Abstract
Few studies have documented the spatial and temporal dynamics of highly invasive species in coral reef benthic communities. Here, we quantified the ecological dynamics of invasion by a corallimorph, Rhodactis howesii, at Palmyra Atoll in the central Pacific. A localized outbreak of this species was first observed following a shipwreck at Palmyra in 1991 and has subsequently spread across hectares, reaching 100% cover in some areas. We examined the spatial and temporal dynamics of this invasion, and its impact on the benthic community, using a combination of permanent photoquadrats and large-scale photomosaic imagery. Our data revealed two distinct patterns in the spatial dynamics of R. howesii on the reef. First, following the removal of the shipwreck in 2013, the cover of the corallimorph in the immediate vicinity of the wreck decreased markedly, with crustose coralline algae (CCA), an important reef-builder, dominating the newly available substrate. However, in contrast to the decline at the epicenter of the invasion, the corallimorph has spread to additional sites around the atoll where increases in abundance have been associated with decreases in hard coral cover. Reductions in percent cover and corallimorph patch size near the epicenter of the outbreak, coupled with increases in cover and patch size and appearance of the corallimorph at other locations around Palmyra, demonstrate the dynamic nature of this “invasion.” Further, we found that the corallimorph settled disproportionately often on patches of turf or CCA cover, but can then overgrow all benthic competitors following establishment. This study provides evidence that R. howesii has the capacity to be highly invasive on coral reefs and highlights the importance of large-scale, long-term monitoring efforts to capture the dynamic nature of such invasions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species invasions can rapidly transform the structure and function of ecosystems, resulting in the loss of biodiversity and productivity, thus impoverishing ecosystems and the human populations that rely on them (Elton 1958; Simberloff and Rejmánek 2011). While mobile, invasive predators such as fishes (Pringle 2011) and reptiles (Wiles et al. 2003) are known to transform communities via top-down mechanisms, sessile invaders such as mussels (Nalepa and Schloesser 1992), clams (Kimmerer et al. 1994), and algae (Boudouresque et al. 1995) transform ecosystems via bottom-up dynamics and direct habitat modifications. On coral reefs, invasion by fleshy, non-calcifying organisms directly undermines the structural integrity and complexity of the ecosystem’s carbonate foundation, resulting in numerous indirect negative impacts (Perry et al. 2008). For example, reef habitat with high corallimorph cover can shift from net accretion to net dissolution, resulting in a loss of overall ecosystem function (Takeshita et al. 2016). Though the effects of fleshy algae have received the greatest attention on coral reefs, similar impacts of non-calcifying sessile animals (e.g., sponges, tunicates, and certain anthozoans) warrant equal attention (Norström et al. 2009).
Community transitions are often catalyzed by large-scale coral mortality resulting from pulse disturbances (Norström et al. 2009) or from species invasions (Conklin and Smith 2005). However, less is known about the stability and long-term effects of these transitions due to lack of spatiotemporal data needed to accurately quantify successional dynamics and changes in community composition. The persistence of species invasions [here defined as the over-proliferation of a species that may or may not be native to the region of study sensu (Warren 2007)] or outbreaks is difficult to assess without long-term monitoring. Here, we make use of a long-term and spatially extensive dataset collected at Palmyra Atoll from 2010 to 2017 to examine the spread of a corallimorph, Rhodactis howesii, and related changes in benthic community composition.
Corallimorph species are exceptional benthic competitors on coral reefs that can rapidly invade and colonize open space (Chadwick-Furman and Spiegel 2000; Muhando et al. 2002). They have a variety of life history strategies and behavioral characteristics that contribute to their competitive success. For example, corallimorphs are able to reproduce sexually and asexually via spawning, budding, clonal replication, and fragmentation (Chadwick and Adams 1991; Chadwick-Furman and Spiegel 2000) and population sizes can double in as little as two months (Chadwick and Adams 1991). In the Red Sea, Rhodactis spp. can tolerate high temperature and irradiance, which affords an additional competitive advantage in shallow back-reef environments (Kuguru et al. 2004; Kuguru et al. 2007). Additionally, the corallimorph R. howesii possesses large nematocysts that have been known to cause significant damage to living coral tissue, enhancing their capacity to outcompete corals and other benthic organisms (Work et al. 2008).
Corallimorphs, and R. howesii in particular, have been shown to be aggressive competitors, and outbreaks have been documented on some coral reefs (Muhando et al. 2002; Work et al. 2008; Crane et al. 2016). This study focuses on the outbreak of R. howesii at Palmyra Atoll, a National Fish and Wildlife Refuge and part of the Pacific Remote Islands Marine National Monument in the central Pacific. Due to its protected status, Palmyra’s reefs are relatively intact, with high cover of reef-building organisms (Smith et al. 2016) and healthy herbivore populations (Sandin et al. 2008; Edwards et al. 2014). The reefs have had minimal human impact due to the lack of a permanent population, aside from a brief occupation by the US military during WWII. However, despite its high level of protection over the last 17 years, a portion of the reef on the shallow terrace has undergone a shift in community structure from a coral to corallimorph-dominated state (Work et al. 2008). This shift was associated with a localized disturbance caused by the grounding of the Hui Feng, a longline fishing vessel, in 1991. R. howesii was initially observed in low abundances at the site of the shipwreck and was generally rare at other sites around the atoll (Work et al. 2008). However, by 2005, the corallimorph population at the wreck site (hereafter referred to as the “Longliner site”) had rapidly expanded, covering over 2 km2 of the reef (Work et al. 2008). While there was no clear link between the wreck and the corallimorph outbreak, the rapid spread and alteration of the benthic community were of particular concern to management agencies. Work et al. (2008) hypothesized that iron leaching from the wreck facilitated corallimorph growth via nutrient input and that removal of the wreck would assist in recovery of the benthic community (Kelly et al. 2011). In light of this, the US Fish and Wildlife Service removed the shipwreck in the winter of 2013 in an effort to reduce the impact of the corallimorph and facilitate reef recovery. This provided a unique opportunity to study the changes in the spatial and temporal trajectories of the corallimorph and recovery of the benthic community associated with the wrecks removal.
The goals of this study were to characterize the spatial and temporal variability in corallimorph abundance and corresponding changes to the benthic communities over a nine-year time window. Specifically, we sought to (1) document the spatial distribution of the corallimorph around the island, (2) examine changes in corallimorph cover and overall benthic community composition, (3) investigate initial establishment and subsequent spread of corallimorph invasions, and (4) explore the large-scale spatial dynamics of corallimorph patches throughout time. The results shed light on the impacts of corallimorphs on benthic coral reef communities, their response to shipwreck removal, and temporal trends in corallimorph dynamics at permanent sites around the atoll. This information can inform future management efforts aimed at monitoring and limiting the expansion of this coral reef invader.
Materials and methods
Distribution of R. howesii at Palmyra Atoll
We used records and images of the occurrence of R. howesii at Palmyra from 2009 to 2017 to document the spatial extent of the population. These data included 27 one-time surveys from a monitoring expedition in 2010, permanent sites established at Palmyra between 2009 and 2012 (details below), and towed-diver surveys following protocol outlined in Work et al. (2008). Further details of the monitoring sites and methods can be found in the supplementary material (ESM Table S1).
Changes in benthic community composition
Changes in benthic community composition around Palmyra Atoll were examined using permanent photoquadrat transects established in 2009 at 4 forereef sites (10 m depth; FR3, FR5, FR7, FR9) and 4 sites on the shallow reef terrace (5 m depth; RT1, RT4, RT10, RT13; Fig. 1). An additional back-reef site (Penguin Spit Middle, PSM) was established in 2010. Transects were 50 m in length and parallel to shore with ten permanent 0.63 m2 photoquadrats positioned every 5 m along the transect and marked with stainless steel eyebolts. Each site was established with one, 50-m transect. The permanent sites were surveyed annually, and the results reflect data collected between late August and early October for each year to minimize any seasonal variation in the benthic community. Percent cover was determined using the image analysis software Photogrid 1.0 (Barott et al. 2012; Smith et al. 2016). Photographs were analyzed by overlaying 100 stratified random points across each picture and identifying the substrate under each point to the finest taxonomic resolution possible (genus for corallimorph, hard corals and macroalgae, functional group for crustose coralline algae (CCA), turf algae, and other invertebrates). Points were then kept consistent from year-to-year to examine variability in percent cover of common benthic functional groups and the corallimorph over time.
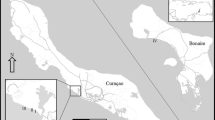
A spatial display of the historical observations of R. howesii around Palmyra Atoll. Reef communities are shown in gray, sand flats in white, and land mass in black a Observed (black circle) and Not Observed (white circle) of the corallimorph Rhodactis howesii at all sites surveyed at Palmyra Atoll from 2009 to 2016. b Higher resolution of the spatial cover of the corallimorph at the Longliner site across the western reef terrace from surveys spanning 2013–2017. c Site names for all long-term monitoring transects discussed throughout manuscript
To examine changes at the epicenter of the outbreak, additional study sites were established in 2013 around the Longliner wreck prior to its removal by the USFWS. Four permanent transects (as described above) were installed along the cardinal directions (N, E, S, W) radiating out from the Longliner wreck itself. Plots were photographed annually and analyzed as described above. To examine changes in the percent cover of corallimorph and other benthic organisms, data from the 4 transects were combined to provide site averages of the dominant functional groups (corallimorph, hard coral, CCA, macroalgae, and turf algae) during the monitoring period from 2013 to 2017.
Initial establishment of R. howesii on benthos
We examined the initial stages of corallimorph establishment to better understand the invasion process. To determine if the corallimorph settled on certain benthic functional groups more frequently than would be expected by chance (based upon the abundance of each group), we recorded the functional group that each individual corallimorph polyp settled upon (hard coral, CCA, macroalgae, turf algae, or soft coral) for every permanent, invaded plot. The proportions of expected settlement substrate were calculated using functional group abundance for each plot. We then used a Chi-squared analysis (R 2.15.1 (R Core Team 2016)) to compare the observed and expected settlement substrate values to determine preference or avoidance of certain benthic functional groups.
We then assessed the ability of the corallimorph to overgrow different benthic functional groups. To accomplish this, we identified each of the randomly stratified points used in the photoquadrat analysis (point locations were held constant through time) that were recorded as corallimorph. We then tracked each point back through time to identify what the point was in the year prior to transitioning to corallimorph. This showed which taxa could be overgrown by the corallimorph and how often it successfully displaced each taxa.
Changes in corallimorph patch dynamics throughout time
Because of the clonal nature of R. howesii, we were interested in measuring how patch dynamics changed over time at a scale larger than what was observed in the photoquadrats. To investigate this, we used large-area imagery (photomosaics) to survey swaths of reef annually from 2012 to 2016. In 2012, a 200 m2 site was established adjacent to the Longliner wreck. In 2013, 16 replicate 100 m2 photomosaic sites were established along the 10-m isobath of the forereef habitat across the north and south shores of the atoll. All plots were established with two stainless steel pins marked by GPS to ensure that the same area of the reef was photographed at each survey. Briefly, a single mosaic consisted of approximately 5000 individual images taken over a 45–60-min dive (Edwards et al. 2017). Analytical processing of the mosaic imagery was conducted using custom algorithms designed by Edwards et al. 2017 in R 3.2 (R Core Team 2016). Further details regarding the collection and processing methods can be found in previous publications (Gracias and Santos-Victor 2000, 2001; Lirman et al. 2007; Edwards et al. 2017).
Changes in corallimorph patch size (defined here as spatially continuous aggregations of corallimorph polyps) were digitized in Adobe Photoshop, and data were analyzed for the Longliner site and the forereef site (FR5) where initial invasion and subsequent growth were observed (as described above). For each survey year (2012–2016), imagery was uploaded to Adobe Photoshop Creative Cloud and the boundaries of all contiguous corallimorph patches were digitized by hand using a Wacom pen tablet (model # CTH-470). The digitized patches were then exported from Photoshop as a single .PNG image file for each year, from each site. The total number of patches, the size of each patch, and total spatial cover were calculated using R 2.15.1 (R Core Team 2016). Significant changes in patch size across years at each site were analyzed using a Kruskal–Wallis rank sum test as the strongly right-skewed data prevented the use of standard parametric approaches. All statistical analyses for the study were conducted in R 2.15.1 (R Core Team 2016).
Results
Distribution of R. howesii at Palmyra Atoll
Based upon atoll wide surveys from 2009 to 2017, the corallimorph was widely observed across the shallow reef terrace, particularly around the Longliner wreck site, with continued spread south across the channel toward the back-reef site (PSM) and across the southern forereef (Fig. 1a, b). Data from photomosaic sites across the atoll showed distant populations of the corallimorph on the forereef up to 6 km away from the invasion epicenter. R. howesii was virtually ubiquitous along the southern forereef, where it was observed at 7 of the 9 sites. In contrast, R. howesii was observed at only 4 of 13 sites along the northern forereef and was completely absent at the 2 easternmost (162°0) and 3 westernmost sites (162°9) (Fig. 1). However, due to logistical difficulties reaching those sites, they have not been re-surveyed since 2010 and it is possible that the corallimorph has now reached the tips of the atoll.
Changes in benthic community composition
The forereef site FR3 had 1% cover of R. howesii when first surveyed in 2009. Corallimorph cover at the site peaked in 2014, reaching a max of 15% cover that has since dropped to 6% in 2017 (Fig. 2c). Hard coral cover has remained high at FR3, with an average of 56.1 ± 1.7% (mean ± SE) (Fig. 2a) from 2009 to 2017. Percent cover of CCA remained relatively stable, with an average of 15.2 ± 1.2% at the site from 2009 to 2017 (Fig. 2b). In contrast, percent cover of turf algae has decreased at FR3, dropping from a high of 14.9 ± 2.7% in 2015 to a low of 7.3 ± 1.5% in 2017 (Fig. 2d).
Change in mean functional group cover± SE at four permanent monitoring sites around Palmyra Atoll from 2012 to 2017 (a–e). Two forereef (FR3, FR5), one reef terrace (RT10), and one back-reef (PSM) are shown. f Final–initial change in cover for each functional group by site from 2009 to 2017. The dashed line represents the removal of the shipwreck from the site
In contrast to FR3, the corallimorph was not recorded in the first survey of site FR5 in 2009, but was present in 2010. R. howesii cover peaked at 27.6 ± 8.3% in 2016 and dropped to 24.6 ± 8.3% in 2017 (Fig. 2c). Hard coral cover steadily decreased at the site from a high of 24.2 ± 4.4% in 2009 to a low of 11.7 ± 3.1% in 2016. A slight increase in hard coral cover was observed in 2017, reaching 13.4 ± 3.8%. CCA and turf algal cover also decreased throughout the monitoring time period (Fig. 2f). CCA peaked at 44.1 ± 3.2% in 2010 and has since dropped to 27.4 ± 5.9% in 2017. Turf algae decreased from 16.4 ± 2.5% to 8.1 ± 3.1% from 2009 to 2017.
Initial invasion of corallimorph in established plots was observed at the reef terrace site RT10, which was established in 2009 with 0% cover. R. howesii invaded a single plot in 2014, peaked with 22% cover in 2015, and has since decreased to 9% cover in 2017. Hard coral cover was the highest at RT10 for all sites, with an average of 59.1 ± 1% from 2009 to 2017. CCA and turf algae decreased at the site, dropping from 13.3 ± 2.2% to 9.6 ± 2.3% and from 29 ± 6.4% to 15.7 ± 4.2%, respectively (Fig. 2). In contrast, macroalgae increased in percent cover at the site from 6.6 ± 1.7% in 2009 to 18.3 ± 3.7% in 2017.
The back-reef site, PSM, was the only site with a continual increase in corallimorph cover throughout time. Initial percent cover was 19 ± 6.7% in 2010 and increased to 43.3 ± 11.1% in 2017. Hard coral cover remained consistent at the site, with an average of 20.1 ± 0.7%. CCA increased in percent cover from 9.2 ± 1.9% in 2010 to 27.6 ± 6.5% in 2017. In contrast, turf and macroalgal cover declined over the monitoring period. Turf algae had an initial cover of 42.4 ± 7.3% in 2010 and a final cover of 6.9 ± 2% in 2017. Macroalgae decreased from 27.7 ± 6.3% in 2010 to 11.8 ± 2.4 in 2017.
At the Longliner site, cover of the corallimorph decreased drastically from an average of 68.5 ± 2.3% in 2013 to 1.5 ± 0.3% in 2017 (Fig. 3c). Cover of CCA increased the most during the monitoring period, starting at 8 ± 1.2% in 2013 and rising to 49.3 ± 1.6% by 2017 (Fig. 3b). Turf and macroalgae showed a general increase until 2016 where they both peaked at 30.1 ± 3% and 28.8 ± 1.1%, respectively (Fig. 3d, e). Since 2016, both turf and macroalgae have dropped in percent cover to 17.7 ± 1.5% and 24.7 ± 1.1%, respectively.
Initial establishment of R. howesii on benthos
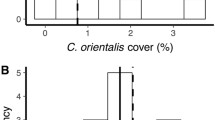
Upon initial invasion of a given plot, R. howesii polyps disproportionately colonized CCA and turf algae, despite accounting for abundance of these functional groups (Fig. 4). Out of the 23 instances where a polyp landed on previously unsettled substrate, 11 settled on CCA, 8 on turf, and 4 on macroalgae. Further, the corallimorph significantly avoided settling on hard coral and macroalgae (χ2 = 16.94, p value 0.0007). However, once the corallimorph was established in a plot, it overgrew all other functional groups. A total of 836 transitions were documented from one taxon to corallimorph between 2009 and 2017 in the monitoring plots. Of those transitions, hard coral and CCA were overgrown 15.6% and 23.3% of the time, respectively. Turf and macroalgae were overgrown 30.9% and 30% of the time. In contrast, soft coral represented only 0.24% of the transitions, making it the least overgrown functional group in our dataset.
Substrate preference of initial corallimorph settlement in non-invaded plots and subsequent overgrowth of function groups post-invasion. a Number of observed and expected settlement events for each functional group. Observed values higher than expected demonstrate preference for habitat type, observed values lower than expected demonstrate avoidance of habitat type. Chi-squared test of observed frequencies indicated significant avoidance of hard coral and macroalgae and selectivity of CCA (χ2 = 16.94, p < 0.01). b Number of transitions from benthic functional group to corallimorph from 2009 to 2017 in all plots
Changes in corallimorph patch dynamics throughout time
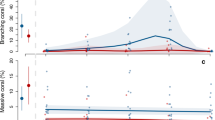
The percent cover of corallimorph in the Longliner photomosaic site declined from 50 to 10% (Fig. 5) between 2012 and 2016, while the number of patches increased by an order of magnitude (232 to 2398). In contrast, the mean patch size decreased from 0.6 m2 in 2012 to 0.2 m2 in 2016, with significant decreases between 2012 and 2014, 2014 and 2015, and 2015 and 2016 (p < 0.001, ESM Table S2, Fig. 5). However, at the more distant forereef site FR5, corallimorph cover increased from 5 to 23% between 2012 and 2016. The increase in cover was accompanied by a significant decrease in mean patch size from 2012 to 2014 (p < 0.001, ESM Table S2), though patch size has not significantly changed since 2014. The number of patches increased from 143 to 636 between 2012 and 2014 (Fig. 5).
Initial and final corallimorph cover at the epicenter of the invasion contrasted with initial colonization and subsequent overgrowth of corals on the forereef. Initial and final photoquadrat from the Longliner North transect (a) in 2013 and 2017. Initial invasion and subsequent growth of the corallimorph in a quadrat from FR5 on the forereef in 2009 to 2017 (b). Black arrow shows initial corallimorph polyps at early stages of invasion. Images show the difference between sites that experienced a decline vs. invasion during the monitoring time period. It is important to note that not all plots at these sites are experiencing the same corallimorph dynamics
Discussion
Ecological dynamics of marine invasive species and their impact on benthic communities are often difficult to assess, as they require data spanning large spatial and temporal scales. Previous studies have documented large-scale, rapid changes in benthic community composition from hard coral to macroalgal cover (Done 1992; Hughes 1994; McCook 1999; Côté et al. 2005). However, few studies have examined less common community transitions where corals are replaced by fleshy-invertebrate taxa such as soft corals, anemones, sponges, or corallimorphs (Norström et al. 2009; Kuguru et al. 2010; Elliott et al. 2016). Additionally, data on the impacts of these outbreaks or invasions on the associated benthos are limited because pre-invasion community structure is often unknown. Here, we present detailed information on the spatial and temporal dynamics of the invasive corallimorph R. howesii and associated impacts on the benthic reef communities of Palmyra Atoll. We show contraction of the population at the initial site of invasion, as well as expansion and spread of the corallimorph at more distant locations around the atoll. Further, while this species more readily invaded space occupied by CCA and turf algae, it appears able to overgrow all other benthic functional groups once established, highlighting R. howesii’s invasive nature.
Interestingly, some sites with high coral cover were more resistant to invasion than others, despite proximity to the initial invasion epicenter. For example, the forereef site just south of the Longliner wreck (FR3, Fig. 1, mean hard coral cover 56.1 ± 1.7%) was established in 2009 and had a single corallimorph polyp in one quadrat along our transect. By 2014, 15 polyps were present in the quadrat and CCA was the only functional group to decline as the corallimorph increased at the site (14.6 ± 1.6% to 11.4 ± 2%). However, by 2017, the corallimorph had dropped back down to a single polyp and CCA had rebounded to 20.7 ± 2.8%, higher than its initial cover (Fig. 2). A similar pattern was observed at RT10, the reef terrace site with the highest coral cover (mean 59.1 ± 1%). R. howesii first invaded a single quadrat in 2014 at 12% cover, increased to 22% (in the single quadrat) by 2015, and declined to 9% by 2017. The only functional group to suffer a concurrent decrease was turf algae, which decreased from 29 ± 6.4% in 2009 to 15.7 ± 5.4% by 2017 (Fig. 2). FR3 and RT10 represent the two sites with the highest initial coral cover, suggesting that spread of the corallimorph was constrained at sites with more intact benthic communities. Additionally, the concurrent declines in CCA and turf algae associated with increased corallimorph cover suggest that these functional groups were more susceptible to overgrowth.
While the growth of the corallimorph was suppressed at FR3 and RT10, it increased in abundance at the forereef site (FR5) and back-reef site (PSM). No corallimorph was recorded when FR5 was first surveyed in 2009, but cover was 1.5 ± 0.2% by 2010 (Fig. 2c). However, the corallimorph steadily increased through time, reaching a site average of 27.6 ± 8.3% in 2016 (Fig. 2c). Unlike FR3 and RT10, where coral cover was unaffected as cover of the corallimorph increased, hard coral cover at FR5 decreased from 24.2 ± 4.1% in 2009 to 13.4 ± 3.8% in 2017. Turf algae cover also decreased by half during this time period (16.4 ± 2.5% to 8.1 ± 3.1%), suggesting that the expansion of the corallimorph at FR5 was mostly at the expense of hard coral and turf algae. The back-reef site (PSM) was first surveyed in 2010 with 19% ± 6.7% initial corallimorph cover. It increased to 43.3 ± 11.1% in 2017, more than doubling over 7 years and reaching 100% cover in some quadrats (Fig. 2). In contrast to FR5, hard coral stayed relatively consistent (20.1 ± 0.7%) throughout the monitoring period and CCA increased from 9.2 ± 1.9% in 2010 to 27.6 ± 6.5% in 2017. Turf and macroalgae showed the greatest declines over the monitoring period (42.4 ± 7.3 to 6.9 ± 2% and 27.7 ± 6.3 to 11.8 ± 2.4%, respectively), suggesting that the corallimorph primarily overgrew algae at PSM. Overall, the corallimorph most easily overgrew turf and macroalgae across all sites, with a significant decline in coral observed solely at FR5. This, combined with the suppression of corallimorph growth at FR3 and RT10, indicates that sites with minimal disturbance and higher coral cover were more resistant to corallimorph expansion.
These trends are likely due to coral defense mechanisms, including sweeper tentacles that can attack and defend against benthic competitors (Lapid et al. 2004; Chadwick and Morrow 2011), making it more difficult for the corallimorph to expand at sites with higher coral cover (e.g., RT10). Trends in initial invasion support this, with colonization occurring primarily on CCA and turf algae, with a strong avoidance of hard coral substrate (Fig. 4). However, at sites with higher levels of disturbance and lower initial coral cover, the corallimorph overgrew hard coral as well, causing tissue necrosis and, in some cases, death (Williams 1991; Langmead and Chadwick-Furman 1999; Work et al. 2008). In fact, the forereef site with the highest corallimorph cover (FR5) was characterized by the most consistent wave energy (Gove et al. 2015). These direct effects of the corallimorph are further exacerbated at sites with high R. howesii cover like FR5 and PSM by indirect effects. For example, the corallimorphs ability to rapidly colonize available space may result in less available substrate for juvenile coral recruits, thus preventing recovery of hard coral communities at these sites. Continued monitoring of the corallimorph at these sites is important, and population control should be considered as a potential mitigation effort around Palmyra given the competitive abilities of R. howesii.
In contrast to the dynamics observed at FR5 and PSM, the corallimorph population at the Longliner wreck site decreased from 2013 to 2017 (Fig. 6). Prior to the wreck removal in 2013, R. howesii covered an average of 68.5 ± 2.3% of the Longliner monitoring transects. Less than a year after the wreck was removed, the corallimorph decreased to 58.2 ± 2.8% and dropped to only 1.5 ± 0.3% cover at the site by 2017 (Figs. 3, 5a). This startling decline in cover (Figs. 5a–d, 6) was accompanied by an increase in CCA from 8 ± 1.2% in 2013 to 49.3 ± 1.6% in 2017 (Fig. 3). This has important implications for recovery of the benthos because CCA is an active reef-builder and a preferred settlement substrate for juvenile corals (Harrington et al. 2004). Increased abundance of CCA also consolidated and cemented loose rubble that was dislodged during wreck removal, creating further substrate for juvenile corals to settle on, and assisting in the recovery of the reef as corallimorph cover declined (Bosence 1983; Littler and Littler 1984; Price 2010).
Contrasting spatial dynamics at the initial epicenter (Longliner) and a forereef site where initial invasion and subsequent expansion were observed (FR5). Photomosaics of the Longliner plot (a–d) and FR5 e–h from 2012 to 2016 with corallimorph patches shown in white and the remainder of the benthos in black. The number of corallimorph patches from 2012 to 2016 is shown in i. Percent cover of corallimorph (out of 200 m2) is shown from 2012 to 2016 for both sites (j). Change in mean patch size from 2012 to 2016 is shown in k. Letters denote significant changes in patch size from year-to-year with a p < 0.001
Turf and macroalgae at the Longliner site also increased after wreck removal, although less drastically than CCA (from 10.5 ± 1% to 17.7 ± 1.5% for turf and 7.9 ± 1.2% to 24.7 ± 1.1% for macroalgae). Turf, macroalgae, and CCA frequently dominate early successional communities given their ability to rapidly colonize available space (Steneck and Dethier 1994). Both turf and macroalgae can have varying impacts on coral communities through both indirect and direct methods of competition. Both can compete with juvenile and adult corals for space on the benthos via physical (shading, abrasion), biological (microbial-mediated feedbacks), or chemical (allelopathic) mechanisms that can influence competitive dominance (Barott et al. 2009; Barott and Rohwer 2012; Vermeij et al. 2009; Birrell et al. 2008; Box and Mumby 2007; Smith et al. 2006; Rasher et al. 2011). However, given the high biomass of herbivores at Palmyra (Hamilton et al. 2014), the turf and macroalgal assemblages are likely controlled via grazing, allowing for continued settlement and growth of CCA and juvenile corals (Cheal et al. 2010).
To further examine the different trends in corallimorph cover between FR5 and the Longliner site (Fig. 6), the spatial dynamics of R. howesii were investigated using large-scale photomosaic images collected from 2012 to 2016 (Fig. 5). Corallimorph cover in the Longliner photomosaic decreased from 51.9% in 2013 to 5.9% in 2016 (Fig. 5a–d, j) and was accompanied by a significant decrease in mean patch size (Fig. 5k). The number of patches at the site showed an increasing trend until 2015, when a fourfold increase was observed in the course of a year (ESM Table S3). Collectively, these data show that the decrease in percent cover of the corallimorph was directly related to the breakup of its patches and the reduction in the overall patch size. These trends may suggest that density-dependent mortality (either resource limitation or disease) contributed to the decline. In contrast, the percent cover of the corallimorph in the photomosaic at FR5 increased from 3.2% to 19.1% cover from 2012 to 2016. This growth was associated with an increase in the number of patches, and a slight but significant increase in mean patch size (Fig. 5e–k, ESM Table S2). These data suggest that larger patches of R. howesii are better able to hold and subsequently increase territory through lateral expansion via budding and fragmentation. In contrast, smaller patches may be more susceptible to edge effects and more prone to further fragmentation and decline. Further monitoring of the large-scale dynamics of the corallimorph at FR5 may provide continued insight into the spatial trends of corallimorph invasion and its patterns of expansion.
The rapid decline in the corallimorph following the wreck removal strongly suggests that some aspect of the removal contributed to the population breakup of the corallimorph. The physical disturbance associated with the wreck removal may have caused scouring, sedimentation, and physical disruption of the habitat directly adjacent to the former wreck, facilitating breakup of corallimorph patches (Fig. 6). Severe physical disturbances have been shown to disrupt invasion dynamics in other systems, potentially altering the dynamics of the invader through large-scale mortality and facilitating recovery of the reef by opening up substrate for settlement by reef-builders (Nyström and Folke 2001; Nyström et al. 2008; Norström et al. 2009; Mouillot et al. 2013).
Many other factors could have contributed to the initial outbreak of R. howesii on Palmyra’s reefs. For example, Work et al. (2008) suggested that a limiting nutrient (e.g., Fe) may have seeped from the wreck and released the corallimorph from bottom-up control. However, this mechanism does not explain the continued spread of the corallimorph around the island post-wreck removal. Rather, the overall distribution of the corallimorph around the atoll mirrors water flow models (Rogers et al. 2017), suggesting that propagule delivery from the Longliner site may have facilitated the expansion to the southern forereef (Work et al. 2018). Furthermore, Palmyra has had an extensive amount of metal debris on land and in the water since military occupation in the 1940–1950s, making the outbreak at the wreck site curious. Previous studies found that iron content in corallimorph tissue was not significantly higher at the Longliner site when compared to other locations around the atoll (Carter 2014). It is also doubtful that R. howesii was brought in as an introduced species on the shipwreck, as it is native to the central Pacific and there were reports of it around the atoll before the wreck occurred (J. Maragos, pers com.). In light of this, it seems likely that an invasive strain may have been brought in on the Hui Feng or that the corallimorph was native to the atoll, but that the disturbance associated with the shipwreck facilitated its growth and expansion and the subsequent disturbance from the ship removal contributed to its current decline.
Although this study focused on the dynamics at Palmyra Atoll, R. howesii has been observed expanding at other sites around the Pacific such as Fagatele Bay National Marine Sanctuary, Helen Reef, and Ulithi Atoll in Micronesia (unpublished data, Crane et al. 2016). Although the decline in corallimorph cover associated with the shipwreck removal is encouraging, the continued growth of the corallimorph at sites like FR5 and PSM (Figs. 2, 5, 6) suggests that the outbreak may continue at more distant sites. However, sites such as RT10 and FR3 have shown decreases in their corallimorph population, suggesting that high coral cover may help prevent the rapid expansion of the corallimorph. These data suggest that sites with lower coral cover and initial stages of corallimorph invasion may benefit from removal and restoration efforts the most. The strong negative impacts of R. howesii merit continued long-term monitoring and conservation efforts to document and mitigate the continued growth and impact on the benthic communities of Palmyra Atoll.
References
Barott KL, Smith JE, Dinsdale E, Hatay M, Sandin SA, Rohwer RL (2009) Hyperspectral and physiological analyses of coral-algal interactions. PLoS ONE 4(11):e8043
Barott KL, Rohwer FL (2012) Unseen players shape benthic competition on coral reefs. Trends in Microbiology 20(12):621–628
Barott KL, Williams GJ, Vermeij MJA, Harris J, Smith JE, Rohwer FL, Sandin SA (2012) Natural history of coral-algae competition across a gradient of human activity in the Line Islands. Mar Ecol Prog Ser 460:1–12
Birrell CL, McCook LJ, Willis BL, Harrington L (2008) Chemical effects of macroalgae on larval settlement of the broadcast spawning coral Acropora millepora. Mar Ecol Prog Ser 362:129–137
Bosence DWJ (1983) Coralline algal reef frameworks. J Geol Soc London 140:365–376
Boudouresque CF, Meinesz A, Ribera MA, Ballesteros E (1995) Spread of the green alga Caulerpa taxifolia (Caulerpales, Chlorophyta) in the Mediterranean: possible consequences of a major ecological event. Sci Mar(BARC) 59:21–29
Box SJ, Mumby PJ (2007) Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar Ecol Prog Ser 342:139–149
Carter AL (2014) Invasion Mechanisms of the corallimorph, Rhodactis howesii, at Palmyra Atoll. UC San Diego
Chadwick NE, Morrow KM (2011) Competition among sessile organisms on coral reefs. Coral Reefs: an ecosystem in transition. Springer, pp 347–371
Chadwick-Furman NE, Spiegel M (2000) Abundance and clonal replication in the tropical corallimorpharian Rhodactis rhodostoma. Invertebr Biol 119:351–360
Chadwick NE, Adams C (1991) Locomotion, asexual reproduction, and killing of corals by the corallimorpharian Corynactis californica. Hydrobiologia 216–217:263–269
Cheal AJ, MacNeil MA, Cripps E, Emslie MJ, Jonker M, Schaffelke B, Sweatman H (2010) Coral-macroalgal phase shifts or reef resilience: Links with diversity and functional roles of herbivorous fishes on the Great Barrier Reef. Coral Reefs 29:1005–1015
Conklin EJ, Smith JE (2005) Abundance and spread of the invasive red algae, Kappaphycus spp., in Kane’ohe Bay, Hawai’I and an experimental assessment of management options. Biol Inva 7:1029–1039
Côté IM, Gill JA, Gardner TA, Watkinson AR (2005) Measuring coral reef decline through meta-analyses. Philos Trans R Soc Lond B Biol Sci 360:385–395
Crane NL, Paddack MJ, Nelson PA, Abelson A, John Rulmal J, Bernardi G (2016) Corallimorph and Montipora Reefs in Ulithi Atoll, Micronesia: documenting unusual reefs. Journal of the Ocean Science Foundation 21:10–17
Done TJ (1992) Phase-Shifts in Coral-Reef Communities and Their Ecological Significance. Hydrobiologia 247:121–132
Edwards CB, Eynaud Y, Williams GJ, Pedersen NE, Zgliczynski BJ, Gleason ACR, Smith JE, Sandin SA (2017) Large-area imaging reveals biologically driven non-random spatial patterns of corals at a remote reef. Coral Reefs. https://doi.org/10.1007/s00338-017-1624-3
Edwards CB, Friedlander AM, Green AL, Hardt MJ, Sala E, Sweatman HP, Williams ID, Zgliczynski B, Sandin SA, Smith JE (2014) Global assessment of the status of coral reef herbivorous fishes : evidence for fishing effects Global assessment of the status of coral reef herbivorous fishes : evidence for fishing effects. Proc R Soc B Biol Sci 280:7–11
Elliott J, Patterson M, Summers N, Miternique C, Montocchio E, Vitry E (2016) How does the proliferation of the coral-killing sponge Terpios hoshinota affect benthic community structure on coral reefs? Coral Reefs 35:1083–1095
Elton CS (1958) The ecology of invasion by plants and animals. Methuen, London, p 181
Gove JM, Williams GJ, McManus MA, Clark SJ, Ehses JS, Wedding LM (2015) Coral reef benthic regimes exhibit non-linear threshold responses to natural physical drivers. Mar Ecol Prog Ser 522:33–48
Gracias N, Santos-Victor J (2000) Underwater video mosaics as visual navigation maps. Comput Vis Image Underst 79:66–91
Gracias N, Santos-Victor J (2001) Underwater mosaicing and trajectory reconstruction using global alignment. 4:2557–2563
Hamilton SL, Smith JE, Price NN, Sandin SA (2014) Quantifying patterns of fish herbivory on Palmyra Atoll (USA), an uninhabited predator-dominated central Pacific coral reef. Mar Ecol Prog Ser 501:141–155
Harrington L, Fabricius K, De’Ath G, Negri A (2004) Recognition and selection of settlement substrata determine post-settlement survival in corals. Ecology 85:3428–3437
Hughes TP (1994) Catastrophes, Phase Shifts, and large-scale degradation of a Caribbean coral reef. Science 265:1547–1551
Kelly LW, Barott KL, Dinsdale E, Friedlander AM, Nosrat B, Obura D, Sala E, Sandin SA, Smith JE, Vermeij MJA, Williams GJ, Willner D, Rohwer F (2011) Black reefs: iron-induced phase shifts on coral reefs. ISME J 6:638–649
Kimmerer WJ, Gartside E, Orsi JJ (1994) Predation by an introduced clam as the likely cause of substantial declines in zooplankton of San Francisco Bay. Mar Ecol Prog Ser 113:81–93
Kuguru BL, Mgaya YD, Öhman MC, Wagner GM (2004) The reef environment and competitive success in the Corallimorpharia. Mar Biol 145:875–884
Kuguru B, Achituv Y, Gruber DF, Tchernov D (2010) Photoacclimation mechanisms of corallimorpharians on coral reefs: Photosynthetic parameters of zooxanthellae and host cellular responses to variation in irradiance. J Exp Mar Bio Ecol 394:53–62
Kuguru B, Winters G, Beer S, Santos SR, Chadwick NE (2007) Adaptation strategies of the corallimorpharian Rhodactis rhodostoma to irradiance and temperature. Mar Biol 151:1287–1298
Langmead O, Chadwick-Furman NE (1999) Marginal tentacles of the corallimorpharian Rhodactis rhodostoma. 2. Induced development and long-term effects on coral competitors. Mar Biol 134:491–500
Lapid ED, Wielgus J, Chadwick-Furman NE (2004) Sweeper tentacles of the brain coral Platygyra daedalea: Induced development and effects on competitors. Mar Ecol Prog Ser 282:161–171
Lirman D, Gracias NR, Gintert BE, Gleason ACR, Reid RP, Negahdaripour S, Kramer P (2007) Development and application of a video-mosaic survey technology to document the status of coral reef communities. Environ Monit Assess 125:59–73
Littler MM, Littler DS (1984) Models of tropical reef biogenesis: the contribution of algae. Prog Phycol Res 3:323–364
McCook LJ (1999) Macroalgae, nutrients and phase shifts on coral reefs: Scientific issues and management consequences for the Great Barrier Reef. Coral Reefs 18:357–367
Mouillot D, Graham NAJ, Villeger S, Mason NWH, Bellwood DR (2013) A functional approach reveals community responses to disturbances. Trends in Ecol & Evo 28(3):167–177
Muhando CA, Kuguru BL, Wagner GM, Mbije NE, Ohman MC (2002) Environmental effects on the distribution of corallimorpharians in Tanzania. Ambio 31:558–561
Nalepa TF, Schloesser DW (1992) Zebra mussels biology, impacts, and control. CRC Press
Norström AV, Nyström M, Lokrantz J, Folke C (2009) Alternative states on coral reefs: Beyond coral-macroalgal phase shifts. Mar Ecol Prog Ser 376:293–306
Nyström M, Graham NAJ, Lokrantz J, Norström AV (2008) Capturing the cornerstones of coral reef resilience: Linking theory to practice. Coral Reefs 27:795–809
Nyström M, Folke C (2001) Spatial resilience of coral reefs. Ecosystems 4:406–417
Perry CT, Spencer T, Kench PS (2008) Carbonate budgets and reef production states: A geomorphic perspective on the ecological phase-shift concept. Coral Reefs 27:853–866
Price N (2010) Habitat selection, facilitation, and biotic settlement cues affect distribution and performance of coral recruits in French Polynesia. Oecologia 163:747–758
Pringle RM (2011) Nile perch. Univ of California Press, Encyclopedia of biological invasions, p 484
Rasher DB, Stout EP, Engel S, Kubanek J, Hay ME (2011) Macroalgal terpenes function as allelopathic agents against reef corals. Proc Natl Acad Sci 108:17726–17731
Rogers JS, Monismith SG, Fringer OB, Koweek DA, Dunbar RB (2017) A coupled wave-hydrodynamic model of an atoll with high friction: mechanisms for flow, connectivity, and ecological implications. Ocean Model 110:66–82
Sandin SA, Smith JE, DeMartini EE, Dinsdale EA, Donner SD, Friedlander AM, Konotchick T, Malay M, Maragos JE, Obura D, Pantos O, Paulay G, Richie M, Rohwer F, Schroeder RE, Walsh S, Jackson JBC, Knowlton N, Sala E (2008) Baselines and degradation of coral reefs in the Northern Line Islands. PLoS One 3:e1548
Simberloff D, Rejmánek M (2011) Encyclopedia of biological invasions. Univ of California Press, p. 792
Smith JE, Brainard R, Carter A, Dugas S, Edwards C, Harris J, Lewis L, Obura D, Rohwer F, Sala E, Vroom PS, Sandin S (2016) Re-evaluating the health of coral reef communities: baselines and evidence for human impacts across the central Pacific. Proc R Soc B Biol Sci 283:20151985
Smith JE, Shaw M, Edwards RA, Obura D, Pantos O, Sala E, Sandin SA, Smriga S, Hatay M, Rohwer FL (2006) Indirect effects of algae on coral: algae-mediated, microbe-induced coral mortality. Ecology letters 9(7):835–845
Steneck RS, Dethier MN (1994) A Functional Group Approach to the Structure of Algal-Dominated Communities. 69:476–498
Takeshita Y, McGillis W, Briggs EM, Carter AL, Donham EM, Martz TR, Price NN, Smith JE (2016) Assessment of netcommunity production and calcification of a coral reef using a boundary layer approach. JGR Oceans. https://doi.org/10.1002/2016JC011886
Vermeij MJA, Smith JE, Smith CM, Vega Thurber R, Sandin SA (2009) Survival and settlement success of coral planulae: Independent and synergistic effects of macroalgae and microbes. Oecologia 159:325–336
Warren CR (2007) Perspectives on the `alien’ versus `native’ species debate: a critique of concepts, language and practice. Prog Hum Geogr 31:427–446
Wiles GJ, Bart J, Beck RE, Aguon CF (2003) Impacts of the Brown Tree Snake: Patterns of Decline and Species Persistence in Guam’s Avifauna. Conserv Biol 17:1350–1360
Williams RB (1991) Acrorhagi, catch tentacles and sweeper tentacles: a synopsis of “aggression” of actiniarian and scleractinian Cnidaria. Hydrobiologia 216–217:539–545
Work TM, Aeby GS, Neal BP, Price NN, Conklin E, Pollock A (2018) Managing an invasive corallimorph at Palmyra Atoll National Wildlife Refuge, Line Islands, Central Pacific. Biol Invasions 20:2197–2208
Work TM, Aeby GS, Maragos JE (2008) Phase shift from a coral to a corallimorph-dominated reef associated with a shipwreck on Palmyra atoll. PLoS One 3:1–5
Acknowledgements
Funding was provided by the US Fish and Wildlife Service through grant USDI-FWS Proposal 20120629, from the Nature Conservancy’s supported monitoring trip to Palmyra in 2010, from NSF award #OCE1316047, from the Gordon and Betty Moore Foundation, and from generous donations from the Scripps family and the Bohn family. We thank the staff of The Nature Conservancy, the US Fish and Wildlife Service, and the Palmyra Atoll Research Consortium for their logistical support. Access to the refuge and field work was conducted under USFWS special use permit #12533-16008 and #12533-16009. This publication is PARC contribution #PARC-140. We would also like to thank Dr. Jim Maragos for early discussions regarding concern over the corallimorph invasion on Palmyra. Further, we thank Gareth J. Williams, Brian Zgliczynski, Zach Caldwell, Amanda Pollock, Kydd Pollock, Nichole Price, Eric Conklin, and Jesse Tootell for their assistance with field work, as well as Amelia Scharp and Carolina Carpenter for analysis of images during post-processing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topic Editor Morgan S. Pratchett
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carter, A.L., Edwards, C.B., Fox, M.D. et al. Changes in benthic community composition associated with the outbreak of the corallimorph, Rhodactis howesii, at Palmyra Atoll. Coral Reefs 38, 1267–1279 (2019). https://doi.org/10.1007/s00338-019-01841-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-019-01841-5