Abstract
Both natural and anthropogenic factors are changing coral-reef structure and function worldwide. Long-term monitoring has revealed declines in the local composition and species diversity of reefs. Here we report changes in coral-reef community structure over 12 yr (2000–2012) at 17 sites and three spatial scales (reef, gulf and country) in the Tropical Eastern Pacific (Panama). We found a significant 4% annual decline in species population sizes at the country and gulf scales, with significant declines ranging from 3 to 32% at all but one reef. No significant temporal change in expected richness was found at the country scale or in the Gulf of Chiriquí, but a 7% annual decline in expected species richness was found in the Gulf of Panama. There was a 2% increase in community evenness in the Gulf of Chiriquí, but no change in the Gulf of Panama. Significant temporal turnover was found at the country and gulf scales and at 29% of the reefs, a finding mostly explained by changes in species abundance, and losses and gains of rare species. Temporal trends in alpha and beta diversity metrics were explained by water temperature maxima, anomalies and variation that occurred even in the absence of a strong El Niño warming event.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, one-third of reef-building corals are at risk of extinction (Carpenter et al. 2008). Many coral reefs have experienced large-scale degradation (Pandolfi et al. 2003) and major changes in community composition (Hughes et al. 2003); 2016 was exceptional because of the strong El Niño event (Normile 2016). Long-term trends in coral species diversity may be complex, reflecting countervailing drivers, with trends in diversity differing at local and regional scales (Gonzalez et al. 2016). Global reef degradation is attributable to warming (Hoegh-Guldberg 1999) and ocean acidification (Kleypas and Yates 2009), while locally it is caused by overfishing (Hughes 1994), sediment runoff (Bellwood et al. 2004; Alvarez-Filip et al. 2013), water pollution (Guzman and Holst 1994), diseases (Francini-Filho et al. 2008) and direct damage to the reef structure through anchoring and trawling (Guzman et al. 1991; Graham et al. 2013). These disturbances are predicted to increase in frequency and severity over the coming century (Hoegh-Guldberg 1999; Cai et al. 2014).
Knowledge of trends in local diversity stems from meta-analysis, in which data are combined from many different studies. Some meta-analyses, in which average trends in local diversity have been estimated for marine systems, report no net change (Dornelas et al. 2014) and even increases in coastal biodiversity (Elahi et al. 2015). The conclusions depend on whether data are collected from areas of low or high human impact (e.g., Elahi et al. 2015) and the temporal scale over which they are analyzed. It is important to note, however, that marine tropical areas are underrepresented in these meta-analyses (Gonzalez et al. 2016).
In contrast, studies that have quantified changes in coral cover over time and space have consistently reported significant declines in the Caribbean (Gardner et al. 2003), the Indo-Pacific (Bruno and Selig 2007) and the Great Barrier Reef (Sweatman et al. 2011; De’ath et al. 2012). However, quantifying coral cover can mask changes in the community composition of coral species (Gardner et al. 2003). Trends in coral diversity can be measured at the population level, according to decreasing or increasing species abundance (Loh et al. 2005), or at the community level (Dornelas et al. 2014), where changes in species composition can be detected by evaluating alpha diversity and spatial and temporal beta diversity. Alpha diversity is measured as the number of species, or expected number of species, within a sample. This measure can be related to the abundance of individual species through various diversity indices (Jost 2006). Spatial beta diversity quantifies the variation in species composition among communities, while temporal beta diversity or turnover quantifies the variation in species composition over time, which can provide a more sensitive method for identifying temporal biodiversity change (Magurran and Henderson 2010; Dornelas et al. 2014).
In this study, we combined data from a multi-reef monitoring survey and a multiscale analysis to test whether changes in coral diversity, measured as species abundance, richness, evenness and turnover, can be detected in coral reefs off Pacific Panama over a 12-yr period, and whether these changes can be attributed to environmental and physical factors. We hypothesize that there have been significant changes in coral diversity in reefs in Pacific Panama, as in other regions, with an expected decline in abundance of species as well as changes in species richness, evenness and turnover. We also hypothesize that these changes have occurred at local (reef) and regional spatial scales. We further hypothesize that the attribution of the potential changes is different for reefs inside and outside marine protected areas due to direct impact to the reef framework. Finally, we hypothesize that environmental factors, such as water temperature anomalies, could have affected all reefs, even in the absence of a strong El Niño–Southern Oscillation (ENSO) event during the study period.
Methods
Study area
The Pacific coast of Panama is divided into two gulfs, the Gulf of Panama (GP) and the Gulf of Chiriquí (GC), which are semi-open areas separated by a ca. 100-km-wide peninsula. Oceanographic differences between the gulfs have shaped the reefs that inhabit them (Toth et al. 2017). The GC presents a nearly constant sea surface temperature of 28 °C, low and constant NO −3 and chlorophyll a concentrations near the surface. In contrast, the GP has seasonal temperature changes of 10 °C and high concentrations of NO −3 , and chlorophyll a in the upper layer during the dry season (December to March) (D’Croz and O’Dea 2007). These conditions promote phytoplankton blooms that can restrict coral growth in the GP (Glynn et al. 2017b). These differences are due to a wind-driven seasonal upwelling that only occurs in the GP (Xie et al. 2005; Fielder and Lavín 2017).
Extensive coral reefs are present in both the GP and the GC (Guzman et al. 2004, 2008; Glynn et al. 2017b). These reefs are composed of low evenness frameworks that extend several kilometers, as is typical in the Eastern Pacific (Cortés et al. 2017); coral diversity tends to be low on continuous coral-reef frameworks compared to discontinuous coral communities from the same region (Guzman et al. 2004, 2008). There are 22 coral species reported in the GC (Guzman et al. 2004) and 19 in the GP (Guzman et al. 2008), with a total of 27 species reported for Pacific Panama (Cortés et al. 2017). Reef framework accumulation is lower in the GP than the GC (Toth et al. 2017). These reefs are located at about 10 m depth and are limited to shallow and clear water due to their association with zooxanthellae Symbiodinium. Reef frameworks rest on basalt foundations; the GC reefs have vertical buildups of 10–12 m, with ages up to 5600 yr (Glynn and Macintyre 1977), and GP reefs have vertical buildup of 5.6–6.1 m and ages of 4500 yr (Maté 2003). The extreme warming event in 1982–1983 affected corals in both the GP and the GC. Water temperature rose to > 29 °C, killing 75% of hermatypic corals in the GC and 85% in the GP (Glynn 1990). Age estimates for the massive corals killed during this event suggested that a disturbance of this magnitude had not occurred in the past 200 yr (Glynn 1990). A similar ENSO event in 1997–1998, in which temperatures rose to 29 °C in the GP and more than 30 °C in the GC for over 3 weeks, was less severe and caused only 13.1% coral mortality in the GC and no mortality in the GP (Glynn et al. 2001b). The presence of extensive but simple coral-reef systems and the history of reef bleaching at the country scale provide an important context for observing temporal changes in coral diversity (Cortés et al. 2017).
Community data
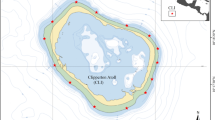
Community data (species % cover yr−1) were collected by the Smithsonian Tropical Research Institute’s Panama Coral Reef Monitoring Network. This initiative monitored 17 coral reefs on the Pacific coast of Panama: seven reefs in the GP and 10 in the GC (Fig. 1a). The initiative, which began with six monitored reefs in 2000, concluded with 17 monitored reefs in 2012; not all reefs were monitored for the same amount of time (Table 1). Monitoring sites were selected based on the existence of a solid reef-building framework, regardless of reef health. The sites chosen include a range of levels of degradation and management. Six of the reefs in the GP were located inside the Marine Special Management Zone of Las Perlas Archipelago. Six of the reefs in the GC were located within Coiba National Park, the largest marine reserve in Pacific Panama, and one (Paridas) was located in the National Marine Park Golfo de Chiriquí (Table 1). Reefs were monitored annually with six permanent belt transects located parallel to the coastline, 10 m long and 10 m apart: three of the transects were located on the reef flat or upper slope (< 5 m) and three on the lower slope or reef base (> 5–12 m). Reefs on which the six transects were within a continuous reef framework were described as “continuous,” while reefs in which at least one of the transects lays within a discontinuous reef framework, interrupted by sand channels and massive coral heads, were descried as “discontinuous” (Table 1). Each survey lasted 1.5 h on average. Each transect was marked at the beginning, middle and end with a permanent metal rod driven into the reef. Ten 1-m2 quadrats were measured along each transect with successive placements of a 1-m2 PVC quadrat divided with strings into a 10 × 10 cm grid (see Guzman et al. 2004, 2008). The percentage cover of each type of sessile organism was estimated as a fraction of a grid square; a 2.5-cm2 colony was one-quarter of a square representing 0.25% cover.
a Location of the 17 monitored reefs in Pacific Panama. b In situ water temperature (°C) in the GP (gray) and the GC (black): mean, maximum (up-pointing triangles), and minimum (down-pointing triangle), number of warm days (> 30 °C), + 1 °C and + 1.5 °C anomalies, and days away from maximum monthly mean (MMM). Solid lines are means and standard deviations are dashed lines
Due to the long debate on morphological versus phylogenetic species identification, and the need for more studies to resolve the taxonomy of common coral species in the area (Combosch and Vollmer 2015), this study followed the Reyes-Bonilla (2002) species checklist, except Psammocora superficialis, which was synonymized with P. profundacella (Benzoni et al. 2010), and Pavona chiriquensis, described in 2001 (Glynn et al. 2001a). Algal cover was divided into four functional groups: calcareous, crustose coralline algae, turf and frondose macroalgae. Octocorals and sponges were recorded to species when possible. Water temperature (wt) loggers (HOBO U22 Temp Pro V2, Onset Computer Corporation) were installed at 10 of the monitored reefs (six in GC and four in CP; Table 1). HOBO stations were programmed to record in situ wt every 30 min. Data were downloaded and stations redeployed annually.
Two approaches were used to analyze temporal trends in the biodiversity of scleractinians in coral reefs off Pacific Panama at three spatial scales (reef, gulf and country). The first approach estimates temporal trends in alpha diversity by looking at changes in species richness and evenness and changes in species abundance (percentage cover). The second approach estimates trends in temporal beta diversity, disentangling the contribution of species replacement and changes in species abundance (percentage cover) and quantifying the contribution of each species to community changes. Independent analyses were done for each reef, grouped by gulf and then pooled together. All analyses were performed using R software version 3.2.2 (R Core Team 2015) unless otherwise indicated.
Temporal trends in alpha diversity
Expected species richness was estimated using species accumulation curves fitted to presence/absence data obtained from the sixty 1-m2 plots on each reef for each year, using the function “specaccum,” available in the vegan R package (Oksanen et al. 2016). Analyses were done using the “random” method, which adds plots in a random order, with 500 permutations per run. The number of unobserved species per year per reef was calculated with the Chao estimator (Chao 1987) using the function “specpool” in the same R package. Expected species richness in 60 plots was regressed against the year for each site. We did not rarify our estimates of species richness across time because equal sample effort (total number of plots) was applied at each census and on every reef. Analysis of temporal trends in estimated species richness for the entire study region (country scale) and for each gulf was conducted with a random intercept nested mixed model in which the intercept was allowed to change for each reef. We used function “lmer” in the nlme package (Pinheiro et al. 2016) to fit the model:
with reefs nested within gulfs as a random factor. We compared the additive model to the interactive model using function “anova” to test whether trends were significantly different between gulfs.
Temporal variation in community diversity was estimated at each site using a Rényi profile (Rényi 1961). The Rényi profile, which summarizes the spectrum of diversity indices from richness to evenness, is an ordering technique designed to rank different communities from low to high diversity, where a higher profile indicates higher overall diversity (Kindt et al. 2006). Rényi profiles were calculated for each year for each reef using the function “renyi” in vegan (Oksanen et al. 2016). The temporal variation for each Rényi diversity index was estimated for each reef; a significant difference in the temporal variation among diversity indices was tested as follows:
with reefs nested within gulfs as a random factor. Hill numbers (Hill 1973) 1 and 2, corresponding to Shannon index (H’) and species evenness, were extracted from the diversity profiles and analyzed individually for temporal trends. Henceforth, we refer to Hill number 1 as Shannon and Hill number 2 as evenness.
A minimum–maximum autocorrelation factor analysis (MAFA) was used to identify and explain common temporal trends in scleractinian species abundance for each reef. MAFA is a type of principal component analysis designed for time series data (Zuur et al. 2007); it estimates the maximum and minimum autocorrelation factor between time intervals to find a common trend (MAFA axis) in a multivariate time series dataset and then correlates it with an explanatory variable. Changes in total coral cover alone do not reveal temporal community changes, as the increase in cover of some species, usually dominant ones, compensates for the decline of others, usually rare ones. The MAFA trend takes into account the temporal variation in individual species within each reef and produces a summarized temporal trend. This approach also allowed us to analyze the trends at different spatial scales (reefs, gulf and country) while taking into consideration the abundance of each species at the smallest scale. The first MAFA trend represents the main underlying pattern in the data (Zuur et al. 2007). This analysis does not create eigenvalues to quantify the importance of each trend, but it does calculate whether the autocorrelation of the axes is significantly different from zero (Zuur et al. 2007). The analysis also quantifies canonical correlations between the MAFA axes (e.g., reef trend) and each of the original variables (e.g., species abundances). The MAFA-dependent variable was annual species percentage cover (for common scleractinian corals only). Explanatory variables were: annual number of days with average wt >29 °C; number of +0.5 °C and +1 °C annual temperature anomalies; number of days with wt > local maximum monthly mean; temperature coefficient of variation; and minimum and maximum wt. Analyses were performed using Brodgar software version 2.7.4. Temperature was only used as an explanatory variable in reefs with in situ data loggers (Table 1).
A hierarchical mixed-effects model with a random intercept was used to estimate the temporal change in the MAFA trend (species abundance) at the gulf and country scales as:
with reefs nested within gulfs as a random factor.
Trends in temporal beta diversity
Temporal change in beta diversity was quantified as the variation in community composition, or species turnover, over survey events (sensu Legendre and Salvat 2015). First, we calculated the Jaccard distance, which measures the dissimilarity between communities, between the last year of data (2012) and every monitored year for each reef (see also Dornelas et al. 2014). The final year was used as the reference year because monitoring began in different years at different reefs, but the last year was common to all reefs. The Jaccard distance between each year and the final year was plotted against time, and a best-fit line was used to calculate the slope of this long-term relationship. The same analysis was done to test for changes in beta diversity annually by calculating the Jaccard distance between each of the consecutive years. This turnover metric includes shifts in species composition and is therefore a more sensitive indicator of community change than alpha diversity (Dornelas et al. 2014). Jaccard indices were calculated using the “vegdist” function, method “Jaccard” in vegan (Oksanen et al. 2016). An estimate of the temporal turnover at the country scale and for each gulf was calculated using a hierarchical mixed-effects model with a random intercept using the Jaccard distance between each year and the last year of data, in the same way as the model for alpha diversity and species abundance.
The total temporal variance in community composition, as well as its components, was calculated following Legendre (2014). This method explains the dissimilarity between sampling times caused by species replacement and abundance differences. The calculation was done by setting Legendre’s R Software function “beta.div.comp” (http://adn.biol.umontreal.ca/~numericalecology/Rcode/) to the quantitative form of the Jaccard-based b-diversity indices (Ruzicka indices in the Podani family), which includes species abundances, not only presence or absence, according to Legendre (2014). The contribution of species replacement and changes in species abundance to the total temporal beta diversity was calculated for each reef. Species contribution to beta diversity (SCBD) for each reef was calculated with Legendre and Caceres (2013) R software function “beta.div” (http://adn.biol.umontreal.ca/~numericalecology/Rcode/). All analyses used the Hellinger transformation, which is appropriate for measuring the variation in community composition data (Legendre and Caceres 2013). SCBD of common species between gulfs was tested for correlation using the “rcorr” function of the Hmisc R package (Harrel and Dupont 2015).
Correlation with temperature parameters
Correlations with temperature parameters were only analyzed for reefs with an in situ HOBO logger (Table 1). Temperature records were analyzed using different approaches to describe coral thermal stress: (1) days when daily wt was above 30 °C (Glynn et al. 2017a); (2) number and magnitude of wt positive anomalies (+ 0.5, + 1, + 1.5 °C) per year, calculated by quantifying the difference between average daily temperature and daily regional long-term mean (December 1971–December 2000), with a 1-degree latitude grid resolution. Long-term mean data were acquired from NOAA (http://www.esrl.noaa.gov/psd/data/gridded/data.noaa.oisst.v2.html); and (3) maximum of the monthly mean (MMM) (Skirving et al. 2006), which is the mean temperature during the warmest month calculated for each site with HOBO devices. The number of days with daily mean wt above MMM was quantified for each reef, as well as the number of days with wt 1 °C above the MMM (sensu Glynn and D’Croz 1990). Additionally, degree-days and minimum, maximum and coefficient of variation were calculated for each year for each reef. All analyses were done in R software version 3.2.2 (R Core Team 2015).
An asymmetric canonical ordination redundancy analysis (RDA), which quantifies the percentage of variation in the response variables explained by the predictor variables in a multivariate dataset (Peres-Neto et al. 2006), tested the effect of temperature on community changes at the reef scale, with biodiversity indices (MAFA trend, expected number of species and Jaccard dissimilarity) as response variables. A model selection tool (vegan R function “ordistep”) was used to select relevant temperature parameters as an explanatory variable. Only sites with more than one species and where temperature loggers were installed (Table 1) were used in the analysis. In the same way, a second RDA was used to test the effect of degree of management, reef type (continuous or discontinuous framework), distance from human settlement, initial species richness and initial coral cover (Table 1) on diversity trends. All reefs were included in the second RDA.
Results
Coral cover during the first monitoring year varied among reefs. The reef with highest cover was San Pedro (GP, 91%), and the reef with the least cover was Canales (GC, 1%). Coral cover on most of the monitored reefs (11 out of 17) was originally less than 50% (Table 2). At the reef scale, annual coral cover change ranged from a 5% decline (GC, Coiba NE) to 3% increase (GC, Montuosa) (Table 2). Total coral cover remained unchanged at the country scale (Pacific Panama) and in the GC and declined by 0.5 ± 0.01% (mean ± SE) per year in the GP (Table 2). Monitored reefs were highly dominated by a single species, and this was maintained over time in all reefs except for Jicarita (GC) and Señorita (GP) (Electronic supplementary material, ESM, Fig. S1). Pocillopora damicornis was the dominant species on 82% of the reefs (Table 1).
Temporal trends in coral alpha diversity
There was no overall temporal trend in expected species richness at the country scale (B = − 0.03, SE = 0.02, p > 0.05); however, the trends were significantly different between the gulfs (χ 2 = 5.18, p < 0.05). The slope of the decline in the GC was not statistically different from zero (B = 0.02, SE = 0.03, p > 0.05), but the slope in the GP indicated a significant decline in expected species richness of 7% yr−1 (B = − 0.07, SE = 0.03, p < 0.05) (Table 3; Fig. 2; ESM Fig. S2). Iguana and Saboga reefs in the GP, both with discontinuous reef frameworks, had significant decreasing trends (Adj. R 2 = 0.30 and 0.32, respectively). The reef at Saboga lost three of seven species: Pavona gigantea, P. varians and Pocillopora elegans, and Iguana lost two of five species: Gardineroseris planulata and Psammocora profundacella (ESM Fig. S1) during the survey period. Although Secas and Jicarita did not have significant declining trends in expected species richness, they did lose some species during the study period, Pocillopora elegans, Porites lobata and Psammocora stellata in Secas and P. stellata in Jicarita.
Rényi diversity profiles showed that most reefs had a steep decline in species richness to evenness, reflecting the strong dominance of a single species, Pocillopora damicornis, on most reefs. The temporal variations in these diversity profiles were significantly different between gulfs and sites (p < 0.001) (ESM Fig. S3). The hierarchical mixed-effects model for Shannon diversity did not reveal statistically significant temporal trends at the country or the gulf scales (both p > 0.05) (Table 3). Evenness, however, significantly increased in the GC (B = 0.02, SE = 0.01, p < 0.05), but no significant trends were found at the country scale and for the GP (p > 0.05 in both cases; Table 3).
Temporal trends in coral abundance
The MAFA analysis revealed an overall decreasing trend in species abundance, representing a 4% annual decline at the country scale (slope = 0.04, SE = 0.008, p < 0.001) and in each gulf (GC: B = 0.04, SE = 0.009, p < 0.001; GP: B = 0.04, SE = 0.01, p < 0.001). In the GP, five reefs had significant changes. Four reefs had significant declining trends: San Pedro (slope = − 0.11, Adj. R 2 = 0.44), Señorita (− 0.08, 0.92), Iguana (− 0.07, 0.92) and Saboga (− 0.06, 0.65), while San Jose (0.15, 0.96) had a significant increasing trend (Table 3; Fig. 3). In the GC, five reefs had significant declining trends in species abundance: Montuosa (slope = − 0.32, Adj. R 2 = 0.788), Secas (− 0.18, 0.95), Cebaco (− 0.17, 0.79), Canales (− 0.15, 0.90) and Coiba NE (− 0.12, 0.55). None of the reefs in the GC had a significant increasing trend (Table 3: Fig. 3). Trend slope was not correlated with the number of years (time points) of data (Pearson correlation, p > 0.05).
Trends in temporal beta diversity
The hierarchical mixed model showed that as time progressed the original composition of the coral communities became more distinct from the community in the final monitoring year, which is represented by an overall declining temporal trend of 2% yr−1 (B = − 0.02, SE = 0.004) (Pacific Panama). The Jaccard distance showed a negative trend of − 0.02 in the GP (SE = 0.005) and − 0.03 (SE = 0.005) in the GC (Fig. 4). There was a significant interaction (p > 0.05), meaning that the trends were significantly different between gulfs. The temporal change in community similarity in the more thermally stable GC was 1.6 times greater than in the more thermally variable GP.
Five reefs had significant declining trends in Jaccard distance over time, meaning that they displayed a greater distance between communities during the first and last monitoring years: Canales (slope = − 0.06, Adj. R 2 = 0.77), Mona (− 0.05, 0.61), Cebaco (− 0.04, 0.71) and Isla Coiba (− 0.02, 0.28) in the GC and Iguana (− 0.0005, − 0.06) in the GP. Only one site, Saboga (GP), had a significant positive trend (0.03, 0.53) (Table 3; Fig. 4). A change in community similarity was not observed on an annual scale when calculating Jaccard distances between consecutive years. The number of years of data was not related to the slope of the trend (Pearson correlation, p > 0.05).
Beta diversity decomposition using the Ruzicka index (quantitative form) showed that temporal changes in community similarity were better explained by changes in the abundance of species (78.7%) than by changes in species replacement (21.2%), with a similar pattern at the reef and gulf levels (Table 3; Fig. 5a, b). However, temporal dissimilarity in community composition was best explained by species replacement in Jicarita (88%) and San Telmo (71%). San Pedro and Cebaco reefs were excluded from this analysis because they only had one species, P. damicornis, during the study period.
Relative contribution of species replacement and changes in abundance (%) to the total temporal beta diversity for the Gulf of Panama (GP) (a) and the Gulf of Chiriquí (GC) (b). Mean and temporal variation in the contribution of each species to temporal beta diversity changes in the GP (c) and the GC (d). Species full names in Table 3
SCBD was correlated with species that were common in both gulfs (n = 11, correlation coefficient = 0.8, p < 0.005). However, in the GC most of the variation was due to changes in P. elegans, while in the GP, changes in temporal community similarity were best explained by six species P. elegans, Psammocora stellata, Pavona frondifera, G. planulata, P. clavus and P. produndacella (Table 4; Fig. 5c,d). Species SCBD index was not correlated with mean relative abundance at the reef level (p > 0.05), meaning that common and rare coral species contributed to community changes in the reefs.
Correlation with temperature parameters
HOBO loggers installed at ten reefs recorded a total of 4918 d of in situ water temperature in the GP and 4273 d in the GC (Table 1; Fig. 1b). The annual number of days above 29 °C ranged 5–212 d in the GC and 0–63 d in the GP. Years with a greater number of warm days were 2002, 2003, 2005, 2006 and 2012, all of which, except 2012, clearly coincided with moderate El Niño years. There were significantly more annual days with wt >29 °C on GC reefs than GP reefs during the study period (Welch two-sample t test, p < 0.001). The mean annual number of temperature anomalies above 0.5 °C degrees ranged 0–65 in the GC and 0–30 in the GP; anomalies above 1 °C ranged 0–37 in the GC and 0–4 in the GP. The GC had significantly more daily temperature anomalies per year than the GP (Welch two-sample t-test, p < 0.001). 2009 had the greatest number of temperature anomalies; this was also an El Niño year.
Changes in diversity metrics were explained by increases in water temperature even in the absence of a strong El Niño event. At the country scale, changes in coral alpha diversity (expected species richness) and abundance (MAFA trend) were correlated with increases in water temperature (anomalies, mean, number of days with wt above 30 °C and degree-days) and its variation (Adj. R 2 = 0.31, p < 0.001) (Table 5; Fig. 6a). MMM, or maximum and minimum temperatures combined, explained 72% of the variation in diversity trends in reefs in the GC (Adj. R 2 = 0.721, p < 0.001) (Table 5; Fig. 6b). The coefficient of variation, number of anomalies, and maximum and mean temperature combined explained 43% of the variation in diversity metrics in reefs in the GP (Adj. R 2 = 0.43, p < 0.001) (Table 5; Fig. 6c).
Redundancy analysis of the correlation between changes in diversity metrics (dashed arrows): species abundance (maximum autocorrelation factor analysis, MAFA), temporal turnover (Jaccard) and expected species richness (Exp.S) with temperature parameters as explanatory variables (solid arrows): anomalies above 1 °C (a1), anomalies above 1.5 °C (a1.5), degree-days (dd), number of days above 30 °C (hot), maximum monthly mean (MMM), variation coefficient (vc), and mean, maximum and minimum water temperature recorded from in situ temperature loggers. Model for a the entire study area, b five reefs in the Gulf of Chiriquí and c three reefs in the Gulf of Panama
The second RDA, testing the possible effect of the initial number of species, initial coral cover, degree of reef protection, distance from human settlement on the response variables and type of reef (continuous or discontinuous framework), did not reveal any significant effects on diversity trends.
Discussion
After 12 yr of coral-reef monitoring on 17 reefs in Pacific Panama, small changes in coral percentage cover at the reef scale did not reveal ecologically important temporal community changes. However, we detected significant changes in multiple diversity metrics at the reef, gulf and country scales. These changes were partially explained by the different temperature parameters associated with warming and temperature fluctuations, even in the absence of strong El Niño or La Niña events. This study found not only declines in coral species’ abundance, but also identified diversity changes associated with the declines in abundance at different spatial scales.
A declining trend in species abundance was found at all spatial scales and equated to a 4% annual decline. A reduction in coral species’ abundance was significant at 59% of the monitored reefs. A significant increase only occurred at San Jose (GP), due to an increase in the already dominant P. damicornis, which was the main reason for a decrease in evenness. This general negative trend was mainly explained by the reduction in abundance of common species (such as P. damicornis); it was present in reefs under all levels of protection, including reefs within the no-take zone of Coiba National Park and at reefs with no protection, such as Cebaco and Islas Secas. Similar results were found on Caribbean coral reefs, which have had significant declines without major changes in species richness or local extinctions (Riegl et al. 2009).
Trends in species alpha diversity varied according to metric and spatial scale. At the country scale, opposing positive and negative trends resulted in no net change in alpha diversity. At smaller scales, however, significant trends were found. There was a 7% annual decline in expected species richness in the GP and a 2% increase in species evenness in the GC. The decrease in expected species richness was the result of the loss of common, but not dominant species (Pavona gigantea, P. varians, Pocillopora elegans, G. planulata, and Psammocora profundacella) and was more evident on Saboga, Iguana and Jicarita reefs with discontinuous frameworks in their reef base and on Secas, which had a continuous framework. These reefs are located in areas under different levels of protection. In marine protected areas, direct human impact to the reef is expected to be low, but the implementation of regulations is uncommon (Guzman et al. 1991) and anthropogenic stress comes indirectly through overfishing of surrounding areas. Iguana Island is a small uninhabited island with a 16-ha reef (Guzman et al. 1991) sheltered from river runoffs and sedimentation. Saboga reef is in front of a human settlement, and impacts to the reef via water pollution and sedimentation are known (Guzman et al. 2008). Most of the studied reefs were dominated by a single species (Pocillopora damicornis). With the loss of rare species and the decline in abundance of the dominant species, communities became more even, which was more evident at Coiba reef.
Changes in community composition were also seen at the three studied scales. The community change in the GC was 1.6% greater than in the GP, where only two reefs, Saboga and Iguana, had significant declines. Temporal diversity changes at most reefs were explained by the overall decrease in abundance showed by the MAFA analysis and beta diversity decomposition. An important contribution to species replacement was seen at reefs in Jicarita, Iguana, San Jose and San Telmo. Although San Jose, San Telmo, Jicarita and Iguana are isolated, remote reefs that are not exposed to threats like human development, they are under considerable pressure from tourism. All of these reefs are also exposed to the indirect effects of nearby fishing and regional temperature fluctuations.
Even in the absence of strong El Niño warming events, which have caused major coral bleaching in the area (Hughes et al. 2003; Glynn et al. 2017a), sporadic and uneven bleaching was observed during this study. The sum of small but frequent positive anomalies, as well as a high MMM and water temperature variation contributed to affect heat-vulnerable or less thermo-tolerant species, which our analyses revealed made greater contributions to community temporal changes.
Warming experiments and monitoring after mass mortality events have demonstrated that massive species, such as Pavona gigantea, Porites lobata, and Pavona clavus, are more tolerant to warming (Hueerkamp et al. 2001) and have faster recovery (Guzman and Cortés 2007) than the branching species Pocillopora damicornis and P. elegans (Hueerkamp et al. 2001). In previous experiments, Pavona gigantea exhibited the greatest resistance to bleaching and Porites lobata showed the greatest recovery, while Pavona clavus showed no recovery (Hueerkamp et al. 2001). In this study, the ecological consequences of this tolerance are reflected in the contribution of each species to community changes, here quantified with the SCBD index. Pavona gigantea was one of the species with the smallest contribution to changes (0.03), compared to the contributions of Porites lobata (0.1), Pavona clavus (0.2) and Pocillopora elegans (0.5).
In conclusion, we found that significant changes in coral diversity and community composition occurred over the course of a decade in reefs off the Pacific coast of Panama at the national, gulf and reef scales even in the absence of a strong warming event or significant coral cover decline. These changes imply alterations to the structural complexity and functioning of the coral communities (Alvarez-Filip et al. 2013). Our capacity to detect and attribute trends depended on the scale analyzed and the metric used, emphasizing the importance of multiscale biodiversity monitoring and quantitative attribution where possible. The estimates of the trends found in this study, however, could be affected by the differences in monitoring start data among reefs, which emphasizes the importance of long-term constant and continuous monitoring programs and the need for temporal baselines (Gonzalez et al. 2016).
We found that communities can diverge rapidly in composition even if a stress threshold is not reached (e.g., coral bleaching threshold). The sum of frequent smaller natural disturbances, in this case warm days, can lead to decreases in species abundance and species replacements (sensu Lamy et al. 2015). If these changes do not occur between similar species, a novel community will be created (Hobbs et al. 2006), which is highly likely to change the ecosystem services the previous community provided (Graham et al. 2014). These types of changes in community composition become especially important when they involve foundation species, such as pocilloporid corals, which, based on these results and on scenarios of ocean warming for the region, are especially threatened.
In this analysis, the level of reef management and protection (no-take zone, protected area, or not protected) did not explain changes in coral species abundance or species turnover. This could be a result of ineffective management in protected areas (Alvarado et al. 2017) or a region-wide suite of anthropogenic impacts (Cortés and Reyes-Bonilla 2017).
Lastly, this study, as well as the previous reports on bleaching, can be used as a reference to understand the shifting baseline of diversity and coral abundance in the region (Pauly 1995). This will help interpret the lasting ecological effects of the high temperatures of the 2015–16 ENSO event (Jacox et al. 2016). Resampling this monitoring network would allow assessment of the effects of temperature stress on communities living close to their environmental tolerance in the GC and communities living in variable, but less stressful environments in the GP. A detailed evaluation of the trends of heat-intolerant coral species is needed, because this study identified them as responsible for the community changes we detected.
References
Alvarado JJ, Aburto-Oropeza O, Abad R, Barraza E, Brandt M, Cantera J, Estrada P, Gaymer CF, Guzmán-Mora AG, Herlan JJ, Maté JL (2017) Coral reef conservation in the Eastern Tropical Pacific. In: Glynn PW, Manzello DP, Enoch AC (eds) Coral reefs of the Eastern Tropical Pacific. Springer, Dordrecht, pp 203–250
Alvarez-Filip L, Carricart-Ganivet JP, Horta-Puga G, Iglesias-Prieto R (2013) Shifts in coral-assemblage composition do not ensure persistence of reef functionality. Sci Rep 3:3486
Bellwood DR, Hughes TP, Folke C, Nyström M (2004) Confronting the coral reef crisis. Nature 429:827–833
Benzoni F, Stefani F, Pichon M, Galli P (2010) The name game: morpho-molecular species boundaries in the genus Psammocora (Cnidaria, Scleractinia). Zool J Linn Soc 160:421–456
Bruno JF, Selig ER (2007) Regional decline of coral cover in the Indo-Pacific: timing, extent, and subregional comparisons. PLoS ONE 8:e711
Cai W, Borlace S, Lengaigne M, Rensch Van, Collins M, Vecchi G, Timmermann A, Santoso A, McPhaden MJ, Wu L, England MH, Wang G, Guilyardi E, Jin FF (2014) Increasing frequency of extreme El Niño events due to greenhouse warming. Nat Clim Chang 4:111–116
Carpenter KE, Abrar M, Aeby G, Aronson RB, Banks S, Bruckner A, Chiriboga A, Cortes J, Delbeek JC, DeVantier L, Edgar GJ, Edwards AJ, Fenner D, Guzman HM, Hoeksema BW, Hodson G, Johan O, Licuanan WY, Livingston SR, Lovell ER, Moore JA, Obura DO, Ochavillo D, Polidoro BA, Precht WF, Quibilan MC, Reboton C, Richards ZT, Rogers AD, Sanciangco J, Sheppard A, Sheppard C, Smith J, Stuart S, Turak E, Veron JEN, Wallace C, Weil E, Wood E (2008) One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 321:560–563
Chao A (1987) Estimating the population size for capture-recapture data with unequal catchability. Biometrics 43:783–791
Combosch DJ, Vollmer SV (2015) Trans-Pacific RAD-Seq population genomics confirms introgressive hybridization in Eastern Pacific Pocillopora corals. Mol Phylogenet Evol 88:154–162
Cortés J, Reyes-Bonilla H (2017) Human influences on Eastern Tropical Pacific coral communities and coral reefs. In: Glynn PW, Manzello DP, Enoch AC (eds) Coral reefs of the Eastern Tropical Pacific. Springer, Dordrecht, pp 203–250
Cortés J, Enochs IC, Sibaja-Cordero J, Hernández L, Alvarado JJ, Breedy O, Cruz-Barraza JA, Esquivel-Garrote O, Fernández-García C, Hermosillo A, Kaiser KL, Medina-Rosas P, Morales-Ramírez A, Pacheco C, Pérez-Matus A, Reyes-Bonilla H, Riosmena-Rodríguez R, Sánchez-Noguera C, Wieters EA, Zapata FA (2017) Marine biodiversity of Eastern Tropical Pacific coral reefs. In: Glynn PW, Manzello DP, Enoch AC (eds) Coral reefs of the Eastern Tropical Pacific. Springer, Dordrecht, pp 203–250
D’Croz L, O’Dea A (2007) Variability in upwelling along the Pacific shelf of Panama and implications for the distribution of nutrients and chlorophyll. Estuar Coast Shelf Sci 73:325–340
De’ath G, Fabricius KE, Sweatman H, Puotinen M (2012) The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc Natl Acad Sci USA 109:17995–17999
Dornelas M, Gotelli NJ, McGill B, Shimadzu H, Moyes F, Sievers C, Magurran AE (2014) Assemblage time series reveal biodiversity change but not systematic loss. Science 344:296–299
Elahi R, O’Connor MI, Byrnes JE, Dunic J, Eriksson BK, Hensel MJ, Kearns PJ (2015) Recent trends in local-scale marine biodiversity reflect community structure and human impacts. Curr Biol 25:1938–1943
Fiedler PC, Lavín MF (2017) Oceanographic conditions of the Eastern Tropical Pacific. In: Glynn PW, Manzello DP, Enoch AC (eds) Coral reefs of the Eastern Tropical Pacific. Springer, Dordrecht, pp 59–84
Francini-Filho RB, Moura RL, Thompson FL, Reis RM, Kaufman L, Kikuchi RK, Leão ZM (2008) Diseases leading to accelerated decline of reef corals in the largest South Atlantic reef complex (Abrolhos Bank, eastern Brazil). Mar Pollut Bull 56:1008–1014
Gardner TA, Côté IM, Gill JA, Grant A, Watkinson AR (2003) Long-term region-wide declines in Caribbean corals. Science 301:958–960
Glynn PW (1990) Coral mortality and disturbances to coral reefs in the Tropical Eastern Pacific. In: Glynn PW (ed) Global ecological consequences of the 1982–1983 El Niño-Southern Oscillation. Elsevier, Amsterdam, pp 141–182
Glynn PW, Macintyre IG (1977) Growth rate and age of coral reefs on the Pacific coast of Panama. In: Proceedings 3rd international coral reef symposium, vol 2, pp 251–259
Glynn PW, D’Croz L (1990) Experimental evidence for high temperature stress as the cause of El Niño-coincident coral mortality. Coral Reefs 8:181–191
Glynn PW, Maté JL, Stemann TA (2001a) Pavona chiriquiensis, a new species of zooxanthellate scleractinian coral (Cnidaria: Anthozoa: Agariciidae) from the Eastern Tropical Pacific. Bull Biol Soc Wash 10:210–225
Glynn PW, Maté JL, Baker AC, Calderón MO (2001b) Coral bleaching and mortality in Panama and Ecuador during the 1997–1998 El Niño-Southern Oscillation event: spatial/temporal patterns and comparisons with the 1982–1983 event. Bull Mar Sci 69:79–109
Glynn PW, Mones AB, Podesta GP, Colbert A, Colgan MW (2017a) El Niño-Southern Oscillation: effects on Eastern Pacific coral reefs and associated biota. In: Glynn PW, Manzello DP, Enochs IC (eds) Coral reefs of the Eastern Tropical Pacific. Springer, Netherlands, pp 251–290
Glynn PW, Alvarado JJ, Banks S, Cortés J, Feingold JS, Jiménez C, Maragos JE, Martínez P, Maté JL, Moanga DA, Navarrete S, Reyes-Bonilla H, Riegl B, Rivera F, Vargas-Angel B, Wieters EA, Zapata FA (2017b) Eastern Pacific coral reef provinces, coral community structure and composition: an overview. In: Glynn PW, Manzello DP, Enochs IC (eds) Coral reefs of the Eastern Tropical Pacific. Springer, Dordrecht, pp 107–176
Gonzalez A, Cardinale BJ, Allington GR, Byrnes J, Endsley KA, Brown DG, Hooper DU, Isbell F, O’Connor MI, Loreau M (2016) Estimating local biodiversity change: a critique of papers claiming no net loss of local diversity. Ecology 97:1949–1960
Graham NA, Cinner JE, Norström AV, Nyström M (2014) Coral reefs as novel ecosystems: embracing new futures. Curr Opin Environ Sustain 7:9–14
Graham NA, Bellwood DR, Cinner JE, Hughes TP, Norström AV, Nyström M (2013) Managing resilience to reverse phase shifts in coral reefs. Front Ecol Environ 11:541–548
Guzman HM, Holst I (1994) Inventario biológico y estado actual de los arrecifes coralinos a ambos lados del Canal de Panamá. Rev Biol Trop 42:493–514
Guzman HM, Cortés J (2007) Reef recovery 20 years after the 1982–1983 El Niño massive mortality. Mar Biol 151:401–411
Guzman HM, Robertson DR, Díaz ML (1991) Distribución y abundancia de corales en el arrecife del Refugio de Isla Iguana, Pacífico de Panamá. Rev Biol Trop 39:225–231
Guzman HM, Guevara CA, Breedy O (2004) Distribution, diversity, and conservation of coral reefs and coral communities in the largest marine protected area of Pacific Panama (Coiba Island). Environ Conserv J 31:111–121
Guzman HM, Benfield S, Breedy O, Mair JM (2008) Broadening reef conservation across the Tropical Eastern Pacific seascape: distribution and diversity of reefs in Las Perlas Archipelago, Panama. Environ Conserv J 35:1–9
Harrell FE, Dupont C Jr, (2015) Hmisc: Harrell miscellaneous. R package version 3.17-0. http://CRAN.R-project.org/package=Hmisc
Hill MO (1973) Diversity and evenness: a unifying notation and its consequences. Ecology 54:427–432
Hobbs RJ, Arico S, Aronson J, Baron JS, Bridgewater P, Cramer VA, Epstein PR, Ewel JJ, Klink CA, Lugo AE, Norton D, Ojima D, Richardson DM, Sanderson EW, Valladares F, Vilá M, Zomora R, Zobel M (2006) Novel ecosystems: theoretical and management aspects of the new ecological world order. Glob Ecol Biogeogr 15:1–7
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshw Res 50:839–866
Hueerkamp C, Glynn PW, D’Croz L, Maté JL, Colley SB (2001) Bleaching and recovery of five eastern Pacific corals in an El Niño-related temperature experiment. Bull Mar Sci 69:215–236
Hughes TP (1994) Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265:1547–1551
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J, Lough JM, Marshall P, Nystöm M, Palumbi SR, Pandolfi M, Rosen B, Roughgarden J (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301:929–933
Jacox MG, Hazen EL, Zaba KD, Rudnick DL, Edwards CA, Moore AM, Bograd SJ (2016) Impacts of the 2015–2016 El Niño on the California current system: early assessment and comparison to past events. Geophys Res Lett 43:7072–7080
Jost L (2006) Entropy and diversity. Oikos 113:363–375
Kindt R, Van Damme P, Simons AJ (2006) Tree diversity in western Kenya: using profiles to characterise richness and evenness. In: Hawksworth DL, Bull AT (eds) Forest diversity and management. Topics in biodiversity and conservation, vol 2. Springer, Dordrecht, pp 193–210
Kleypas JA, Yates KK (2009) Coral reefs and ocean acidification. Oceanography 284:118–120
Lamy T, Legendre P, Chancerelle Y, Siu G, Claudet J (2015) Understanding the spatio-temporal response of coral reef fish communities to natural disturbances: insights from beta-diversity decomposition. PLoS ONE 10:e0138696
Legendre P (2014) Interpreting the replacement and richness difference components of beta diversity. Glob Ecol Biogeogr 23:1324–1334
Legendre P, Cáceres M (2013) Beta diversity as the variance of community data: dissimilarity coefficients and partitioning. Ecol Lett 16:951–963
Legendre P, Salvat B (2015) Thirty-year recovery of mollusc communities after nuclear experimentations on Fangataufa atoll (Tuamotu, French Polynesia). Proc R Soc Lond B Biol Sci 282:20150750
Loh J, Green RE, Ricketts T, Lamoreux J, Jenkins M, Kapos V, Randers J (2005) The Living Planet Index: using species population time series to track trends in biodiversity. Philos Trans R Soc Lond B Biol Sci 360:289–295
Magurran AE, Henderson PA (2010) Temporal turnover and the maintenance of diversity in ecological assemblages. Philos Trans R Soc Lond B Biol Sci 365:3611–3620
Maté JL (2003) Corals and coral reefs of the Pacific coast of Panama. In: Cortés J (ed) Latin American coral reefs. Elsevier Science, Amsterdam, pp 387–418
Normile D (2016) El Niño’s warmth devastating reefs worldwide. Science 352:15–16
Oksanen JF, Blanchet G, Friendly M, Kindt R, Legendre P (2016) Vegan: community ecology package. R package version 2.4-0. http://CRAN.R-project.org/package=vegan
Pandolfi JM, Bradbury RH, Sala E, Hughes TP, Bjorndal KA, Cooke RG, Warner R (2003) Global trajectories of the long-term decline of coral reef ecosystems. Science 301:955–958
Pauly D (1995) Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol Evol 10:430
Peres-Neto PR, Legendre P, Dray S, Borcard D (2006) Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87:2614–2625
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2016) nlme: linear and nonlinear mixed effects models. R package version 3.1-128. http://CRAN.R-project.org/package=nlme
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rényi A (1961) On measurements of entropy and information. In: Neyman J (ed) Proceedings of the 4th Berkeley symposium on mathematical statistics and probability. University of California Press, Berkeley, USA
Reyes-Bonilla H (2002) Checklist of valid names and synonyms of stony corals (Anthozoa: Scleractinia) from the eastern Pacific. J Nat Hist 36:1–13
Riegl B, Purkis SJ, Keck J, Rowlands GP (2009) Monitored and modeled coral population dynamics and the refuge concept. Mar Pollut Bull 58:24–38
Skirving WJ, Strong AE, Liu G, Liu C, Arzayus F, Sapper J, Bayler E (2006) Extreme events and perturbations of coastal ecosystems. In: Richardson LL, LeDrew EF (eds) Remote sensing of aquatic coastal ecosystem processes. Springer, Netherlands, pp 11–25
Sweatman H, Delean S, Syms C (2011) Assessing loss of coral cover on Australia’s Great Barrier Reef over two decades, with implications for longer-term trends. Coral Reefs 30:521–531
Toth LT, Macintyre IG, Aronson RB (2017) Holocene reef development in the Eastern Tropical Pacific. In: Glynn PW, Manzello DP, Enochs IC (eds) Coral reefs of the Eastern Tropical Pacific. Springer, Dordrecht, pp 177–202
Xie SP, Xu H, Kessler WS, Nonaka M (2005) Air–sea interaction over the Eastern Pacific warm pool: gap winds, thermocline dome, and atmospheric convection. J Climate 18:5–20
Zuur A, Ieno EN, Smith GM (2007) Analyzing ecological data. Springer Science & Business Media, Berlin
Acknowledgements
The authors thank Carlos Guevara, Marcela Herrera, Jorge Morales, Carlos Vega, Aldo Croquer and Carlos Sanhil for assistance in the field. The Nature Conservancy and the Environmental Science Program of the Smithsonian Tropical Research Institute sponsored the monitoring project to HMG. Thanks to the Government of Panama for financial support to CG during data analysis and manuscript preparation (IFARHU-SENACYT) and to the Panamanian Ministry of Environment for providing the permits to work in protected areas. AG acknowledges the support of the Canada Research Chair program, a Killam Fellowship and an NSERC Discovery grant. AG and CG acknowledge the support of the Québec Centre for Biodiversity Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Biology Editor Dr. Andrew Hoey
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gomez, C.G., Gonzalez, A. & Guzman, H.M. Multiscale change in reef coral species diversity and composition in the Tropical Eastern Pacific. Coral Reefs 37, 105–120 (2018). https://doi.org/10.1007/s00338-017-1637-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-017-1637-y