Abstract
Scleractinian corals typically reproduce sexually as either gonochoric (separate male and female) or hermaphroditic (producing both eggs and sperm) colonies. The Caribbean pillar coral Dendrogyra cylindrus has been classified as gonochoric, but multi-year spawning observations at a Florida Keys site revealed incidences of hermaphroditism. Separate clonal colonies (ramets) of a single genet released either male or female gametes. Furthermore, 22% of observed ramets produced both eggs and sperm within different regions of a single colony. Over multiple years, one ramet switched from female to hermaphrodite, one from male to hermaphrodite, and one from hermaphrodite to male. Proposed evolutionary mechanisms include size- or age-based energy allocation, environmental energy allocation, or chemically induced change in a single-sex region. Because of the low population density of D. cylindrus in the Florida Keys, sexual partners are scarce, and hermaphroditism may be a strategy to yield higher rates of successful sexual reproduction. The findings also have implications for future restoration efforts aiming to strategically outplant individuals to maximize in situ fertilization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reproduction of scleractinian coral colonies can occur asexually (primarily through fragmentation) as well as sexually through egg–sperm fertilization (Harrison and Wallace 1990). Though fragmentation can provide frequent and rapid opportunity for new colony growth (Highsmith 1982), sexual reproduction provides important opportunities for long-range dispersal of larvae as well as potential for genetic recombination. Sexual reproduction is usually accomplished by the release of gametes (eggs and/or sperm) into the water column at a synchronized time based on solar (van Woesik et al. 2006), wind (van Woesik 2010), lunar (Richmond and Jokiel 1984), and diel (Brady et al. 2009) cycles, as well as genetic (Levitan et al. 2011) and possibly chemical cues. For many species, this process occurs in the hours after sunset, in the days following the full moon, of the warm water months (Szmant 1986).
Reproductive strategies of coral species are generally classed as gonochoric or hermaphroditic. In gonochoric species, a colony produces either sperm or eggs that, in broadcast spawners, are released in synchrony into the water column where fertilization occurs. In hermaphroditic species, both eggs and sperm are released by a colony, usually in buoyant bundles that break apart upon reaching the surface. Though self-fertilization can occur (Heyward and Babcock 1986), many coral species are self-incompatible and require interaction with gametes from a different genet for fertilization to be successful. Of 393 coral species examined by Baird et al. (2009), 31% (122 species) were gonochoric, though the proportion was over 50% in the Western Atlantic. There are, however, exceptions to this binary system. At least 27 coral species have mixed reproductive styles (see reviews in Harrison 2011; Guest et al. 2012; additional species described in Rinkevich and Loya 1987; Hoke 2007; Loya and Sakai 2008). These include species in which sperm-producing individuals also produce sterile eggs, species that are sequentially hermaphroditic (including bidirectional switching), and species in which some colonies exhibit simultaneous hermaphroditic qualities.
The pillar coral Dendrogyra cylindrus is a scleractinian coral found in mostly low abundance throughout the Greater Caribbean. It is a member of the family Meandrinidae, but is the only species within its genus. Its 2008 IUCN (International Union for Conservation of Nature) “vulnerable” listing (Aronson et al. 2008) and 2014 “threatened” listing under the Endangered Species Act (NOAA 2014) prompted increased efforts to study its distribution, life history, and reproductive potential. Histological samples from Puerto Rico identified D. cylindrus as a gonochoric spawner with one probable spawning event per year (Szmant 1986). Gonochoric spawning was confirmed nearly 30 yr later with both males and females observed in the Florida Keys (Neely et al. 2013) and Curacao (Marhaver et al. 2015). Subsequent years of observation and genetic work in the Florida Keys suggest that D. cylindrus is not strictly gonochoric, but can be hermaphroditic where ramets (colonies) of a single genet can be of opposite sex and where a single ramet (colony) can release both eggs and sperm.
Methods
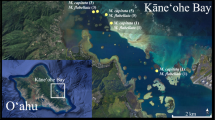
Dendrogyra cylindrus colonies were observed during three spawning seasons (2012–2014) at Pillar Coral Forest, a site 7.5 km offshore of Key Largo in the Upper Florida Keys (Fig. 1). The site lies in 3–5 ms of water and was dominated by D. cylindrus. Within the site, 165 distinct colonies were identified over a 20 m by 80 m geographic area. A colony was defined as one or more pillars with continuous live tissue or distinct connective skeleton. All colonies were mapped, measured for diameter and height using a rigid 0.5-m stick, visually assessed for percentage of live coral coverage, and photographed for repeatable identification; a subset of colonies was tagged to further aid in identification. Differences in ramet size and percent live cover between genders were analyzed using one-way ANOVAs (α = 0.05, Shapiro–Wilk normality and Brown–Forsythe equal variance test conditions satisfied). Spatial cluster analysis on gender was conducted using Ripley’s K-function with ten distance bands and a confidence interval derived from 99 permutations.
Tissue was sampled from 21 colonies haphazardly selected across the site using a combination of methods: either clipping small pieces of tissue or conducting a biopsy of two or three polyps using the syringe technique (Kemp et al. 2008). All samples were preserved in 95% non-denatured ethanol, and DNA was extracted using the QIAGEN DNeasy Blood & Tissue Kit. Eleven species-specific microsatellite markers developed by the Baums Laboratory were amplified with high power to distinguish multi-locus genotypes following protocols developed by Chan et al. (2018). In brief, PCR products were visualized using an ABI3730 (Applied Biosystems) automated DNA sequencer with the Gene Scan 500-Liz internal size standard. GeneMapper Software 5.0 (Applied Biosystems) was used to analyze electropherogram peaks and designate allele sizes. GenAlEx version 6.1 (Peakall and Smouse 2006) was used to construct multi-locus genotypes. If two samples had the same alleles at all 11 markers, then they were considered ramets of the same genet.
Spawning observations were conducted at the site between 2100 and 2200 h following the August full moons of 2012, 2013, and 2014 (Fig. 2). In 2012 and 2013, three to four divers swam haphazardly around the site using weighted glow sticks to mark and assign genders to colonies based on the release of sperm and/or eggs. In 2014, the majority of these previously marked colonies as well as additional adjacent colonies were targeted for observation by four divers. The number of colonies monitored per diver varied based on available personnel, but never exceeded eight closely spaced colonies.
Nights of observation and spawning at the site. Circles and times represent the August full moon in local time. Crosses represent nights during which colonies were observed but no spawning occurred. Clouds represent dribbles of gametes (small clouds) and gamete releases exceeding 50% of live tissue area (large clouds). Dates without clouds or crosses were not observed
Results and discussion
Over 3 yr of spawning observations at this Upper Keys site, divers documented gamete release by 18 permanently tagged D. cylindrus colonies (Table 1). Ten of these were observed spawning in two separate years, and one was observed spawning all three consecutive years. During 2012, six tagged colonies were observed releasing eggs and two colonies were documented releasing sperm. In 2013, six colonies were observed releasing eggs only, one colony released sperm only, and two colonies released eggs from one pillar and sperm from another pillar on the same colony. In 2014, observed colony spawning included five females, five males, and three hermaphrodites that released sperm and eggs from different pillars of the same colony. In addition, the 2014 observations allowed for comparisons with previously observed spawning colonies to show: (1) one colony that released only eggs in 2012 released both eggs and sperm in 2014, (2) one colony that released only sperm in 2013 released both eggs and sperm in 2014, and (3) one colony that released both eggs and sperm in 2013 released only sperm in 2014 (Table 1).
Microsatellite analysis of 21 colonies sampled across this site revealed that all were genetically identical, strongly suggesting that the site as a whole is one genet. Of the 21 samples, 14 were from colonies that were observed to spawn (Table 1).
A single genet at this site was thus composed of male, female, and hermaphroditic ramets. Further, ramets within this genet spawned as both males and females over the course of multiple years (sequential hermaphroditism) as well as within the same night (simultaneous hermaphroditism). Incidence of hermaphroditic colonies either within a single spawning event or over multiple years of spawning observation at this site was 22% (4 of 18 colonies).
Ramet gender was not significantly related to percent live cover (F2,15 = 1.22. p = 0.32). On average, hermaphrodites had larger maximum base diameters than males or females, but this trend was not significant (Fig. 3; F2,15 = 2.65; p = 0.10). Ramet gender was not clumped spatially within the site (Ripley’s K-function analyses across 10 distance bands were all within the 99% confidence intervals). Rather, males, females, and hermaphrodites were interspersed throughout the area of observation (Fig. 4).
Spatial plot of all colonies at the primary study site (Pillar Coral Forest—Upper Florida Keys). Black colonies were not actively observed spawning. Pink colonies were only observed releasing eggs, blue colonies were only observed releasing sperm, and yellow colonies were observed releasing both eggs and sperm over the 3 yr of spawning observations (2012–2014)
Though coral species are generally defined as being either gonochoric or hermaphroditic, there are exceptions to this rule. The incidence of hermaphroditism in gonochoric species has been documented in 12 scleractinian coral families, but to date only one other study has documented this phenomenon within the family Meandrinidae (Hoke 2007 with Dichocoenia stokesii).
Studies documenting the proportion of hermaphroditic colonies within a gonochoric coral species or population generally note a low incidence, often specific to one site or subpopulation as opposed to being common throughout the range of the species (review in Guest et al. 2012). Based on other D. cylindrus spawning events on the Florida Reef Tract, this appears to be the case for D. cylindrus as well. Observations at three other clonal sites in the Florida Keys between 2014 and 2016 found that ramets within a genet released only single-sex gametes (Fig. 1). This included nine colonies at a single site in Dry Tortugas National Park (2013 and 2014: only males observed), approximately 50 colonies at a Middle Keys site (2015 and 2016: only males observed), and approximately 100 colonies at an additional Upper Keys site (2016: only females observed). Several mechanisms are proposed for this selective occurrence of hermaphroditism in D. cylindrus.
Sequential hermaphroditism is the process of individuals switching gender within their lifetime. This is often explained by age-specific fecundity in which certain life stages favor increased reproductive success of either males or females (Ghiselin 1969; Warner 1975). The investment in time and energy reserves required for egg production within corals outweighs that for sperm, suggesting that older, larger colonies would benefit by producing eggs while younger, smaller individuals could still participate in genetic recombination by producing sperm. This has been demonstrated in at least two coral species in the Red Sea. Small Fungia scutaria colonies were predominantly male, while large ones were female (Kramarsky-Winter and Loya 1998), and Stylophora pistillata colonies were exclusively male at first reproduction, but older colonies showed increased proportions of hermaphrodites (Rinkevich and Loya, 1987). In S. pistillata, sequential hermaphroditism was bidirectional; older dying colonies reverted to sperm production. However, our study cannot confirm that age or size necessarily governs the sex of D. cylindrus. Age is not easily quantifiable as all our observed colonies are derived from asexual reproduction with no obvious “parent” colony. In regards to size, smaller colonies trended toward being female, which would counter the traditional idea of sequential hermaphrodism as a form of requiring large energy reserves for egg production, but this trend was not significant. Additionally, the other observed sites with similar combinations of large and small ramets showed only single-sex gamete release.
Another hypothesis suggests that energy reserves and subsequent gamete production are not driven by colony age/size, but by colony health and stress. Porites porites has been found to be male-dominated or hermaphroditic in polluted water, but with more balanced male-to-female ratios in clear water (Tomascik and Sander 1987). As with size or age, energy allocation toward survival rather than reproduction could be affected by stress or health, potentially leaving colonies to preferentially produce sperm in high-stress years or environments while allocating reserves to eggs in more conducive environments. Our data are not sufficient to address this hypothesis; however, the site where hermaphroditism was observed suggests that stress would have to vary on spatial scales < 10 m. Based on high mortality from disease and a hyperthermal bleaching event in 2014, it is likely that the observed D. cylindrus colonies’ energy reserves depleted over the 3 yr of coral spawning observations. Two of the 18 observed colonies shifted toward sperm production over the 3-yr period (one female to hermaphrodite and one hermaphrodite to male), but one that released only sperm in 2013 also released eggs in 2014. Regardless, our small sample size and lack of continuity in individual colony data cannot confirm that these shifts are related to colony health. A disease outbreak on the Florida Reef Tract beginning in 2014 (Precht et al. 2016) led to complete mortality of the study site/genotype, precluding further spawning observations at this site. Nevertheless, the possibility that the species is capable of shifting its sexual strategy to accommodate depleted energy reserves should be considered.
A third possibility is that individuals may switch sexes in an environment that is dominated by one gender. Spawning in corals may be influenced by conspecific chemical cues as well as environmental ones (Levitan et al. 2011). In the absence of cues from the opposite sex, particularly over decadal or longer time scales, colonies may somehow trigger a change to the other sex to allow for crossing with individuals previously of the same gender, or even for “self-fertilization” within the genet. Laboratory fertilization of eggs and sperm collected from clonal ramets at this study site did exhibit low rates of fertilization and larval development (but not settlement), suggesting that self-fertilization may be possible. However, evidence from the other observed sites suggests that if sex switching is possible, the process is not widespread within the region.
The ability of an individual genet and/or colony to release both eggs and sperm has implications for the reproductive capability of D. cylindrus. By retaining the capability to produce eggs, sperm, or both as large mature colonies, the species may increase its chances of producing gametes that can encounter conspecifics for successful cross-fertilization. However, the opportunities for this in the Florida Reef Tract are limited. Sites with multiple colonies are usually monoclonal, and the genetically distinct individuals are often separated by several kilometers, further decreasing the likelihood of successful fertilization and recruitment (Chan et al. 2018). Even with hermaphroditic reproduction, fertilization rates are likely very low or nonexistent, rendering this species reproductively extinct on the Florida Reef Tract. This is further evidenced by the lack of D. cylindrus juveniles seen on the Florida Reef Tract (Miller 2000–2011).
Nevertheless, this observation of hermaphroditic individuals and the potential for differential gamete release is important for consideration in restoration projects, particularly those that consider relocation or outplanting of individuals with a goal of increasing in situ fertilization potential. Examining patterns or changes in gamete production over longer time frames and in response to different energy reserves may help resolve the varying mechanisms and better inform these management decisions.
References
Aronson R, Bruckner A, Moore J, Precht B, Weil E (2008) Dendrogyra cylindrus. The IUCN Red List of Threatened Species 2008:e.T133124A3582471. http://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T133124A3582471.en
Baird AH, Guest JR, Willis BL (2009) Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu Rev Ecol Evol Syst 40:551–571
Brady AK, Hilton JD, Vize PD (2009) Coral spawn timing is a direct response to solar light cycles and is not an entrained circadian response. Coral Reefs 28:677–680
Chan AN, Lewis C, Neely KL, Baums IB (2018) Fallen pillars: the past, present, and future population dynamics of a rare, specialist coral-algal symbiosis. bioRxiv. https://doi.org/10.1101/365650
Ghiselin MT (1969) The evolution of hermaphroditism among animals. Q Rev Biol 44:189–208
Guest JR, Baird AH, Goh BPL, Chou LM (2012) Sexual systems in scleractinian corals: an unusual pattern in the reef-building species Diploastrea heliopora. Coral Reefs 31:705–713
Harrison PL (2011) Sexual reproduction of scleractinian corals. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Netherlands, pp 59–85
Harrison PL, Wallace CC (1990) A review of reproduction, larval dispersal and settlement of scleractinian corals. In: Dubinsky Z (ed) Ecosystems of the world 25: Coral reefs. Elsevier, Amsterdam, pp 133–196
Heyward AJ, Babcock RC (1986) Self- and cross-fertilization in scleractinian corals. Mar Biol 90:191–195
Highsmith RC (1982) Reproduction by fragmentation in corals. Mar Ecol Prog Ser 7:207–226
Hoke SM (2007) Gametogenesis and spawning of the elliptical star coral, Dichocoenia stokesi (Cnidaria: Scleractinia) in Southeast Florida. Master’s thesis, Nova Southeastern University
Kemp D, Fitt W, Schmidt G (2008) A microsampling method for genotyping coral symbionts. Coral Reefs 27:289–293
Kramarsky-Winter E, Loya Y (1998) Reproductive strategies of two fungiid corals from the northern Red Sea: environmental constraints? Mar Ecol Prog Ser 174:175–182
Levitan DR, Fogarty ND, Jara J, Lotterhos KE, Knowlton N (2011) Genetic, spatial, and temporal components of precise spawning synchrony in reef building corals of the Montastraea annularis species complex. Evolution 65:1254–1270
Loya Y, Sakai K (2008) Bidirectional sex change in mushroom stony corals. Proc R Soc Lond B Biol Sci 275:2335–2343
Marhaver KL, Vermeij MJA, Medina MM (2015) Reproductive natural history and successful juvenile propagation of the threatened Caribbean Pillar Coral Dendrogyra cylindrus. BMC Ecol 15:9
Miller SM (ed) (2000-2011) Quick Look Reports and Data Summaries. CMS/UINCW, Key Largo, FL. http://people.uncw.edu/millers/CoralReef_QuickLooks.htm
Neely KN, Lunz KS, Macaulay KA (2013) Simultaneous gonochoric spawning of Dendrogyra cylindrus. Coral Reefs 32:813
NOAA (2014) Endangered and Threatened Wildlife and Plants: Final Listing Determinations on Proposal To List 66 Reef-Building Coral Species and To Reclassify Elkhorn and Staghorn Corals. US Federal Registry 79:53851–54123
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Precht WE, Gintert BE, Robbart ML, Fura R, van Woesik R (2016) Unprecedented Disease-Related Coral Mortality in Southeastern Florida. Sci Rep 6:31374
Richmond RH, Jokiel PL (1984) Lunar periodicity in larva release in the reef coral Pocillopora damicornis at Enewetak and Hawaii. Bull Mar Sci 34:280–287
Rinkevich B, Loya Y (1987) Variability in the pattern of sexual reproduction of the coral Stylophora pistillata at Eilat, Red Sea: A long term study. Biol Bull 173:335–344
Szmant AM (1986) Reproductive ecology of Caribbean reef corals. Coral Reefs 5:43–54
Tomascik T, Sander F (1987) Effects of eutrophication on reef-building corals III. Reproduction of the reef-building coral Porites porites. Mar Biol 94:77–94
van Woesik R (2010) Calm before the spawn: global coral spawning patterns are explained by regional wind fields. Proc R Soc Lond B Biol Sci 277:715–722
van Woesik R, Lacharmoise F, Köksal S (2006) Annual cycles of solar insolation predict spawning times of Caribbean corals. Ecol Lett 9:390–398
Warner RR (1975) The adaptive significance of sequential hermaphroditism in animals. Am Nat 109:61–82
Acknowledgements
This research was enabled by NOAA Award NA 10NMF4720029, US Fish and Wildlife Service grants program (FA F13AF01085), and Florida’s Wildlife Legacy Initiative (State Wildlife Grants CFDA No. 15.634) to the Florida Fish and Wildlife Conservation Commission and a Florida Fish and Wildlife Conservation Commission subcontract (SW13059) to Pennsylvania State University. It was conducted under permit FKNMS-2013-085-A1 from the Florida Keys National Marine Sanctuary. We are grateful to staff and volunteers from the Florida Aquarium, Keys Marine Laboratory, Florida International University, Florida Fish and Wildlife Research Institute’s South Florida Research Lab, SUNY at Buffalo, and Dry Tortugas National Park for observations at other locations. Thanks also to two external reviewers for comments that improved this manuscript. Chan was supported by the National Science Foundation (NSF) Graduate Research Fellowship Program under Grant No. DGE1255832. The conclusions drawn in this work are those of the authors and do not necessarily reflect the views of the NSF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Topic Editor Anastazia Banaszak
Rights and permissions
About this article
Cite this article
Neely, K.L., Lewis, C., Chan, A.N. et al. Hermaphroditic spawning by the gonochoric pillar coral Dendrogyra cylindrus. Coral Reefs 37, 1087–1092 (2018). https://doi.org/10.1007/s00338-018-1730-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-018-1730-x