Abstract
Spatial heterogeneity plays an important role in consumer–resource interactions. It arises from variability in the underlying distribution of the resource and/or the consumer, as well as the habitat in which the consumer–resource interaction occurs. In some cases, the resource is the habitat, especially when the habitat is biogenic (e.g., kelp, corals, seagrasses). In these systems, the resulting dynamics can be particularly rich because the consumer–resource interactions are coupled with changes in the habitat (i.e., resource) that are due to the consumer–resource interaction. In Moorea, French Polynesia, two corallivorous snails, Coralliophila violacea and Drupella cornus, feed and live on massive Porites corals. Here, I (1) document the spatial patterns of the snails among sites, within sites, and on corals; (2) examine the drivers of small-scale aggregations by testing the attraction of the snails to chemical cues coming from conspecifics and corals; (3) test the effects of aggregations of snails on coral growth by manipulating snail density. The distributions of both snails were highly heterogeneous among sites across the island, and both species were spatially aggregated both among and within corals. The source of chemical attraction that caused the small-scale clustering differed between the two snails. D. cornus was attracted to conspecifics and to corals damaged by conspecifics, whereas C. violacea was attracted to damaged corals (regardless of the cause). Increasing snail density caused a linear decline in coral growth that was similar for the two snail species. The combination of the clustered spatial pattern of both snail species and their negative effects on coral growth could lead to important feedbacks in which high densities of snails reduce coral cover in localized areas and create spatial dynamics that affect the spatial distributions of both corals and snails across the reef.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spatial distribution of organisms over a landscape is often highly variable. In some cases, these spatial distributions arise from underlying variation in the habitat (e.g., vegetation distributions are linked to soil type and available nutrients, John et al. 2007) or predators (e.g., African ungulates occupy areas rarely used by predators, Thaker et al. 2011). In other cases, the heterogeneity results from intrinsic movement patterns (e.g., plants with lower seed dispersal distances exhibit more clustering than plants that disperse over greater distances, Seidler and Plotkin 2006). Finally, the spatial distributions also can result from underlying heterogeneity in the physical characteristics of the environment that define the habitat (e.g., pools and riffles in streams). In many systems, the habitat is dynamic and will change in response to changing environmental conditions. Changes in the habitat will often influence the distribution of the occupants as well. While a static habitat might support a fairly consistent spatial distribution of organisms, organisms that use a dynamic habitat will be constantly altering their distribution as the habitat changes.
Many habitats are comprised of biogenic structures (e.g., kelp, corals, seagrasses, and trees). In these systems, some of the organisms that occupy the habitat are also consumers of it. Thus, the habitat and resource are the same, and the dynamics of the consumer–resource interaction become intrinsically linked to the spatial and temporal dynamics of the habitat within which the consumer and resources interact. When the occupant is a consumer of the habitat, habitat loss is not solely due to stochastic events and environmental factors, but can also occur due to the activity of the consumer, particularly in sites with high densities of consumers.
One system in which these coupled dynamics of habitat and consumers may be particularly important is coral reefs. Corals are a biogenic habitat that hosts a variety of symbionts whose effects potentially drive coral dynamics. Some of these symbionts have positive effects on the coral. For example, Trapezia crabs protect corals from predators (Glynn 1980; Pratchett et al. 2000; Pratchett 2001; McKeon et al. 2012; McKeon and Moore 2014) and other stressors (Stewart et al. 2006; Stier et al. 2010, 2012). Other symbionts negatively affect corals. For example, many organisms that use coral as habitat are corallivores and consume living coral tissue (Rotjan and Lewis 2008).
Corallivory is an important driver of overall reef health and affects coral mortality, disease, and recruitment. Outbreaks of corallivores such as the seastar, Acanthaster planci, and the gastropod, Drupella cornus, can cause widespread mortality of corals. For example, A. planci reduced coral cover by 90% in a 2.5-yr period in Guam (Chesher 1969), and D. cornus reduced coral cover by 75% on areas of backreef at Nigaloo Reef in Western Australia (Turner 1994b). Additionally, diseases can be transmitted by corallivores (e.g., white band is transmitted by Coralliophila abbreviata, Williams and Miller 2005), and corallivores prevent recovery after bleaching (Rotjan et al. 2006; Bruckner et al. 2017). Finally, corallivory affects community composition through processes such as the preferential predation of newly settled (Penin et al. 2010) and juvenile (Lenihan et al. 2011) corals. Because corals provide food and habitat for many corallivores, corals and their symbionts provide the opportunity to investigate feedbacks between consumers and their resource/habitat and the resulting effects on spatial patterns and dynamics.
The present study focuses on two corallivorous gastropods, Drupella cornus and Coralliophila violacea. Both gastropods feed on coral tissue and can play an important role in reef dynamics, especially when they occur at high densities. These two snails are major contributors to coral mortality in Hawaii (Couch et al. 2014), and both species are among the most abundant gastropods across the Indo-Pacific, occurring in high numbers in Kenya (e.g., McClanahan 1990) and the Red Sea (Zeid et al. 2004; Al-Horani et al. 2011).
Drupella cornus feeds by scraping coral tissue with its radula, leaving behind large, white feeding scars. This snail is associated with widespread coral decline (Boucher 1986; Shafir et al. 2008; Turner 1994b), and while they prefer faster-growing branching corals (e.g., Acropora and Pocillopora) (Taylor 1978; Shafir et al. 2008; Schoepf et al. 2010; Al-Horani et al. 2011), they also feed on slower-growing mounding corals such as Porites or even temporarily on mushroom corals (Hoeksema et al. 2013; Moerland et al. 2016) when preferred prey are scarce. These snails are often found in large aggregations, possibly due to increased settlement near adult conspecifics (Turner 1994a) and due to chemicals in the mucus released by damaged (Kita et al. 2005).
Coralliophila violacea does not directly kill the coral polyps from which it feeds. Instead, it slowly removes nutrients, leaving behind small, circular scars. Because of this feeding behavior, this snail is known as a “prudent sessile feeder” (Oren et al. 1998). As it feeds, it creates carbon sinks as nearby polyps transport nutrients to where the coral tissue is being damaged by the snail. This allows the snail to feed in one area for long time periods. It is frequently found on Porites corals (Taylor 1978; Soong and Chen 1991). While there are relatively few studies of C. violacea, related species in the Caribbean, (C. abbreviata, C. caribaea, and C. galea), are fairly well studied and contribute to widespread decline of Acropora palmata (e.g., Brawley and Adey 1982; Miller 2001; Baums et al. 2003) and are spatially clustered (Potkamp et al. 2016).
Because these C. violacea and D. cornus both consume and live on similar corals (when preferred prey of Drupella is rare), yet feed (and thus, likely affect the coral) through different mechanisms, they provide an interesting opportunity for a comparative investigation of the drivers and consequences of spatial patterning in consumer populations. Here, I address three questions: (1) How are D. cornus and C. violacea distributed in space and do patterns of aggregation exist at multiple spatial scales: island-wide, among reef sites within the island, among quadrats within a reef site, and within corals? (2) What chemical cues drive the aggregations of these two species at smaller spatial scales? and (3) How does this variation in local density (resulting from aggregation) affect coral growth? To address these questions, I used a combination of field surveys and experiments conducted in Moorea, French Polynesia.
Methods
Spatial distributions of D. cornus and C. violacea
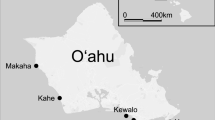
To quantify spatial patterns among corals and sites, I surveyed 8 areas in the Moorea lagoon (Fig. 1) in June and July 2011. The lagoon of Moorea is shallow (up to approximately 3 m depth), and following the A. planci outbreak in 2008 (Kayal et al. 2011), the dominant corals were massive Porites species and Porites rus. I selected sites with at least 1250 m2 of live coral that represented the lagoon habitats of Moorea. Of the sites surveyed, two (1 and 5) were located directly behind the reef crest. Three sites were backreef sites (3, 7, and 8), and three were on the fringing reef (2, 4, and 6). At each site, I surveyed ten 25 m by 0.5 m belt transects oriented perpendicular to shore. The first transect was haphazardly placed within a site, and all following transects were approximately 5 m from and parallel to the first transect. Each transect consisted of 0.5 m by 0.5 m quadrats placed over live coral centered along the transect. Each quadrat was centered on the transect and laid contiguous to the previous quadrat or whenever live coral was encountered (if it was preceded by sand or a dead coral). Within each quadrat, I recorded the coral species and number of snails (C. violacea and Drupella spp.) and visually estimated the percent of live coral tissue in the quadrat. In total, I surveyed 80 transects, with 4–24 quadrats per transect (depending on the amount of live coral cover in the transect).
Location of sampling sites for surveys of snail abundance in the lagoon of Moorea, French Polynesia. Sites 1 and 5 were directly behind the reef crest, sites 3,7, and 8 in the lagoon’s backreef, and 2, 4, and 6 on the fringing reef. The map was generated using ggmap (Kahle and Wickham 2013), with Google Earth imagery, copyright 2018 TerraMetrics
To model the distribution of snails across multiple spatial scales, I used generalized linear mixed effects models using the glmmADMB package in R (Skaug et al. 2011). I modeled the abundance of each snail species as a function of coral cover (area of Porites rus, massive Porites, and branching corals). Because C. violacea is only found on Porites rus and massive Porites, I did not include branching corals in the analysis of C. violacea abundance.
Analysis to explore spatial variation outside of coral cover consisted of two components: incorporation of spatial variation among sites and within sites (represented in nested random effects), and tests for statistical overdispersion (or spatial clustering) via fits of various count-based distributions. To account for spatial variation at the multiple spatial scales represented in the hierarchical survey (quadrat, transect, and site), I included site and transect as nested random effects. These random effects not only prevent pseudoreplication, but the presence of random effects at a given level indicates that snails were aggregated across that spatial scale. I used a likelihood ratio test to determine the significance of the random effects (clustering at multiple spatial scales) by comparing a model with the best fit distribution with and the corresponding model without the random effects. To test for spatial clustering outside of the hierarchical variation captured by the random effects, I compared a model with (negative binomial distribution, NBD) and without (Poisson) statistical overdispersion. I also included zero-inflated versions of the two distributions. I selected the best model using AIC and tested the significance of overdispersion using a likelihood ratio test. Statistical overdispersion (or spatial clustering) can occur at any spatial scale and is captured by the overdispersion term (k) in the negative binomial model or as additional variation in the Poisson model.
To determine spatial patterns on a single coral, I conducted a separate survey on fringing reefs (near sites 2 and 6 from the 2011 survey) in 2014. To obtain independent values of nearest neighbor distances, I searched reef sites for corals with more than two snails (91 corals with Drupella and 107 corals with Coralliophila) at two fringing reef sites, haphazardly selected a snail, and measured the distance from shell to shell of its nearest neighboring conspecific. I then compared the distribution of those distances to the expected distribution of nearest neighbors (derived in ESM), assuming snails were distributed randomly in space (“counts” follow a Poisson distribution), and assuming snails were clustered in space (“counts” follow NBD). I selected the best model (Poisson vs. NBD) using AIC and tested for goodness of fit using a multinomial likelihood ratio test. While this approach is similar to methods such as Clark–Evans that would use nearest neighbor distances to describe the spatial pattern of snails on a single coral, this approach uses a single nearest neighbor distance from multiple corals to describe the spatial distribution of snails at the spatial scale of a single coral, but across multiple corals.
Chemical cues
To test for the attraction of the two snails to chemical cues from conspecifics, corals, and coral damage, I conducted laboratory choice trials with four options in each trial. I conducted these experiments in an aquarium with four chambers 15 cm by 10 cm extending from a central area. Each chamber was separated from the central area by a mesh barrier placed 5 cm into the side chamber. An overhead flow system delivered water to the back of each chamber, which exited the system from the floor of the central area; thus, water flowed from each chamber into the central area. I ran three different experiments, each providing four options to the snails (one in each of the four chambers). The first experiment offered “snails” (i.e., two snails), “coral,” (i.e., one undamaged coral), “snails + coral” (i.e., two snails consuming a coral), and an “empty” chamber. The second experiment offered combinations of snails and corals, but in all cases the snails were placed in mesh sacks to prevent feeding during the trial. In this experiment, options were “snails + snail scar” (i.e., two snails and a coral fed upon by snails), “snail scars” (i.e., a coral fed upon by snails with no snails present), “snails + artificial scar” (i.e., two snails and a coral damaged with a waterpik), and “artificial scar” (i.e., a coral damaged by a waterpik without snails). The final experiment offered only corals with options of “coral” (i.e., undamaged coral), “artificial scar,” and “snail scar.”
I collected C. violacea, D. cornus, and juvenile massive Porites from the Moorea lagoon 3 d prior to their use in an experiment. For the 3 d prior to the start of the experiments, I held snails and corals together in plastic containers with mesh sides to enable water flow and placed corals not receiving snail damage directly in the water table fed by unfiltered seawater in a shaded outdoor area. Half an hour before the start of the experiment, I removed snails from the corals and used a waterpik to create artificial scars similar in size to the natural scars.
I placed the appropriate corals and snails into their chambers (behind the mesh barriers) and placed the test snail in the center of the middle area. If the test snail showed no movement after 30 min, it was replaced. After 2 h (preliminary observations indicated that few snails move after this time), I recorded the final location of the test snail, removed the corals and snails, and wiped down each aquarium. Each snail was only used in one trial, and only snails that made a choice were included in the analyses: 40/56 C. violacea and 55/63 D. cornus made a choice in the first experiment, 19/28 C. violacea and 25/32 D. cornus made a choice in the second experiment, and 18/20 C. violacea and 23/25 D. cornus made a choice in the third experiment.
To analyze the data, I used a multinomial exact test followed by pairwise comparisons with Bonferonni-adjusted p values for the first two experiments. For the third experiment, I used a prior contrasts and a multinomial exact test to address three questions: (1) Are snails attracted to coral (“empty” vs. the other three treatments), (2) are snails attracted to damaged coral (undamaged vs. damaged corals), and (3) are snails attracted to a certain kind of damage (artificial vs. natural damage)?
Effect of snail abundance on coral growth
To test for effects of aggregation on coral growth, I varied the density of snails on juvenile corals. If aggregations (or higher densities of snails) had greater consequences for corals than the additive effects of an individual snail, I hypothesized the effect of local density on coral growth would be nonlinear. I tested the effect of snail density on the growth of juvenile massive Porites in a laboratory experiment lasting for 23 d conducted from July–August 2011 (C. violacea) and June–July 2012 (D. cornus). For each experiment, I collected 40 juvenile colonies (approximately 2 cm to 3 cm in diameter) from pavement sites near the reef crest on the north shore of Moorea, French Polynesia, and transported them to the Richard B. Gump field station. I collected the snails from fringing reef sites, and starved them for 3 d prior to the experiment.
I attached corals to plastic mesh with Zspar epoxy and weighed them using the buoyant mass technique (Davies 1989). I then randomly assigned corals to a treatment of 0, 1, 2, or 3 snails and placed the snails directly on the coral in large tubs with a flow through system of unfiltered seawater covered by shade cloth. Midday irradiance at this location is approximately 896 (A Brown, pers. Comm.). Cages (C. violacea) and tethers (D. cornus) kept the snails on their assigned corals. For the C. violacea experiment, all corals, regardless of treatment, were placed in a cage that completely surrounded the coral. Throughout each experiment I monitored the snails and replaced any that were missing or dead. At the end of each experiment I weighed the corals and measured the surface area using the foil technique (Marsh Jr 1970). I standardized the growth of each coral by dividing its change in mass by its surface area. In both experiments, I excluded several corals from the analysis due to death prior to the conclusion of the experiment. These corals were distributed across all treatments. Six corals died in the C. violacea experiment, (3 that had 1 snail, 1 that had 2 snails, and 2 with 3 snails), and 3 corals died in the D. cornus experiment (1 each of a control coral, coral with 1 snail and coral with 2 snails). Despite the experiments occurring during different years and with different snail containment methods due to logistical constraints, corals in the control treatment grew very similarly. Therefore, I combined the experiments and analyzed the data using a linear regression and tested for nonlinearities by comparing linear, exponential, and quadratic models and selecting the best model using AICC.
Results
Spatial distributions of D. cornus and C. violacea
Based on the best fit models (Table 1), both species were aggregated spatially at all spatial scales, but different coral species led to some of this variation. C. violacea are found on both Porites rus, and massive Porites (Table 2), although only P. rus was a significant contributor to their distribution (z = 2.49, p = 0.013 and z = 0.67, p = 0.50 for P. rus and massive Porites, respectively). For D. cornus, the area of branching corals was the only significant coral cover type (z = 3.15, p = 0.0016). Thus, part of the pattern of aggregation was attributable to the availability of these substrates. However, even after accounting for variation in coral cover, the nested sampling random effect was significant for both snails (G2(3) = 146.6, p < 0.001, G2(3) = 18.6, p = 0.0003 for C. violacea and D. cornus, respectively). C. violacea showed more variation among sites than transects within a site (random effects variance estimate of 1.97 vs 0.34), and the opposite was true for D. cornus (variance estimate of 0.37 vs 0.90). Additionally, both snail species exhibited non-random variation across all spatial scales as the negative binomial model was a better fit than the Poisson models (Table 1). The overdispersion parameter was also significant for both snails (k = 1.06, G2 = 2984.1, p < 0.001, and k = 0.21, G2 = 162.8, p < 0.001, for C. violacea and D. cornus, respectively), demonstrating that both species were aggregated due to factors other than coral cover or the differences between site and transect locations accounted for by the random effects. For C. violacea, but not for D. cornus model fit was improved by including a parameter for excess zeros (Table 1), suggesting patterns of aggregation that were even more extreme than could be captured by the negative binomial model alone.
At the smallest spatial scale (on a coral), most snails were very near to a conspecific. 69% of D. cornus and 58% of C. violacea were ≤ 2 cm from the nearest conspecific (Fig. 2). For both snails, the expectation derived from a negative binomial distribution was the best fit, but in neither cases was it a good fit (\( G^{2} = 7.6*10^{ - 6} ,\, p < 0.0001 \) and \( G^{2} = 1.36*10^{ - 13} ,\, p < 0.0001 \) for D. cornus and C. violacea, respectively). This poor goodness of fit was driven by the high frequencies of snails < 2 cm from their nearest neighbor. Thus, snails were even more aggregated than expected by the negative binomial as including overdispersion (variance > mean) in counts failed to adequately describe the observed clustering demonstrated by the number of snails touching the nearest neighboring conspecific.
Distributions of nearest neighbor distances in a D. cornus and b, C. violacea on corals on the north shore of Moorea, French Polynesia. Observed values are shown by black points (all values ≥ 7 are grouped), and expected values under complete spatial randomness (Poisson—green), and spatial clustering (negative binomial—blue). The Poisson and negative binomial distributions are not truncated, while the final class for observed contains all values greater than 7 cm. The x axis represents the lower range of the distance bin. 92 D. cornus and 107 C. violacea are represented in the figure
Chemical cues
Both snail species preferentially moved toward chemical cues. In the first experiment (Fig. 3a), both species exhibited significant preference (D. cornus, p < 0.0001, C. violacea, p = 0.0001), with both species showing the lowest use of the empty chamber. D. cornus preferred the combination of conspecifics and coral above all other options. C. violacea also preferred this combination over the chamber with only snails, although the use of the snail-only chamber did not differ significantly from that with coral alone. In the second experiment (Fig. 3b), in which every chamber contained a coral, neither snail species showed a significant preference (D. cornus, p = 0.48, C. violacea, p = 0.50).
Proportion of snails attracted to corals, conspecifics, and damage in a lab choice experiment in Moorea, French Polynesia. Only snails that made a choice among the provided options were included in this figure. In experiment 1, 40/56 C. violacea and 55/63 D. cornus made a choice. In experiment 2, 19/28 C. violacea and 25/32 D. cornus made a choice, and in experiment 3, 18/20 C. violacea and 23/25 D. cornus made a choice. Error bars represent ± 1 standard error
In the third experiment, both species showed significant preference, albeit for different treatments (Fig. 3c). Because this experiment was analyzed using contrasts, I present the preference of each snail species to those three contrasts (coral vs. no coral, damaged coral vs., undamaged coral, and corals damaged by conspecifics vs. artificially damaged with a waterpik). D. cornus was attracted to corals (p = 0.0005), preferred damaged over undamaged corals (p = 0.012), and preferred corals damaged by conspecifics over corals that were artificially damaged (p = 0.021). In contrast, C. violacea chose chambers with corals (p = 0.0001), did not show significant preference for damaged vs. undamaged corals (p = 0.096, although this effect is in the same direction as observed for D. cornus), but (unlike D. cornus), preferred coral damaged artificially over corals damaged by conspecifics (p = 0.0034). Thus, the two snail species showed opposite responses to naturally damaged vs. artificially damaged corals. This result contrasts with the result in experiment 2 (e.g., compare “snail scar” with “artificial scar”).
Effect of snail abundance on coral growth
Coral skeletal growth declined linearly with snail density, and there was little evidence of nonlinear effects (Fig. 4). Neither the quadratic model (AICC = 416.27, ΔAICC = 364.29) nor the exponential model (AICC = 174.79, ΔAICC = 954.32) provided a better fit to the data than the linear model (AICC = − 779.65). The addition of each snail resulted in an approximately 17% reduction in the growth of juvenile massive Porites (F1,67 = 11.2, p = 0.0013). Additionally, there was no detectable difference in the effect of the two snail species (F1,67 = 0.0044, p = 0.953). Despite the experiments occurring in different summers, the growth rates of the control corals in both experiments were very similar to each other. Similarly, only the corals in the C. violacea experiment were caged, indicating methodological differences between summers had a negligible effect on coral growth.
Snails decrease coral growth with increasing snail abundance. Results from laboratory experiments conducted in Moorea, French Polynesia in 2011 (C. violacea) and 2012 (D. cornus) also fail to demonstrate a difference in effect between the two snail species. Error bars represent ± 1 standard error and the line the best fit from a linear regression
Discussion
Both C. violacea and D. cornus are clustered in space at multiple spatial scales, and are attracted to chemical cues (damaged corals for C. violacea and both conspecifics and conspecific damage for D. cornus) that likely contribute to these aggregations. Corals grow more slowly in areas where snails are aggregated, and although the snails feed through different mechanisms, they have the same approximate negative effect on coral growth. The average body size of two snails differed by less than 5 mm, so differences in feeding rates would not be expected due to large differences in body size and metabolic demands.
The aggregation of Drupella in space is consistent with previous work. Studies on the Great Barrier Reef (Cumming 1999) and Nigaloo Reef (Turner 1994a) found aggregations of Drupella spp. across sites, although sites at Nigaloo Reef encompassed an area greater than that included in this study from Moorea (sites were 50–100 km apart compared to up to 30 km in Moorea) Additionally, studies in Kenya show increased Drupella abundance in disturbed and overfished areas (McClanahan 1997; 1990) and studies in the Red Sea document higher densities in areas disturbed by divers (Guzner et al. 2010). However, a study conducted in the Red Sea did not find significant differences in density across sites (Schoepf et al. 2010), although these sites were much nearer to one another (within 320 m2) than those in this study.
Multiple mechanisms likely drive differences in snail distribution at small scales (on a single coral) and larger scales (among corals or sites). Clustering of D. cornus among corals and sites is likely due, in part, to the recruitment patterns of D. cornus. Juvenile D. cornus often settle near adults (Turner 1994a), and groups of D. cornus located near each other are closely related, suggesting that groups of larvae settle together (Johnson et al. 1993). Aggregations of D. cornus are also expected on branching corals, because D. cornus prefers branching corals over mounding corals such as massive Porites. While this preference likely played an important role in aggregations (as shown by the magnitude and significance of the coral cover effect), the better fit of the NBD distribution demonstrates aggregation outside of this preference. Additionally, many of these preferred branching corals are either not present or in very low abundance in Moorea due to an A. placni outbreak (Kayal et al. 2011). At smaller spatial scales, previous studies have shown an attraction of Drupella to conspecifics (Schoepf et al. 2010), but did not test for attraction to corals damaged by conspecifics (Fig. 3).
The heterogeneity in C. violacea distributions is likely driven by different factors than those that drive variation in D. cornus. C. violacea does not have much spatial genetic structure at a small scale (Lin and Liu 2008), so the clustering of C. violacea is likely due to settlement of a genetically similar cohort. Additionally, plenty of the preferred species for C. violacea, massive Porites (Soong and Chen 1991) and Porites rus, are present in Moorea, so aggregations are not due to patches of preferred corals.
Feeding strategy may also influence species distribution patterns. Because C. violacea feed by creating carbon sinks (Oren et al. 1998), the snails likely choose areas of the coral where this could be accomplished easily. Previous studies show carbon sinks were not observed around a single snail (Oren et al. 1998), but only in areas of multiple conspecifics or in coral growth areas (such as at colony margins and damaged areas). Therefore, C. violacea likely choose areas with large aggregations of conspecifics or damaged areas of coral. The pattern observed in the choice experiments is consistent with this idea. The combination of snails and corals was the most popular choice in the first experiment (Fig. 3), and corals with artificial scars were the most popular choice of the third experiment. While there was no preference in the second experiment, all corals were damaged, so if damage is the most important cue, rather than the conspecifics, it is plausible that no type of damage would be preferred. A potentially confounding factor in these experiments is that all corals were removed from the reef with a chisel, and even after sitting in the water table to recover, likely gave off some resulting cues from handling. However, these cues were likely much less than the signals from fresh scars. A related species, C. abbreviata has shown preference for corals with damaged tissue as well (Bright et al. 2015).
The responses of D. cornus to chemical cues were much clearer and demonstrate the importance of conspecifics in shaping distributional patterns. In all choice experiments, options involving conspecifics (either their presence or the damage they create) were chosen most often (Fig. 3), and snails could detect damage by conspecifics even in their absence (Fig. 3c). This attraction to conspecifics was previously described by Schoepf et al. (2010), where both adults and juveniles preferred conspecifics over food. My study extends these results and shows that snails are more attracted to conspecifics over undamaged coral and even to corals damaged by conspecifics over coral damaged artificially.
Both snails had extremely similar negative, linear effects on coral growth (Fig. 4). These similar effects were surprising, especially because D. cornus creates a much larger scar than C. violacea, suggesting that they might have had larger per capita effects. Instead, the equality of their per capita effects suggests that even though D. cornus consumes more tissue, C. violacea has a comparable effect because it creates a carbon sink that likely reduces the resources available for growth over an area much larger than the scar it creates. The rate of recovery of a coral might differ however, as C. violacea does not permanently remove or damage as much tissue as D. cornus. Additionally, the lack of a difference in control coral growth or a difference in snail species indicates methodology or annual differences did not lead to the observed differences.
Finally, the interaction between snail aggregations (due to either conspecifics or coral damage) and the effect of the snails on coral growth could cause important feedbacks in the system at multiple spatial scales. On a coral, this could increase spatial heterogeneity through variation in growth rates across the surface of the coral (high where snails are absent and low in the vicinity of snail aggregations), which would increase topographic complexity of the colony. If snails move to areas of damage and high conspecifics, I might expect larger scale variation in coral growth rates linked to the presence of snails. Among corals and among sites, these localized effects could lead to heterogeneity on these spatial scales as well, leading to patchiness in coral growth rate and survival. Similarly, other researchers have also noted the potential for feedback loops due to coral tissue damage and increases in corallivory and disease transmission (Guzner et al. 2010).
As snails deplete the resource, they also remove their habitat, making themselves more vulnerable to predation. As a result, snails will eventually move from depleted areas of the coral to new areas, forming new aggregations. Additionally, during outbreaks with high amounts of coral mortality, there could also be subsequent die-offs of snails. Through the interplay of these interactions, there could be spatial patterns that emerge from these dynamic “hot spots” of localized increases in snail density. These patterns are also observed in host–parasitoid systems. For example, parasitoids of tussock moths cause pattern formation during outbreaks. The extent of localized reductions in the host is limited by the dispersal distance of the parasitoid (Wilson et al. 1999). Host–parasitoid systems are, in some ways, analogous to systems where a biogenic habitat is consumed by an organism. For example, in the snail–coral example, snails do not parasitize their host, but they do require the coral for both food and shelter. If the coral dies, the snails are limited in their ability to redistribute themselves over the reef. It is likely then that I might expect a similar creation of dynamic patches of healthy and unhealthy corals to emerge from long-term interactions between consumers and the biogenic habitat they occupy.
References
Al-Horani FA, Hamdi M, Al-Rousan SA (2011) Prey selection and feeding rates of Drupella cornus (Gastropoda: Muricidae) on corals from the Jordanian coast of the Gulf of Aqaba, Red Sea. Jordan J Biol Sci 4:191–198
Baums I, Miller M, Szmant A (2003) Ecology of a corallivorous gastropod, Coralliophila abbreviata, on two scleractinian hosts. ii. feeding, respiration and growth. Marine Biology 142:1093–1101
Boucher LM (1986) Coral predation by muricid gastropods of the genus Drupella at Enewetak, Marshall Islands. Bull Mar Sci 38:9–11
Brawley SH, Adey WH (1982) Coralliophila abbreviata: a significant corallivore! Bull Mar Sci 32:595–599
Bright AJ, Cameron CM, Miller MW (2015) Enhanced susceptibility to predation in corals of compromised condition. PeerJ 3:e1239
Bruckner AW, Coward G, Bimson K, Rattanawongwan T (2017) Predation by feeding aggregations of Drupella spp. inhibits the recovery of reefs damaged by a mass bleaching event. Coral Reefs 36:1181–1187
Chesher RH (1969) Destruction of Pacific corals by the sea star Acanthaster planci. Science 165:280–283
Couch C, Garriques J, Barnett C, Preskitt L, Cotton S, Giddens J, Walsh W (2014) Spatial and temporal patterns of coral health and disease along leeward Hawaiian island. Coral Reefs 33:693–704
Cumming R (1999) Predation on reef-building corals: multiscale variation in the density of three corallivorous gastropods, Drupella spp. Coral Reefs 18:147–157
Davies PS (1989) Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar Biology 101:389–395
Glynn PW (1980) Defense by symbiotic crustacea of host corals elicited by chemical cues from predator. Oecologia 47:287–290
Guzner B, Novplansky A, Shalit O, Chadwick NE (2010) Indirect impacts of recreational scuba diving: patterns of growth and predation in branching stony corals. Bull Mar Sci 86:727–742
Hoeksema B, Scott C, True J (2013) Dietary shift in corallivorous Drupella snails following a major bleaching event at Koh Tao, Gulf of Thailand. Coral Reefs 32:423–428
John R, Dalling JW, Harms KE, Yavitt JB, Stallard RF, Mirabello M, Hubbell SP, Valencia R, Navarrete H, Vallejo M, Foster RB (2007) Soil nutrients influence spatial distributions of tropical tree species. Proceedings of the National Academy of Sciences 104:864–869
Johnson MS, Holborn K, Black R (1993) Fine-scale patchiness and genetic heterogeneity of recruits of the corallivorous gastropod Drupella cornus. Marine Biology 117:91–96
Kahle D, Wickham H (2013) ggmap: Spatial Visualization with ggplot2. The R Journal 5(1):144–161
Kayal M, Lenihan HS, Pau C, Penin L, Adjeroud M (2011) Associational refuges among corals mediate impacts of a crown-of-thorns starfish Acanthaster plancoutbreak. Coral Reefs 30:827–837
Kita M, Kitamura M, Koyama T, Teruya T, Matsumoto H, Nakano Y, Uemura D (2005) Feeding attractants for the muricid gastropod Drupella cornus, a coral predator. Tetrahedron Lett 46:8583–8585
Lenihan HS, Holbrook SJ, Schmitt RJ, Brooks AJ (2011) Influence of corallivory, competition, and habitat structure on coral community shifts. Ecology 92:1959–1971
Lin TY, Liu LL (2008) Low levels of genetic differentiation among populations of the coral-inhabiting snail Coralliophila violacea (Gastropoda: Coralliophilidae) in regions of the Kuroshio and South China Sea. Zool Stud 47:17
Marsh JA Jr (1970) Primary productivity of reef-building calcareous red algae. Ecology 51:255–263
McClanahan T (1990) Kenyan coral reef-associated gastropod assemblages: distribution and diversity patterns. Coral Reefs 9:63–74
McClanahan, TR (1997) Dynamics of Drupella cornus populations on Kenyan coral reefs Proc 8th lnt Coral Reef Sym 1: 633-638
McKeon CS, Moore JM (2014) Species and size diversity in protective services offered by coral guard crabs. PeerJ Prepr 2:e574
McKeon CS, Stier AC, McIlroy SE, Bolker BM (2012) Multiple defender effects: synergistic coral defense by mutualist crustaceans. Oecologia 169:1095–1103
Miller M (2001) Corallivorous snail removal: evaluation of impact on Acropora palmata. Coral Reefs 19:293–295
Moerland MS, Scott CM, Hoeksema BW (2016) Prey selection of corallivorous muricids at koh tao (Gulf of Thailand) four years after a major coral bleaching event. Contrib Zool 85:291–310
Oren U, Brickner I, Loya Y (1998) Prudent sessile feeding by the corallivore snail, Coralliophila violacea on coral energy sinks. Philos Trans R Soc Lond B Biol Sci 265:2043–2050
Penin L, Michonneau F, Baird AH, Connolly SR, Pratchett MS, Kayal M, Adjeroud M (2010) Early post-settlement mortality and the structure of coral assemblages. Mar Ecol Prog Ser 408:55–64
Potkamp G, Vermeij MJ, Hoeksema, BW (2016) Host-dependent variation in density of corallivorous snails (Coralliophila spp.) at curaçao, southern caribbean. Mar Biodivers: 1–9
Pratchett MS (2001) Influence of coral symbionts on feeding preferences of crown-of-thorns starfish Acanthaster plancin the western Pacific. Mar Ecol Prog Ser 214:111–119
Pratchett M, Vytopil E, Parks P (2000) Coral crabs influence the feeding patterns of crown-of-thorns starfish. Coral Reefs 19:36–36
Rotjan RD, Lewis SM (2008) Impact of coral predators on tropical reefs. Mar Ecol Prog Ser 367:73–91
Rotjan RD, Dimond JL, Thornhill DJ, Leichter JJ, Helmuth B, Kemp DW, Lewis SM (2006) Chronic parrotfish grazing impedes coral recovery after bleaching. Coral Reefs 25(3):361–368
Schoepf V, Herler J, Zuschin M (2010) Microhabitat use and prey selection of the coral-feeding snail Drupella cornus in the northern Red Sea. Hydrobiologia 641:45–57
Seidler TG, Plotkin JB (2006) Seed dispersal and spatial pattern in tropical trees. PLoS Biol 4:e344
Shafir S, Gur O, Rinkevich B (2008) A Drupella cornus outbreak in the northern gulf of eilat and changes in coral prey. Coral Reefs 27:379–379
Skaug H, Fournier D, Nielsen A, Magnusson A, Bolker B. (2011) glmmADMB: Generalized Linear Mixed Models Using AD Model Builder. http://glmmadmb.r-forge.r-project.org, http://admb-project.org. R package version 0.7
Soong K, Chen JL (1991) Population structure and sex-change in the coral-inhabiting snail Coralliophila violacea at Hsiao-Liuchiu. Taiwan. Marine Biology 111:81–86
Stewart HL, Holbrook SJ, Schmitt RJ, Brooks AJ (2006) Symbiotic crabs maintain coral health by clearing sediments. Coral Reefs 25:609–615
Stier A, McKeon C, Osenberg C, Shima J (2010) Guard crabs alleviate deleterious effects of vermetid snails on a branching coral. Coral Reefs 29:1019–1022
Stier AC, Gil MA, McKeon CS, Lemer S, Leray M, Mills SC, Osenberg CW (2012) Housekeeping mutualisms: do more symbionts facilitate host performance? PLoS One 7:e32079
Taylor JD (1978) Habitats and diet of predatory gastropods at Addu Atoll, Maldives. J Exp Mar Bio Ecol 31:83–103
Thaker M, Vanak AT, Owen CR, Ogden MB, Niemann SM, Slotow R (2011) Minimizing predation risk in a landscape of multiple predators: effects on the spatial distribution of african ungulates. Ecology 92:398–407
Turner S (1994a) Spatial variability in the abundance of the corallivorous gastropod Drupella cornus. Coral Reefs 13:41–48
Turner SJ (1994b) The biology and population outbreaks of the corallivorous gastropod Drupella on Indo-Pacific reefs. Oceanogr Mar Biol Ann Rev 32:461–530
Williams DE, Miller MW (2005) Coral disease outbreak: pattern, prevalence and transmission in Acropora cervicornis. Mar Ecol Prog Ser 301:119–128
Wilson WG, Harrison SP, Hastings A, McCann K (1999) Exploring stable pattern formation in models of tussock moth populations. J Anim Ecol 68:94–107
Zeid MA, Kotb MM, Hanafy MH (2004) The impact of the corallivore gastropod Coralliphilia violacea on coral reefs at El-Hamrawain, Egyptian Red Sea coast. Egyptian Journal of Biology 1:124–132
Acknowledgements
I thank Julie Zill, Angela Mulligan, and Morgan Farrell for field assistance and the Osenberg laboratory for helpful discussions. Funding was provided by National Science Foundation OCE-1130359, the QSE3 IGERT program (Grant No. 0801544), and the French American Cultural Exchange (via their support of the “Ocean Bridges” program). This project was conducted at the Richard B. Gump South Pacific Field Station.
Author information
Authors and Affiliations
Corresponding author
Additional information
Topic Editor Michael Berumen
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hamman, E.A. Aggregation patterns of two corallivorous snails and consequences for coral dynamics. Coral Reefs 37, 851–860 (2018). https://doi.org/10.1007/s00338-018-1712-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-018-1712-z