50 cm) are positioned along the permanent transect at approximately the same position at the 1-m mark and photographed with a high-resolution SLR digital camera annually.
Data collection
We used image analysis to determine spatiotemporal changes in the density of parrotfish bite scars and Lithophaga boreholes on the dominant reef-building coral, massive Porites, across the fringing reefs from a 10-yr dataset of benthic photographs. We examined a total of 715 photographs taken in 2006, 2008, 2010, 2011, 2013, and 2016 at three LTER sites characteristically dominated by massive Porites (Fig. 1a; Edmunds 2018). Distinct parrotfish bite scars and Lithophaga bores are visible on massive Porites in the photographs (Fig. 1b). In 350 photographs, massive Porites colonies were present; these photographs were analyzed to investigate hypothesized spatiotemporal patterns in the dynamics between internal and external bioeroders in relation to nutrient supply (Fig. 1c). All images were processed using standardized techniques adapted from Maher et al. (2018) using ImageJ (Schindelin et al. 2012). A ruler was used to set the scale in ImageJ to 1 cm, and every massive Porites colony was outlined to estimate its 2D live surface area within the photoquadrat. The number of distinct parrotfish bite scars and Lithophaga bores was recorded for each live massive Porites coral using the Cell Counter plugin for ImageJ and normalized to live colony surface area for analyses. The percent cover of massive Porites was determined using all MCR LTER photographs during this time period (n =715 photographs total) using CoralNet software with a stratified random point distribution with 100 points and a confidence threshold of 95% for computer automation (Beijbom et al. 2015).
Site map of Moorea, French Polynesia, example time series photograph, and conceptual diagram depicting the hypothesized relationship between nitrogen supply, Lithophaga colonization, and parrotfish bioerosion. a Map of Moorea, French Polynesia depicting the three LTER sites used in this study. b Photograph of massive Porites containing parrotfish bite scars and Lithophaga bores (photograph credit: Peter Edmunds). c As reefs shift from oligotrophic to eutrophic conditions, massive Porites colonies may become colonized with more Lithophaga borers, which may increase both the likelihood and intensity of bioerosion by parrotfish resulting in an increase in macro- and external bioerosion on eutrophic reefs
We used the MCR LTER time series of percent tissue nitrogen (% N) in the brown macroalga Turbinaria ornata at each site to examine the relationship between macroborer densities and nitrogen (N) availability (Carpenter 2018). The nutrient content of macroalgae is often used as a proxy for ambient nutrient conditions as these macroalgae integrate nutrients over a relatively long time frame (i.e., weeks to months) (Atkinson and Smith 1983). Both field surveys and experiments show that algae in consistently enriched environments typically have higher tissue nutrients (e.g., Burkepile and Hay 2009; Vega Thurber et al. 2014). The MCR LTER collects the brown macroalga T. ornata annually at LTER fringing reef sites (Carpenter 2018). T. ornata integrates N into its tissues for ~ 3 months, providing more information about nutrient conditions at sites than water samples, which are ephemeral (Lin and Fong 2008). Briefly, in this study, T. ornata were brought back to laboratory where the epiphytes were brushed off and fronds were removed 5 cm below the apex of the thallus (n = 10 fronds per site). The algae were dried at 60 °C for ~ 48 h or until constant weight and processed for CHN at University of California Santa Barbara’s analytical laboratory (for detailed methods see Carpenter 2018). Tissue CHN data were not available in 2006, so these data were not included in the analysis.
Data on fish abundance from the MCR LTER time series were used to evaluate how parrotfish density varied across site and year. SCUBA divers estimated the abundance and length of fishes on permanent 50 × 5 m belt transects running parallel to shore (n = 4 per site) between 0900 and 1600 h local time at ~ 10 m depth in the fringing reef (Brooks 2018). We included Chlorurus microrhinos, Chlorurus spilurus, Scarus frenatus, and Scarus ghobban parrotfishes that were > 10 cm in total length in our analysis because they are known corallivores and were large enough for their bites to scrape or excavate calcium carbonate (Cole et al. 2008; Rotjan and Lewis 2008).
In situ ground truthing of Lithophaga bores and parrotfish bite scars
Counts of Lithophaga bores or parrotfish bite scars from corals could differ based on whether a given colony is examined in situ on the reef by a snorkeler versus image analysis of a 2D photograph of the colony. To explore potential variation in the data generated by these two methods, we collected paired counts of Lithophaga bores and parrotfish bite scars from individual massive Porites colonies using in situ and image analysis approaches. These data were collected at two fringing reef sites in August and September of 2018 (Site 1: 17° 29′ 1.31″ S, 149° 49′ 0.16″, Site 2: 17° 29′ 24.41″ S, 149° 49′ 33.80″ W, depths 1–3 m) on the north shore of Moorea. A total of 19 colonies were examined per reef site (n = 38 colonies total). For in situ counts, a 50 × 50 cm quadrat was placed on a randomly selected massive Porites colony and Lithophaga bores and parrotfish bite scars were recorded over its entire surface. This same coral colony was then photographed with a 10-cm size standard using an Olympus TG-4 camera in plain view for subsequent blind image analysis. The same observer analyzed these photographs and the time series photographs.
Data analysis
General or generalized linear models were used to test for mean differences in Lithophaga, parrotfish, and parrotfish bite scar densities and percent cover of massive Porites by site and year and their interaction (i.e., two-way ANOVA). We took transect-level averages for massive Porites cover and summed across transects for parrotfish density. Parrotfish density was log (x + 1) transformed to meet assumptions of normality. A gamma hurdle model (i.e., two-way ANOVA based on a binomial and gamma distribution) was used for Lithophaga and bite scar densities because they were highly zero-inflated and non-normal. We used linear regression to test for a relationship between parrotfish density and parrotfish scar density using the site and year means.
We used a simple linear regression to test the relationship between Lithophaga densities and % N in tissues of T. ornata averaged across site by year. Tissue N data were not collected in 2006; thus, these three data points were excluded from the linear regression. To test the relationship between Lithophaga density and parrotfish bite scars on Porites, we first used a logistic regression to test whether Lithophaga density affected the probability of a coral being eroded by parrotfish. We then used a linear model to test the relationship between log-transformed Lithophaga and parrotfish bite scar densities when both were present. We also tested for a quadratic relationship between Lithophaga and parrotfish bite scar densities (i.e., Lithophaga density + Lithophaga density2), but the quadratic term was not statistically significant and, thus, was removed from the model. This two-step approach was used because both the Lithophaga and bite scar data were highly zero-inflated. Site and year were included as crossed random effects in the logistic and linear models to account for repeated measures. Lastly, linear regressions were used to explore correlations between log-transformed Lithophaga and parrotfish bite scar counts based on snorkel observations in situ and image analysis.
Normality and homoscedasticity of residuals were assessed visually for all models using quantile–quantile plots and boxplots by site and year, respectively. All analyses were conducted in the program R (R Development Core Team 2017), and mixed effects models were analyzed using the lme4 package (Bates et al. 2015). All code and raw data used in this analysis are available at https://github.com/njsilbiger/MCRBioerosion (Silbiger et al. 2019).
Results
We observed 2420 Lithophaga bores and 1883 parrotfish bite scars. Lithophaga densities and Porites cover were both highly variable across sites and years and had a significant interaction term (Lithophaga density site × year: χ2 = 8.6, P = 0.013; Fig. S1a and Porites cover site × year: F2,84 = 21.85, P < 0.0001; Fig. S1b). Massive Porites cover was highest at LTER 1 in 2006 (~ 13%) before it declined over time to ~ 2–4% cover by 2011, whereas at LTER 3 and 4, cover was relatively low and stable throughout the study period at ~ 2–4%. Lithophaga densities were consistently the highest at LTER 3, with an average of 0.09 ± 0.01 SE cm−2 on live massive Porites across all years: 4 times higher than LTER 4 and an order of magnitude higher than LTER 1. Parrotfish bite scar density on Porites varied by site (scars: χ2 = 7.03, P = 0.03; Fig. S1c) and year (scars: χ2 = 6.74, P = 0.01 Fig. S1c). Parrotfish density also varied by site (F2,50 = 8.19, P < 0.001; Fig. S1d) and year (F5,50 = 5.94, P < 0.001), but we did not observe a significant interaction term (F10,50 = 0.39, P = 0.9). We also did not observe a relationship between parrotfish density and parrotfish scar density (F1,16 = 0.25, P = 0.6, R2 = 0.15). Parrotfish scar densities were the highest at LTER 3, with an average of 0.08 ± 0.009 SE cm−2 of live coral across all years.
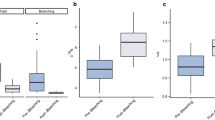
The high spatiotemporal variability in Lithophaga densities may be influenced in part by nitrogen (N) inputs, as evident by the significant positive relationship between % N in algal tissue and mean Lithophaga densities (F1,13 = 6.92, P = 0.02, R2 = 0.3; Fig. 2). Notably, LTER 3 consistently had the highest Lithophaga densities through time and also had the highest algal tissue % N content across all years and (LTER 3: 0.85 ± 0.03%; LTER 1: 0.77 ± 0.02%; LTER 4: 0.77 ± 0.02%; mean ± SE). The elevated Lithophaga densities at sites with higher N input were correlated with an increased likelihood of parrotfish bite scars. Specifically, higher Lithophaga densities significantly increased the probability of a coral having a parrotfish bite scar (χ2 = 5.73, P = 0.017; Fig. 3a), where the odds of a parrotfish bite scar increased by 2.19 with every increase of 0.5 Lithophaga cm−2 on live massive Porites (Fig. 3a). When only considering colonies with Lithophaga borers, there was also a significant positive relationship between Lithophaga density and the density of parrotfish bite scars, where parrotfish bite scars increased by 1.5 for every increase in Lithophaga cm−2 on live massive Porites (F1,33 = 45.2, P < 0.0001, marginal R2 = 0.23, conditional R2 = 0.25; Fig. 3b).
Relationship between nitrogen availability and Lithophaga spp. densities. Dots and whiskers are % tissue N ± SE (x) and mean borers cm−2 ± SE (y) for each site (LTER 1, 3, 4) × year (2008, 2010, 2011, 2013, 2016; n = 15 total). Data for algal tissue % N were not available in 2006 from the MCR LTER; thus, three data points were excluded from the analysis. Predictions are best fit line ± 95% confidence intervals from a simple linear regression. Percent N of Turbinaria ornata were used as a proxy for nitrogen availability at each site
Models testing probability and densities of parrotfish bite scars as a function of Lithophaga spp. density. a The density of Lithophaga spp. significantly increased the probability of a coral having a parrotfish bite scar. Blue and red hash marks in the rug are the raw data (n = 939) and prediction lines are model fit ± 95% confidence intervals. Year and site were included in the logistic model as crossed random effects. Data were scaled for the analysis and back transformed for this figure. b When bite scars were present, parrotfish bite scar density significantly increased with the density of Lithophaga spp. Both Lithophaga spp. and parrotfish bite scar densities were log-transformed for the analysis. Black dots are the raw data (n = 170) and prediction lines are model fit ± 95% confidence intervals. Year and site were included in the both models as crossed random effects
Lastly, we tested the efficacy of our image analysis by comparing Lithophaga and parrotfish bite scar densities counted using image analysis to data collected in situ. For parrotfish bites, there was a significantly positive relationship between parrotfish bite scars from image analysis and parrotfish bite scars counted in situ (F1,36 = 50.64, P < 0.001, R2 = 0.58, y =0.59x + 1.7; Fig. S2a). Similarly, for Lithophaga bore holes, there was a positive relationship between bore holes counted from images and those counted in situ (F1,36 = 48.8, P < 0.001, R2 = 0.58, y =1.2x − 0.20; Fig. S2b), although the image analysis slightly underestimated parrotfish bite scars at low densities.
Discussion
Our study demonstrates positive relationships among nitrogen enrichment, infestation with macroborers, and external bioerosion experienced by corals. Our results indicate that massive Porites colonies on more eutrophic reefs are likely to have higher densities of Lithophaga bivalves (Fig. 2), which increases both the probability of these colonies being bitten by parrotfishes (Fig. 3a) and the density of parrotfish bite scars (Fig. 3b). In fact, an increase of 0.5 lithophagid bivalves cm−2 on a massive Porites colony more than doubled the odds of being bitten (Fig. 3a). Thus, increases in nitrogen supply may strengthen the dynamics between external bioeroders and macroborers, likely resulting in elevated bioerosion on eutrophic reefs.
Our results demonstrate that massive Porites infestation by Lithophaga is correlated with increased external bioerosion by parrotfishes in Moorea. Similarly, parrotfishes in the Caribbean also appear to target corals with increased macroborer densities (Rotjan and Lewis 2005), suggesting that this relationship between external bioeroders and macroborers may be widespread (e.g., generalized across ocean basins). However, more research testing these relationships is needed to determine how common this relationship is on reefs. Additionally, these ecological processes likely occur at different time scales. For example, parrotfish bite scars on Porites spp. heal within 2–3 months (Bak and Stewardvanes 1980; Bonaldo et al. 2011; Welsh et al. 2015), while Lithophaga populations likely have a lagged response to nutrient availability on the order of months to years. Despite this, several studies show strong correlations between nutrient concentrations and internal bioeroder densities (Sammarco and Risk 1990; Scott and Cope 1990; Le Grand and Fabricius 2011). Only a few studies have examined the relationship between nutrient supply and internal and external bioeroder dynamics, and these studies offer several potential mechanisms that could be driving the patterns in our study.
First, parrotfish may be directly targeting macroborers in live coral due to the high nutritional content in macroborer tissues (Rotjan and Lewis 2005), or Lithophaga excretion may make the surrounding coral tissue richer in nitrogen (Mokady et al. 1993), possibly increasing parrotfish bioerosion. However, empirical evidence for these hypotheses is limited. Second, lithophagid infestation weakens the skeletal matrix (Scott and Risk 1988), which could focus parrotfish bioerosion on these colonies as they select for weaker structures (Littler et al. 1989; Chazottes et al. 2017). Third, the density of Lithophaga bivalves may covary with some other characteristics of the coral that influence bioerosion. For instance, in the Eastern Tropical Pacific, Porites evermanni consistently has a higher density of lithophagid bivalves than P. lobata, which induces local triggerfish to prey heavily on P. evermanni (Boulay et al. 2014). It is challenging to identify the massive Porites complex to species using image analysis, and thus different cryptic species of Porites may be driving some of the variation in our Lithophaga densities in Moorea. Fourth, parrotfish may be targeting endolithic and epilithic autotrophs (e.g., cyanobacteria). Most parrotfishes are microphages that consume microscopic benthic autotrophs as these dietary items are high in protein (Clements et al. 2017). Indeed, the abundance of microboring autotrophs, such as cyanobacteria, does increase with higher nutrient availability, resulting in higher microbioerosion rates on nutrient rich reefs (Perry and Harborne 2016). Lithophaga excretion may facilitate these autotrophs by increasing the availability of nutrients in neighboring coral tissues. The strong correlation we observed between Lithophaga densities and parrotfish scars strongly suggests that parrotfish are likely targeting either Lithophaga or the enriched coral tissue surrounding their bore holes. Given that lithophagid bivalves contribute substantially to internal bioerosion (Glynn 1997; Glynn and Manzello 2015) and that parrotfishes are the main drivers of external bioerosion on many reefs (Perry and Harborne 2016), we recommend that future studies explicitly test the mechanisms that drive increased bioerosion on corals with high lithophagid abundances using complementary methodologies (e.g., isotopic analysis, DNA metabarcoding).
We observed fairly high external bioerosion pressure on the massive Porites colonies in our study. The densities of parrotfish bite scars on massive Porites colonies at our fringing reef sites (mean range: 0.04–0.08 scars cm−2) are similar to those reported on an inner shelf reef (mean range: 0.004–0.07 bite scars cm−2; Bonaldo et al. 2012) and mid-shelf reef (mean range: 0.006–0.1 bite scars cm−2; Bonaldo and Bellwood 2011) within the Great Barrier Reef. This pattern is interesting given that fish corallivory and bioerosion rates tend to be higher on offshore reefs characterized by lower nutrients than nearshore, eutrophic reefs (Bonaldo et al. 2014). Although we lack data on parrotfish bite scar densities for offshore massive Porites colonies, we can speculate as to why we see such high bioerosion pressure on colonies inhabiting nearshore reefs. While bioeroding parrotfish abundance did change spatially and temporally (Fig. S1d), we did not see a relationship between parrotfish abundance and parrotfish bite scar density, suggesting that the observed patterns in bite scar densities was likely not driven by parrotfish abundance. Yet, massive Porites has been declining in the Moorea fringing reef habitats for the past decade (Fig. S1b), which may increase bioerosion pressure on the remaining colonies. Bonaldo and Bellwood (2011) found that as massive Porites cover declines, bioerosion pressure increases. Similar trends have been observed in the Caribbean when preferred coral species decline (Burkepile 2012).
We demonstrated that elevated nitrogen supply is correlated with elevated Lithophaga densities on massive Porites (Fig. 2). In Moorea, various anthropogenic sources (e.g., land-based pollution, stream inputs, and submarine groundwater discharge) deliver high concentrations of nutrients into the lagoon, resulting in a mosaic of nutrient hotspots across fringing reef habitats (Haßler et al. 2019). This elevated nutrient supply often increases primary productivity (Fabricius 2005), which supports higher densities of filter feeders, like Lithophaga spp. (Scott and Cope 1990). Nutrient loading also weakens coral skeletal structure (Caroselli et al. 2011; Mwachireya et al. 2016; Rice et al. 2019), which could make corals more susceptible to infestation by macroborers in eutrophic reefs. Notably, environmental parameters other than nutrients (e.g., pH, temperature, etc.) also affect patterns of bioeroder densities and bioerosion rates (e.g., Le Grand and Fabricius 2011; Davidson et al. 2013; Silbiger et al. 2014; Enochs et al. 2016; Silbiger et al. 2016, 2017). These parameters may covary or interact with nutrients (Manzello et al. 2008; DeCarlo et al. 2015; Prouty et al. 2017; Silbiger et al. 2018) and could contribute to the macroborer patterns in this study. However, our results are consistent with other studies that have shown higher abundances of macroborers on more eutrophic reefs (e.g., Sammarco and Risk 1990; Le Grand and Fabricius 2011; but see Chazottes et al. 2017).
Image analysis of time series photographs can be a useful tool for quantifying macroborer abundances for mounding and encrusting coral morphologies (Maher et al. 2018). The correlation between abundances recorded in situ by snorkelers and through image analysis demonstrates that these two methods produce comparable results for both Lithophaga bores and parrotfish bite scars (Fig. S2). A previous study comparing in situ counts and image analysis of an internal bioeroding barnacle found that, on average, image analysis was more conservative than in situ counts (Maher et al. 2018). Similarly, in this study, for massive Porites colonies with higher Lithophaga abundances, photograph counts were more conservative than snorkeler counts (Fig. S2b). However, photograph counts overestimated the number of Lithophaga boreholes in about a third of all observations. These overestimations typically occurred on Porites colonies with lower Lithophaga abundances (Fig. S2b). There are two possible explanations for this pattern. First, snorkelers conducting in situ counts may have underestimated Lithophaga on colonies when they were rare, due to challenging field conditions and breath-holding time constraints associated with free diving. Second, other bioeroders could have been misidentified as Lithophaga in some photographs, artificially inflating reported Lithophaga densities in photograph counts. False positives for Lithophaga from photographs are unlikely, however, since we only observed Lithophaga or vermetid boreholes in the photographs, and Lithophaga spp. have characteristically distinct boreholes that differ from vermetid molluscs in Moorea (Fig. S3).
Although in situ counts of Lithophaga and parrotfish bite scars more accurately estimate densities (Fig. S2), in situ quantification is often time intensive and costly (e.g., dive time on SCUBA surveys, ability to deploy and retrieve calcium carbonate blocks or to core reef substrate). Thus, analyzing existing time series images can help evaluate how macroborer abundances change temporally and spatially in live coral. This study was limited in scope to assessing Lithophaga boreholes on live massive Porites colonies. We did not assess abundances on dead substrate; therefore, our analysis likely underestimates total Lithophaga densities on these reefs. In addition, we were unable to monitor individual coral colonies over time due to slight variability in photoquadrat placement from year to year. Had we been able to quantify individual colonies over time we likely would have had more power to address temporal patterns in bioeroder densities. Research moving forward should couple image analysis methods with in situ methods to quantify macroborer densities in both live and dead coral and determine how these densities may translate to bioerosion rates.
External bioerosion by herbivorous fishes is a major contributor to bioerosion on many reefs (Tribollet et al. 2002; Hoey and Bellwood 2008; Bonaldo et al. 2014). Thus, it is important to study the environmental and biological drivers that may facilitate this process. Our study found strong evidence that macroborers can increase external bioerosion on reef-building corals and that nitrogen supply positively influenced lithophagid abundances. Together, these processes likely amplify the total calcium carbonate excavated from these colonies via bioerosion. Future studies should quantify how these processes impact bioerosion rates for common reef-building corals. As internal bioerosion rates are predicted to increase under future ocean conditions (e.g., ocean acidification: Wisshak et al. 2012, 2013; Andersson and Gledhill 2013; Silbiger and Donahue 2015; DeCarlo et al. 2015; nutrient enrichment: Prouty et al. 2017; Lubarsky et al. 2018), there is a critical need to better understand the relationship between internal and external bioeroders and how anthropogenic forcing may facilitate particular bioeroding taxa.
References
Andersson AJ, Gledhill D (2013) Ocean acidification and oral reefs: effects on breakdown, dissolution, and net ecosystem calcification. In: Carlson CA, Giovannoni SJ (eds) Annual Review of Marine Science, vol 5. Annual Reviews, Palo Alto, pp 321–348
Atkinson MJ, Smith SV (1983) C:N: P ratios of benthic marine plants. Limnol Oceanogr 28:568–574
Bak RPM, Stewardvanes Y (1980) Regeneration of superficial damage in the scleractinian corals Agaricia agaricites F. purpurea and Porites astreoides. Bull Mar Sci 30:883–887
Bates D, Machler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Beijbom O, Edmunds PJ, Roelfsema C, Smith J, Kline DI, Neal BP, Dunlap MJ, Moriarty V, Fan TY, Tan CJ, Chan S, Treibitz T, Gamst A, Mitchell BG, Kriegman D (2015) Towards Automated Annotation of Benthic Survey Images: Variability of Human Experts and Operational Modes of Automation. Plos One 10:22
Bonaldo RM, Bellwood DR (2011) Parrotfish predation on massive Porites on the Great Barrier Reef. Coral Reefs 30:259–269
Bonaldo RM, Krajewski JP, Bellwood DR (2011) Relative impact of parrotfish grazing scars on massive Porites corals at Lizard Island, Great Barrier Reef. Mar Ecol-Prog Ser 423:223–233
Bonaldo RM, Welsh JQ, Bellwood DR (2012) Spatial and temporal variation in coral predation by parrotfishes on the GBR: evidence from an inshore reef. Coral Reefs 31:263–272
Bonaldo RM, Hoey AS, Bellwood DR (2014) The ecosystem roles of parrotfishes on tropical reefs. In: Hughes RN, Hughes DJ, Smith IP (eds) Oceanography and Marine Biology: An Annual Review, vol 52. Crc Press-Taylor & Francis Group, Boca Raton, pp 81–132
Boulay JN, Hellberg ME, Cortes J, Baums IB (2014) Unrecognized coral species diversity masks differences in functional ecology. Proc R Soc B-Biol Sci 281:7
Brooks AJ (2018) MCR LTER: Coral Reef: Long-term Population and Community Dynamics: Fishes, ongoing since 2005
Burkepile DE (2012) Context-dependent corallivory by parrotfishes in a Caribbean reef ecosystem. Coral Reefs 31:111–120
Burkepile DE, Hay ME (2009) Nutrient versus herbivore control of macroalgal community development and coral growth on a Caribbean reef. Mar Ecol-Prog Ser 389:71–84
Caroselli E, Prada F, Pasquini L, Nonnis Marzano F, Zaccanti F, Falini G, Levy O, Dubinsky Z, Goffredo S (2011) Environmental implications of skeletal micro-density and porosity variation in two scleractinian corals. Zoology 114:255–264
Carpenter R (2018) MCR LTER: Coral Reef: Macroalgal CHN, ongoing since 2005
Carreiro-Silva M, McClanahan TR, Kiene WE (2005) The role of inorganic nutrients and herbivory in controlling microbioerosion of carbonate substratum. Coral Reefs 24:214–221
Chazottes V, Hutchings P, Osorno A (2017) Impact of an experimental eutrophication on the processes of bioerosion on the reef: One Tree Island, Great Barrier Reef, Australia. Mar Pollut Bull 118:125–130
Clements KD, German DP, Piche J, Tribollet A, Choat JH (2017) Integrating ecological roles and trophic diversification on coral reefs: multiple lines of evidence identify parrotfishes as microphages. Biol J Linnean Soc 120:729–751
Cole AJ, Pratchett MS, Jones GP (2008) Diversity and functional importance of coral-feeding fishes on tropical coral reefs. Fish Fish 9:286–307
Davidson TM, de Rivera CE, Canton JT (2013) Small increases in temperature exacerbate the erosive effects of a non-native burrowing crustacean. Journal of Experimental Marine Biology and Ecology 446:115–121
DeCarlo TM, Cohen AL, Barkley HC, Cobban Q, Young C, Shamberger KE, Brainard RE, Golbuu Y (2015) Coral macrobioerosion is accelerated by ocean acidification and nutrients. Geology 43:7–10
Dunn JG, Sammarco PW, LaFleur G (2012) Effects of phosphate on growth and skeletal density in the scleractinian coral Acropora muricata: A controlled experimental approach. Journal of Experimental Marine Biology and Ecology 411:34–44
Edinger EN, Limmon GV, Jompa J, Widjatmoko W, Heikoop JM, Risk MJ (2000) Normal coral growth rates on dying reefs: are coral growth rates good indicators of reef health? Mar Pollut Bull 40:404–425
Edmunds PJ (2018) MCR LTER: Coral Reef: Long-term Population and Community Dynamics: Corals, ongoing since 2005. In: LTER MCR (ed)
Enochs IC, Manzello DP, Kolodziej G, Noonan SHC, Valentino L, Fabricius KE (2016) Enhanced macroboring and depressed calcification drive net dissolution at high-CO2 coral reefs. Proc R Soc B-Biol Sci 283:8
Fabricius KE (2005) Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar Pollut Bull 50:125–146
Glynn PW (1997) Bioerosion and coral-reef growth: a dynamic balance. In: Birkeland C (ed) Life and death of coral reefs. Chapman & Hall
Glynn PW, Manzello DP (2015) Bioerosion and coral reef growth: a dynamic balance Coral reefs in the Anthropocene. Springer, pp 67-97
Haßler K, Dähnke K, Kölling M, Sichoix L, Nickl A-L, Moosdorf N (2019) Provenance of nutrients in submarine fresh groundwater discharge on Tahiti and Moorea, French Polynesia. Applied Geochemistry 100:181–189
Hoey AS, Bellwood DR (2008) Cross-shelf variation in the role of parrotfishes on the Great Barrier Reef. Coral Reefs 27(1):37–47
Holmes KE, Edinger EN, Hariyadi LG, Risk MJ (2000) Bioerosion of live massive corals and branching coral rubble on Indonesian coral reefs. Mar Pollut Bull 40:606–617
Hutchings P (2008) Role of polychaetes in bioerosion of coral substrates. Springer-Verlag, Berlin
Hutchings PA (1986) Biological destruction of coral reefs: a review. Coral Reefs 4:239–252
Le Grand HM, Fabricius KE (2011) Relationship of internal macrobioeroder densities in living massive Porites to turbidity and chlorophyll on the Australian Great Barrier Reef. Coral Reefs 30:97–107
Lin DT, Fong P (2008) Macroalgal bioindicators (growth, tissue N, δ15 N) detect nutrient enrichment from shrimp farm effluent entering Opunohu Bay, Moorea, French Polynesia. Mar Pollut Bull 56:245–249
Littler MM, Taylor PR, Littler DS (1989) Complex interactions in the control of coral zonation on a Caribbean reef flat. Oecologia 80:331–340
Lubarsky KA, Silbiger NJ, Donahue MJ (2018) Effects of submarine groundwater discharge on coral accretion and bioerosion on two shallow reef flats. Limnol Oceanogr 63:1660–1676
Maher RL, Johnston MA, Brandt ME, Smith TB, Correa AMS (2018) Depth and coral cover drive the distribution of a coral macroborer across two reef systems. Plos One 13:17
Manzello DP, Kleypas JA, Budd DA, Eakin CM, Glynn PW, Langdon C (2008) Poorly cemented coral reefs of the eastern tropical Pacific: Possible insights into reef development in a high-CO2 world. Proc Natl Acad Sci U S A 105:10450–10455
Mokady O, Bonar DB, Arazi G, Loya Y (1993) Spawning and development of three coral-associated Lithophaga species in the Red Sea. Mar Biol 115:245–252
Mwachireya SA, McClanahan TR, Cote IM, Hartwick BE (2016) Increased terrestrial perturbations modify skeletal properties and mechanical strength of hard corals. Environment and Natural Resources Research 6:153
Perry CT, Harborne AR (2016) Bioerosion on modern reefs: Impacts and responses under changing ecological and environmental conditions. Springer, Dordrecht
Perry CT, Murphy GN, Kench PS, Smithers SG, Edinger EN, Steneck RS, Mumby PJ (2013) Caribbean-wide decline in carbonate production threatens coral reef growth. Nature Communications 4:7
Prouty NG, Cohen A, Yates KK, Storlazzi CD, Swarzenski PW, White D (2017) Vulnerability of coral reefs to bioerosion from land-based sources of pollution. J Geophys Res-Oceans 122:9319–9331
R Development Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rice MM, Ezzat L, Burkepile DE (2019) Corallivory in the Anthropocene: Interactive effects of anthropogenic stressors and corallivory on coral reefs. Frontiers in Marine Science 5:525
Rose CS, Risk MJ (1985) Increase in Cliona delitrix infestation of Montastrea cavernosa heads on an organically polluted portion of the Grand Cayman fringing reef. Mar Ecol-Pubbl Stn Zool Napoli 6:345–363
Rotjan RD, Lewis SM (2005) Selective predation by parrotfishes on the reef coral Porites astreoides. Mar Ecol-Prog Ser 305:193–201
Rotjan RD, Lewis SM (2008) Impact of coral predators on tropical reefs. Mar Ecol-Prog Ser 367:73–91
Sammarco PW, Risk MJ (1990) Large-scale patterns in internal bioerosion of Porites: cross continental shelf trends on the Great Barrier Reef Mar Ecol-Prog Ser 59:145–156
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Scott PJB, Risk MJ (1988) The effect of Lithophaga (Bivalvia, Mytilidae) boreholes on the strength of the coral Porites lobata. Coral Reefs 7:145–151
Scott PJB, Cope M (1990) Tolo revisited: a resurvey of the corals in Tolo Harbor and Channel Hong Kong six years and half a million people later
Silbiger NJ, Donahue MJ (2015) Secondary calcification and dissolution respond differently to future ocean conditions. Biogeosciences 12:567–578
Silbiger NJ, Donahue MJ, Brainard RE (2017) Environmental drivers of coral reef carbonate production and bioerosion: a multi-scale analysis. Ecology 98:2547–2560
Silbiger NJ, Moeller HV, Rice MM (2019) njsilbiger/MCRBioerosion: Macroborer presence on corals increases with nutrient input and promotes parrotfish corallivory, Zenodo
Silbiger NJ, Guadayol O, Thomas FIM, Donahue MJ (2014) Reefs shift from net accretion to net erosion along a natural environmental gradient. Mar Ecol-Prog Ser 515:33–44
Silbiger NJ, Guadayol O, Thomas FIM, Donahue MJ (2016) A novel μCT analysis reveals different responses of bioerosion and secondary accretion to environmental variability. Plos One 11:20
Silbiger NJ, Nelson CE, Remple K, Sevilla JK, Quinlan ZA, Putnam HM, Fox MD, Donahue MJ (2018) Nutrient pollution disrupts key ecosystem functions on coral reefs. Proc R Soc B-Biol Sci 285:9
Tribollet A, Golubic S (2005) Cross-shelf differences in the pattern and pace of bioerosion of experimental carbonate substrates exposed for 3 years on the northern Great Barrier Reef, Australia. Coral Reefs 24:422–434
Tribollet A, Golubic S (2011) Reef bioerosion: agents and processes. Springer, Dordrecht
Tribollet A, Decherf G, Hutchings PA, Peyrot-Clausade M (2002) Large-scale spatial variability in bioerosion of experimental coral substrates on the Great Barrier Reef (Australia): importance of microborers. Coral Reefs 21:424–432
Tribollet A, Langdon C, Golubic S, Atkinson M (2006) Endolithic microflora are major primary producers in dead carbonate substrates of Hawaiian coral reefs. J Phycol 42:292–303
Vega Thurber RL, Burkepile DE, Fuchs C, Shantz AA, McMinds R, Zaneveld JR (2014) Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Global Change Biology 20:544–554
Weinstein DK, Maher R, Correa AMS (2019) Bioerosion. In: Loya Y, Puglise, K.A., Bridge, T. (ed) Mesophotic coral ecosystems. Springer International Publishing
Welsh JQ, Bonaldo RM, Bellwood DR (2015) Clustered parrotfish feeding scars trigger partial coral mortality of massive Porites colonies on the inshore Great Barrier Reef. Coral Reefs 34:81–86
Wisshak M, Schonberg CHL, Form A, Freiwald A (2012) Ocean acidification accelerates reef bioerosion. Plos One 7:8
Wisshak M, Schonberg CHL, Form A, Freiwald A (2013) Effects of ocean acidification and global warming on reef bioerosion-lessons from a clionaid sponge. Aquatic Biology 19:111–127
Acknowledgements
We are very grateful for the decade of work by Dr. Peter Edmunds in taking these annual benthic photoquadrats, which allowed this study to be possible. We are also grateful for the decade of CHN data and fish abundance data collected by Dr. Robert Carpenter and Dr. Andrew Brooks, respectively. We thank Daniel Baldwin for assistance with the collection of data in the field. This material is based upon work supported by the U.S. National Science Foundation under Grant (OCE#16-37396) as well as a generous gift from the Gordon and Betty Moore Foundation. Research was completed under permits issued by the French Polynesian Government (Délégation à la Recherche) and the Haut-commissariat de la République en Polynésie Francaise (DTRT) (Protocole d’Accueil 2005-2018). Grants from the National Science Foundation (#1635798 to AMSC, #1547952 to DEB), NSF Graduate Research Fellowship to both MMR (#1650114) and RLM (#1314109-DGE), and the Worster Award to MMR supported this work. This work represents a contribution of the Moorea Coral Reef (MCR) LTER Site and is CSUN Marine Biology contribution #289. Data and code used in this paper are available on https://github.com/njsilbiger/MCRBioerosion.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Topic Editor Andrew Hoey
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rice, M.M., Maher, R.L., Correa, A.M.S. et al. Macroborer presence on corals increases with nutrient input and promotes parrotfish bioerosion. Coral Reefs 39, 409–418 (2020). https://doi.org/10.1007/s00338-020-01904-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-020-01904-y