Abstract
This study of the Kanna calcareous spring fen on Saaremaa, the largest island of Estonia, elucidates its history of fen development and vegetation diversity over the last 9,200 years. Pollen, spores, non-pollen palynomorphs, macrofossils, loss-on-ignition and humification index analyses were carried out to reconstruct fen succession, vegetation development, environmental changes and human impact. Hierarchical clustering, ordination analysis and linear regression were applied to examine the vegetation composition and richness patterns through time and to identify the potential environmental drivers underlying these patterns. Our results suggest reverse mire development from bog to fen, a rare occurrence and contrary to typical mire autogenic succession from groundwater fed to rainwater fed. Kanna developed as a small bog for the first 2,000 years from 9,200 to 7,200 cal yrs bp. Changes to the hydrological regime around 7,200 cal yrs bp, due to a warmer and drier climate and land uplift, caused a change from an ombrotrophic to a minerotrophic environment. Typical spring fen characteristics developed ca. 5,000 cal yrs bp and continued until ca. 400 cal yrs bp, when the fen was fed by calcareous mineral-rich groundwater and reached very high floristic diversity with various calciphilous and relict plant taxa. We conclude that general changes in the Kanna fen succession, vegetation community and diversity are associated with climatic changes. The present high diversity of the fen is a result of a long-term stable fen environment, which may have been even higher in the past. However, the pollen richness has decreased during the last 400 years, possibly due to human or natural factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Springs and spring fens are characterized by a continuous flow of cold water, uniform in temperature and rich in oxygen and minerals. The water in spring fens seeps up through the ground or flows on the peat surface, thereby enabling the growth of specialized vegetation. The invertebrate fauna is often specific to this habitat and the flora rich in northern taxa (IMEUH 2007). The water of calcareous spring fens is strongly alkaline, which leads to a specifically calciphilous vegetation and often to precipitation of carbonates or tufa (Rodwell 2016). Calcareous spring fens in Europe are classified into three biotopes by the Council Directive 92/43/EEC (Council of the European Communities 1992), calcareous fens with Cladium mariscus and species of the Caricion davallianae (code 7210), alkaline fens (code 7230), and petrifying springs with tufa formation (Cratoneuron) (code 7200).
Calcareous fens are hotspots of biodiversity (Hájková et al. 2012a), yet their area in Europe has declined by 50% during the last 50 years (Rodwell 2016). In many places they have disappeared from the agricultural landscape, having been selectively damaged and destroyed by artificial drainage, which has caused mineralization of nutrients in the fen sediment and the spread of nutrient-demanding plants. Fens can also be negatively affected to some extent by overly intensive livestock grazing (Breitsameter et al. 2017), which is a critical scientific and resource management issue for nature conservation (Wolf and Cooper 2015).
The decrease in the distribution and area of calcareous fens is continuing even in nature reserves due to overall eutrophication of the landscapes, water table decline and lack of management (Nilsson 2016). Calcareous fens hold high conservation priority in Europe owing to their increasing rarity and declining quality (Šefferová Stanová et al. 2008).
Many of the remaining calcareous fens are located in nature reserves. There are also numerous restoration projects on fens and their biodiversity (Bragg and Lindsay 2003; Minayeva et al. 2017). Restoration is done mainly by re-wetting and management by mowing, cutting shrubs or extensive cattle stocking (Breitsameter et al. 2017). The complex nature of fen reconstruction requires background knowledge from several different scientific fields. To date most fen research concentrates on contemporary processes or relatively short-term effects of management, water chemistry and recent climate change (Jordan et al. 2007; Rozbrojová and Hájek 2008; Laitinen et al. 2011; Völlm and Tanneberger 2014; Priede et al. 2016).
Spring fens are well suited to palaeoecological studies. The permanently wet conditions in fens help to preserve pollen, spores and plant macroremains, which results in sediment records that offer insights on the long-term development of fens and their surrounding landscapes throughout their entire history. Although the palaeoecology of calcareous rich fens in central Europe has been relatively well studied (Hájek et al. 2011; Hájková et al. 2012a, b, 2013, 2015; Pidek et al. 2012; Jamrichová et al. 2014; Galka et al. 2017, 2018), there are few such studies from northern Europe (but see Galka et al. 2016; Väliranta et al. 2017).
Estonia can be considered the leading country in Europe in terms of density of calcareous spring fens (Šefferová Stanová et al. 2008), which occupy approximately 900 ha (ca. 0.02% of the state area). About half of Estonian spring fens are located on Saaremaa, mostly along the slopes of the Saaremaa keskkõrgustik (west Saaremaa upland) (Paal and Leibak 2011). More than one-third of previously known spring fens have disappeared or become degraded (Paal and Leibak 2011). Numerous rare plant species are associated with spring fens in Estonia, such as Rhinanthus osiliensis, Selaginella selaginoides, Saxifraga hirculus, Dactylorhiza incarnata, D. baltica, D. russowii and Epipactis palustris. Many fen plants such as Juncus subnodulosus, Selaginella selaginoides, Pinguicula alpina, Cladium mariscus are considered to be relicts of former climatic conditions (Ilomets et al. 2010). Several palaeoecological studies on Saaremaa have focused on the history of vegetation development, shore displacement and human impact, for example from Surusoo (Veski 1996; Poska and Saarse 2002), Vedruka (Poska and Saarse 2002), Pitkasoo (Königsson and Poska 1998) and Jõhvikasoo (Hansson et al. 1996). However, all these studies examined present-day bogs rather than fens.
The primary aim of this study was to study the development of spring fens by using palaeo-reconstructions from the Kanna calcareous spring fen in Viidumäe looduskaitseala (Viidumäe nature reserve), Saaremaa, Estonia, to reconstruct its historical development, vegetation succession and vegetation diversity patterns. We used palaeoecological data from pollen and spores, macrofossils, non-pollen palynomorphs (NPPs) and physical characteristics of the sediment core. In addition, we aimed to better understand the main processes and environmental factors, for example grazing, drainage and climate change, that are likely to have affected the particular development of this fen.
Study area
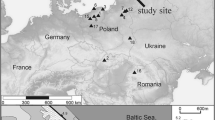
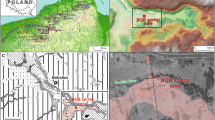
Kanna spring fen (22.096721°E; 58.325031°N) is situated in Viidumäe looduskaitseala (nature reserve) in the western part of Saaremaa (Fig. 1) on the slope of the higher part of the Saaremaa keskkõrgustik (west Saaremaa upland), 29 to 32 m a.s.l. The highest point of the nature reserve is 59 m, and lies on the highest and oldest part of the island, which emerged from the sea in the Baltic basin at about 11,600 years bp (Saarse and Vassiljev 2010). Saaremaa lies on the western edge of the East European Shield with the bedrock composed entirely of Silurian carbonate rocks, primarily limestone. Large parts of the island surface are either covered by glacial sediments from the last Ice Age or reworked by waters of the Baltic Sea (Saarse et al. 2009).
The climate on Saaremaa is more oceanic than the Estonian mainland. The end of spring and beginning of summer are the driest periods with 34 to 67 mm of precipitation out of a 550–820 mm yearly average. The mean air temperature on the western coast of Saaremaa is − 3.5 to − 4 °C in February, 3°–4° warmer than in eastern Estonia. The mean July temperature is ca. 19 °C (Riigi Ilmateenistus 2018). The nature reserve that surrounds Kanna spring fen, which was founded in 1957, covers 2,598 ha. About 85% of the reserve is covered with woods in which Pinus sylvestris, Picea abies, Betula pubescens, Alnus glutinosa and Quercus robur are currently the most common trees. These woods are scattered among traditionally managed meadows, wooded meadows, fens and bogs. The reserve is situated along the coastal escarpment of the former Ancylus Lake (the stage of the Baltic Sea ca. 10,700–9,800 cal bp, with a maximum water level here about 31.5 m) and the spring fens are located at the foot of a relatively steep slope (25°–30°) from which the calcareous ground water flows. Due to the diverse natural conditions, the nature reserve is extremely rich in plant taxa. Approximately 700 species of vascular plants grow in the reserve (about half of the total Estonian flora), of which 58 are designated as protected in Estonia, including Rhinanthus osiliensis (Saaremaa yellow rattle), an endemic species growing only in spring fens on Saaremaa (Talve et al. 2014).
The highest and oldest part of Saaremaa also has the oldest human settlements on the island. Loona, an early Neolithic settlement, covered by a large Bronze Age stone cist grave field and burials from the 13th century ad, is situated ca. 4 km west of the Kanna site. There is also a pre-Viking and early Viking sacrificial site dating to ad 600–900, 2 km south of the fen (Mägi et al. 2014). These archaeological sites prove that Saaremaa was inhabited relatively soon after its emergence from the sea and was one of the most densely populated regions in Estonia in the 11th–13th centuries ad (Jänes-Kapp et al. 2007). The closest farms to the Kanna site are approximately 500 m from it and were inhabited until the mid 20th century; the hay meadows of Tammiku farm are still not overgrown by woods. The closest currently inhabited farms in the villages of Viidu, Viki and Vedruka are ca. 2 km away in different directions.
The Kanna site is a typical example of spring fens in western Saaremaa with vegetation belonging to the Scorpidio-Schoenetum association according to Paal (1997). The local vegetation is dominated by Schoenus ferrugineus. The subdominant taxa include other Cyperaceae (Carex panicea, C. hostiana, Eriophorum latifolium), Poaceae (Phragmites australis, Molinia caerulea), Juncus subnodulosus and Menyanthes trifoliata. There are numerous forbs growing in low frequencies, Pinguicula vulgaris, Drosera anglica, D. rotundifolia, Parnassia palustris, Epipactis palustris, Dactylorhiza incarnata etc.
Materials and methods
Sampling
The sediment was sampled in the part of Kanna fen with the thickest peat layer in October 2016 (Fig. 1c). Three replicate peat cores were obtained with lengths of 228, 230 and 231 cm, using a Russian-type peat sampler. The cores were described visually in the field, wrapped in plastic, transported to the laboratory and stored at 4 °C.
Three samples from Tauber pollen traps, installed and used according to the Pollen Monitoring Programme (PMP) guidelines (Hicks et al. 1996), were analysed to associate the sedimentary pollen data with the pollen rain from modern vegetation. In addition, four moss polster samples were collected following the sampling protocol by Bunting et al. (2013).
Chronology
The age-depth graph of the Kanna core was drawn using the OxCal 4.2.4 deposition model (Bronk Ramsey 2008, 2009) and the IntCal13 calibration curve (Reimer et al. 2013), combining lithological boundaries, dates from spherical fly ash particle (SFAP) analysis and radiocarbon dates from accelerator mass spectrometry (AMS). Altogether 11 AMS radiocarbon dates were obtained from terrestrial macrofossils, plant remains, wood twigs, bark pieces, charcoal, and bulk peat samples by the Poznań Radiocarbon Laboratory (Table 1).
In order to improve the age-depth model in the upper 17 cm of the core, we used an indirect dating method of spherical fly-ash particles (SFAP), the by-product of high-temperature combustion of fossil fuels, which are an important indicator of environmental pollution and atmospheric deposition from industrialization (Zhang et al. 2011). The sample preparation followed the method of Renberg and Wik (1985) to remove organic matter with H2O2. For the fen peat, we also used 3 M HCl treatment to remove carbonates (Rose 1990). A known amount of Lycopodium spores was added for the SFAP concentration calculations. SFAP particles together with Lycopodium spores were counted with a light microscope at × 125 magnification. The distribution of the SFAP concentration was compared with the known fossil fuel combustion history to obtain dates (Alliksaar 2000; Heinsalu et al. 2007).
Analysis of peat physical properties
Loss on ignition (LOI) was used to measure the organic matter content of the sediment (OM). This method provides one of the most widely used simple physical lines of proxy evidence in palaeolimnology (Dean 1974; Boyle 2004) and has been found to be a remarkably good summarising proxy for many changes in different ecosystems. In addition, water, mineral matter (MM) and carbonate (CC) contents were estimated. Altogether 225 samples, representing the complete core at 1 cm intervals, were used for analysis. All heating and weighing procedures followed the protocols by Matthiesen et al. (2005) and Hoogsteen et al. (2015). Peat humification, an indicator of the degree of peat breakdown or decomposition, was estimated for every 5 cm of the sediment core at Eesti Geoloogiakeskus (Geological Survey of Estonia). The analyses were carried out using the centrifuging method designed for determination of the disintegration degree of the peat according to the Russian peat standard GOST 10650-71 (Malterer et al. 1992).
Pollen and non-pollen palynomorph (NPP) analysis
For pollen analysis, 1 cm3 subsamples of material were taken at 5 cm intervals from most of the core, but the uppermost 10 cm was studied at 1 cm intervals due to particular interest in modern disturbances related to fen drainage or grazing. The samples were treated with HCl and 10% KOH followed by the standard acetolysis method (Berglund and Ralska-Jasiewiczowa 1986; Fægri and Iversen 1989). Altogether 53 samples were examined under a light microscope with magnifications of × 250, × 400 and × 1,000. Approximately 1,000 pollen grains were counted from each sample (min = 920, max = 1,115). The pollen collection at the Department of Geology at Tallinn University of Technology and relevant literature (Reille 1992; Beug 2004) were used to assist identification. Various types of non-pollen palynomorphs (NPPs) such as Trichocladium, Arcella sp., Assulina sp., Podospora, Neurospora and Habrotrocha angusticollis etc., were recorded together with the pollen and identified using existing literature and published photographs (Ohenoja 1995; van Geel 2001; Cugny et al. 2010; Miola 2012; López-Vila et al. 2014; Shumilovskikh et al. 2015). Spores and NPPs were excluded from the pollen count, but their percentages were calculated in relation to the pollen sum (Chambers et al. 2011a). In addition to pollen and NPPs, microscopic charcoal particles were noted and categorized in two groups, 10–100 µm and > 100 µm.
A detailed pollen diagram that includes all the identified taxa and spores was created using TILIA v.1.7.16 (ESM; Grimm 2011). In addition to the sedimentary samples, four moss polster and three Tauber trap samples were analysed to characterize the present-day pollen rain.
Macrofossil analysis
For macrofossil analysis, the peat sequence was cut into 10 cm thick layers, from which samples of 5 cm3 were taken. The material was soaked until it was wet, transferred to a Petri dish and scanned with a stereo microscope at × 20–120 magnification and/or light microscope at 160 ×. Fruits and better preserved vegetative tissues were examined with a light microscope and identified using atlases (Dombrovskaya et al. 1959; Katz et al. 1977) and a personal reference collection. Additionally, the whole of each sample was searched for the seeds. The percentages of plant vegetative remains were estimated as the average from five random views of three Petri dishes (in total 15 views) during the scans (Mauquoy et al. 2010). The plant and peat type systematics follow Levesque et al. (1988). The percentage volume of peat composition was estimated based on three main vegetative types of remains, lignosae (comprising woody parts or taxa with hard lignified tissues, especially stems), herbaceae (herbaceous plants), and muscinae (modern Bryophyta).
Background climate data
To test the climate forcing effect (the effect of the difference between sun energy absorbed by the Earth and energy radiated back into space, thus warming or cooling of climate) on vegetation, we used climate data from a transient simulation experiment with the ECBilt-CLIO-VECODE climate model, forced by annually changing values of orbital parameters, atmospheric CO2 and CH4 concentrations, ice sheet surface albedo (the proportion of sunlight that is reflected rather than being absorbed), topography and melt flux (Renssen et al. 2009). The ECBilt-CLIO-VECODE temperature reconstructions cover the world at 5.6° × 5.6° spatial resolution and monthly temporal resolution (Renssen et al. 2009). Local polynomial regression fitting (LOESS) with a span of 0.1 was used to estimate temperatures for those years analysed for pollen and other sedimentary proxies. The average modelled summer (May–August) and winter (December–February) temperatures for western Estonia were used. Temperatures were expressed as the difference from the pre-industrial mean simulated in the climate model (250–550 cal bp).
Pollen richness
Pollen richness is usually calculated by rarefaction with respect to established numbers of counted pollen grains (Birks and Line 1992). Andersen-transformed richness, by which the dominant tree taxa are down-weighted to reduce the bias from their high pollen production on the richness estimate, has shown higher correlations with vegetation richness than the traditional rarefied richness (Felde et al. 2016; Reitalu et al. 2019) and was therefore used in our study. The pollen sums of common tree taxa were multiplied by the Andersen transformation factors (Andersen 1970) and rarefaction was performed on the transformed sum. We also used the traditional rarefaction-based pollen richness estimate to compare with earlier pollen richness studies.
Statistical analysis
CONISS, temporally constrained hierarchical clustering (Grimm 1987) based on Bray–Curtis vegetation dissimilarity (Faith et al. 1987) was used to estimate stratigraphic zones in the pollen data. The broken stick method (Jackson 1993) was used to evaluate the number of significant stratigraphic clusters. Principal component analysis (PCA) with Hellinger-transformed pollen percentages was used for unconstrained ordination. Hellinger transformation together with PCA provides a Euclidean distance based method suitable for ecological data (Legendre and Gallagher 2001). The number of significant PCA axes was estimated based on the broken stick method. We used the envfit() function, which fits environmental factors onto an ordination (Oksanen et al. 2017), to investigate the association of pollen data with environmental variables and non-pollen palynomorphs. In addition, the envfit() function uses permutations to test the significance of each of the environmental variables in relation to the ordination, thereby providing an r2 estimate (squared correlation coefficient) that reflects the strength of the association between the variable and the ordination axes.
In order to study which environmental parameters best explain the trend in pollen richness, we used linear regression analysis with the following explanatory parameters: LOI data (water, OM, MM, CC), humification, charcoal, the proportion of cultivated plants, sedimentation rate and modelled summer and winter temperatures. To clarify which of the explanatory variables significantly explained pollen richness in the core, we used a backward selection of variables in the multiple regression in which only significant variables (p < 0.05) remained in the model. The temporal autocorrelation of the residuals was tested using the autocorrelation function (ACF) from the nlme package (Pinheiro et al. 2019).
The community ecology package vegan (Oksanen et al. 2017), Quaternary science package rioja (Juggins 2017) and R environment v. 3.4.4 (R Core Team 2014) were used for the other statistical analyses.
Results
Chronology and lithology
The Kanna core consisted of homogeneous, highly decomposed peat and plant macrofossils. The bottom 5 cm of sediment was rich in coarse sand accompanied by organic material. The presence of calcareous tufa was observed on the top of the fen surface as well as in water pools and rivulets, but there were no visible traces of tufa within the sediment core.
The age-depth model for Kanna was constructed based on eight radiocarbon dates and three SFAP reference level derived ages (Table 1). The radiocarbon date at 40 cm (Poz-89179) was a clear outlier with a much older age than expected and was thus removed from the age-depth model. The date at 10 cm (Poz-99538) was ca. 40 years younger than suggested by SFAP and was also considered as an outlier. The dates at 25 and 40 cm (Poz-99538, Poz-99569) had reverse ages. The Poz-99538 date seems to be too old compared with the SFAP derived age and it suggests that there must be an about 1,000 year long hiatus in peat accumulation above 25 cm depth. That is however, not supported by the sediment lithology which shows continuous peat accumulation. Turney et al. (2000) reported that Carex macrofossils could have anomalously old radiocarbon ages and therefore Poz-99538 is considered as an outlier.
Based on the age-depth model (Fig. 2), the accumulation of peat at Kanna started ca. 9,200 cal bp and overall, it was generally uniform and continuous at a rate of 0.1–2.1 mm year−1. The top 15 cm showed faster peat accumulation at 1.3–2.1 mm year−1.
Physical properties of the peat
The sediment sequence contained on average 89.9% water, with four major water-based phases (Fig. 3). At the beginning of peat accumulation ca. 9,200 to 7,200 cal bp, water content is 90% on average and decreases around 7,200 cal bp. The second phase until 5,000 cal bp shows a decrease in water content reaching some of its lowest values. The maximum water content was found between ca. 5,000 to 3,000 cal bp, followed by a gradual drop to the present day. Organic matter (OM) values are on average 86.6% of the dry mass, and show an opposite trend to water content, except during the last 400 years, when there are the lowest (79.3%) and highest (91.2%) values. A decrease in OM occurs around ca. 7,000 cal bp. Mineral matter (MM) variability is 8–20% with a low variation from 8 to 11% occurring from 4,000 to 1,000 cal bp. The highest values of 20% MM occur ca. 50 to 80 cal bp and the highest variation of 8–20% is found during the last 100 years. The carbonate content (CC) in the sediment sequence is only 1.3% on average. The highest CC (3.6%) is at the top of the sediment core and tufa was also observed on the surface of the site during fieldwork. The period from 4,000 to 1,000 cal bp is a phase of comparatively high CC (1–2.5%), which coincides with the lowest MM content.
Physical properties and background environmental conditions in Kanna spring fen. Sediment carbonate content (CC), mineral matter (MM), organic matter (OM), water content (Water) based on loss on ignition (%LOI). Peat humification, sediment accumulation rate (AR), charcoal, cultivation (proportion of cereal pollen). Temperature, average deviations of modelled summer (May–August) and winter (December-February) values from pre-industrial mean (Renssen et al. 2009), and stratigraphic zones based on hierarchical clustering of pollen data from the peat core
The average peat humification of Kanna fen is 22%, with the lowest values (17%) occurring during the first phase of the site’s development between 9,000 and 8,000 cal bp. A gradual increase in humification began around 400 cal yrs bp, reaching > 30% in the top 10 cm of the core.
There is a notable peak in charcoal dust at around 2,500 cal bp and relatively high values up to 800 cal bp.
Pollen analysis results
Hierarchical clustering of pollen data and the broken stick model have distinguished three significant (p < 0.05) pollen stratigraphic (assemblage) zones, KAN1–3 in the pollen diagram from Kanna (Fig. 4, ESM).
During the earliest period from 9,200 to 7,200 cal bp (zone KAN1), the local landscape was colonized mainly by pine woods, with Pinus pollen representing 50 to 70% of the total. In contrast to other zones, pollen percentages of low growing perennial shrubs, Calluna vulgaris, Ledum and other Ericales, and of the deciduous shrub Myrica gale were high. This zone was characterized by a notable peak of Sphagnum spores, which together with Tilletia sphagni indicate that Sphagnum was present locally.
Zone KAN2 spans the longest time period from ca. 7,200 to 400 cal bp and is characterized by a rapid decline in Pinus, with pollen percentages down to 30%. Pine was replaced by deciduous trees characteristic of warmer conditions, such as Alnus glutinosa/incana, Tilia cordata, Ulmus glabra/laevis and Quercus robur. Pollen proportions of Ericales, Calluna and Ledum decrease and those of a variety of other pollen types increase, indicate a gradual replacement of these shrubs by herbaceous vegetation. The zone is characterised by the presence of various insect pollinated and calciphilous taxa such as Potentilla (most probably P. erecta) and Parnassia palustris. The first signs of cultivation associated with the appearance of cereal pollen around 3,500 cal bp appear here, followed by a gradual but variable increase in pollen types which indicate human impact.
Zone KAN3 (from ca. 400 to 0 cal bp) is characterized mainly by taxa indicative of human activity. Pinus pollen is dominant again, comprising 60% of total pollen. The peaks of Juniperus pollen indicate pasture in the landscape. The appearance of Secale, Triticum and Hordeum at the end of KAN2 indicates cereal growing and farming. A number of ruderal taxa, such as Artemisia, Chenopodiaceae and Apiaceae, attain their highest values here, accentuating evidence of human activity. Also, Poaceae pollen levels decrease slightly, while Cyperaceae increase sharply to the highest values among the non-arboreal pollen.
In the following text, these zones are referred to as KAN1—bog phase, KAN2—fen phase and KAN3—modern phase.
Non-pollen palynomorphs (NPP)
The pollen slides from Kanna also revealed several different types of testate amoebae, fungi, zoological remains and charcoal (Fig. 5).
The earliest stratigraphic zone (bog phase, from ca. 9,200 to 7,200 cal bp), was characterized by peaks of various NPPs, most notably the peak of fungal hyphae. Pinus pollen together with dark-coloured hyphae was recorded only in this stage. This time period was rich in testate amoebae, especially testaceans of Assulina muscorum, Arcella type and Trigonopyxia arcula.
During the natural fen phase, (ca. 7,200 to 400 cal bp), testate amoebae are largely absent, except for Centropyxis. There is a noticeable peak of charcoal around 2,000 cal bp. Ascospores of Gelasinospora, Podospora and Coniochaeta and the good tree cover indicator Trichocladium (HdV-572) are also present. Fungal hyphae have relatively lower values than in the earlier phase.
Towards more recent time (modern phase, ca. 400 cal bp to present), testate amoebae increase, with the re-appearance of Arcella type and the highest values of Centropyxis type. Loricae of the rotifer Habrotrocha angusticollis are one of the most notable NPPs found from the last hundred years, coinciding with a rise in fungal hyphae.
Macrofossil analysis
The high level of peat decomposition limited the number of identifiable taxa found in the macrofossil analysis (Fig. 6). Based on this, the peat composition does not show high variability during its accumulation period and the three main phases which characterize local vegetation development can be recognised. The lower part of the core (the bog phase, ca. 9,200 to 7,200 cal bp) consisted mainly of Sphagnum moss peat including some Carex remains. Sphagnum peat was absent in the next zone (fen phase, ca. 7,200–400 cal bp), which was dominated by 80% herbaceous peat and ~ 20% wood remains. The herbaceous peat in this phase contained Carex spp. and Cladium mariscus; the wood consisted of Pinus sylvestris and Betula pubescens/pendula. The sediment of the modern phase (20–0 cm, ca. 400 cal bp to present) was comprised of herbaceous peat throughout, mainly composed of Carex spp. and with a presence of Comarum palustre.
Pollen richness
At the beginning of sediment accumulation in the early Holocene (ca. 9,200 cal bp), the pollen richness is relatively low, with an increasing trend (Figs. 4, 7). The highest pollen richness occurs from ca. 7,000 to 1,000 cal bp, during the natural fen phase. Throughout most of the period covered by the site, the richness is considerably higher than the average for Estonia (Fig. 7), but the rapid decrease in richness towards the present day is contrary to the general richness trend there.
Rarefied richness [pollen sum of 411−E(T411)] representing the average diversity in Estonia (Reitalu et al. 2015) and at Kanna
The results of linear regression with backward selection show that pollen richness is significantly positively associated with winter temperature, summer temperature and the amount of charcoal dust (Table 2).
Principal component analysis (PCA)
The broken stick method shows two significant axes for the PCA which explain 52 and 24% of the total variation in the pollen data, respectively (Fig. 8). The results show that taxa such as Pinus, Juniperus, Cyperaceae and cultivated plants (Secale, Triticum, Hordeum) are positively associated with the first PCA axis, but most of the thermophilous taxa, Corylus, Tilia, Ulmus, Quercus and Fagus, are negatively associated. Juniperus, Picea, Quercus, Chenopodiaceae and Parnassia are positively associated with the second PCA axis, whereas the low-growing perennial shrubs Calluna, Ericales, Myrica-type and Ledum are negatively associated.
Results of ordination by PCA, representing samples in clusters from the hierarchical clustering analysis; pollen taxa with selected environmental variables superimposed on the PCA plot. Aster, Asteraceae; Bet, Betula; Call, Calluna vulgaris; Chenopod, Chenopodiaceae; Clad, Cladium mariscus; Cory, Corylus; Cyper, Cyperaceae; Eric, Ericaceae; Frax, Fraxinus; Hipp, Hippophae rhamnoides; Hord, Hordeum; Hum/Cann, Humulus/Cannabis-type; Junip, Juniperus; Led, Ledum; Myr, Myrica gale; Parn, Parnassia; Pic, Picea; Pin, Pinus; Plant, Plantago m/m; Pot, Potentilla; Quer, Quercus; Ran, Ranunculus; Sec, Secale; Solan, Solanum dulcamara; Succ, Succisa; Sphag, Sphagnum; Til, Tilia; Trit, Triticum; Ulm, Ulmus; Umbelif, Apiaceae; Urt, Urtica
Of the environmental variables, organic matter (OM), and humification are positively associated with the first PCA axis, whereas carbonate content (CC), water content and mean summer temperature show negative associations (Fig. 9). The mineral matter (MM) and mean summer temperature are negatively associated with the second PCA axis. Positive associations with the second axis are shown by OM, CC and mean winter temperature. All the tested environmental variables have significant associations with pollen ordination (p < 0.05). The strongest associations with the PC axis are for mean winter temperature (r2 = 0.90, p < 0.001), summer temperature (r2 = 0.73, p < 0.001) and humification (r2 = 0.50, p < 0.001).
The associations between pollen ordination and NPPs show that 14 NPPs are significantly associated with the PC axes (Fig. 9). Among the NPPs, testate amoebae, Habrotrocha angusticolis, Centropyxis type and fungal hyphae have a positive association with PC1, but charcoal, Neurospora and Trichocladium are negatively associated with it. Assulina muscorum, Arcella-type, the remaining testate amoebae and fungal hyphae show negative associations with PC2. Habrotrocha angusticollis (r2 = 0.31, p < 0.001), Centropyxis (r2 = 0.39, p < 0.001) and charcoal (r2 = 0.29, p < 0.001) show the strongest associations with the PC axes.
Discussion
Our study provides a fine example of how a bog can change into a fen due to a unique combination of climatic, topographic and hydrological conditions. The geographically closest comparable published research describing such fen development and vegetation succession is from Apšuciems mire on the southeast Baltic coast of Latvia (Gałka et al. 2016), which developed as did our site, due to active water level changes, at Kanna in a depression on the slope of the Saaremaa upland around 9,200 cal bp.
The only comparable study related to calcareous fen development in Estonia is the unpublished work by Helle Mäemets and Kersti Kihno, in which two short cores (< 1 m) and one long one (2.5 m) were analysed for pollen and are mentioned in Ratas and Kokovkin (1989). This resulted in a pollen diagram for arboreal taxa in which non-arboreal taxa were noted only as presence or absence. Unfortunately, the original count data have been lost, thus impeding direct comparisons with our study. However, the beginning of sediment accumulation at approximately 9,000 cal bp is consistent with our study. Although well-chosen plant macrofossils are considered reliable dating material (Hatté and Jull 2013), the age-depth model reconstruction for the top 40 cm of the Kanna core was complicated, as the samples close to the surface provided inconsistent dates. Having an outlier in a dating series is a common phenomenon, but fen environments can have complicating factors, such as surface flooding, periodic droughts, root intrusion or possible trampling by grazing animals, which might cause dates to deviate from the norm (Väliranta et al. 2014; Wolf and Cooper 2015). According to the date Poz-99538, there might be a hiatus in peat accumulation (Table 1), however, this possibility was dismissed since it disagreed with the SFAP-derived age. In addition, the hiatus would span 1,000 years, which is not supported by the sediment lithology. First, there is continuous peat accumulation and no corresponding discontinuity in other data as for example in mineralisation or erosion, like that recorded by Hájková et al. (2015). Secondly, there are no climatic reasons to suspect a hiatus, which would be more usual in the mid Holocene as a result of the warmer and dryer climate then (Rybníček and Rybníčková 1987; Ammann et al. 2013). In our case, the possible hiatus is during the period when the climate was getting wetter (Hammarlund et al. 2003).
Overall, the Kanna age-depth model appears to be reliable and fits with existing knowledge of vegetation and coastal development in this region.
Bog phase (ca. 9,200–7,200 cal yrs bp)
The beginning of sediment accumulation in the fen around 9,200 cal bp corresponds to the time of the former Initial Litorina Sea (Berglund et al. 2005), when the local water level at Kanna dropped to between 15 and 17 m a.s.l. (present level) (Saarse et al. 2009). This decrease in the water level and loss of water contact between the depression on the slope and the Initial Litorina Sea resulted in the emergence of an isolated water body. The isolation and increasing role of precipitation then led to the development of ombrotrophic vegetation (fed by precipitation rather than ground water) there, as suggested by the pollen and spore assemblage including Calluna vulgaris, other ericaceous taxa and the great abundance of Sphagnum (Fig. 4). It is a typical scenario with the Sphagnum mosses forming the main part of the ombrotrophic peat, as in a raised bog (Clymo 1963), and it fully agrees with the results of the macrofossil analysis (Fig. 5), demonstrating that at the beginning of its accumulation the peat was comprised mainly of Sphagnum moss (up to 80%). Analysis of NPPs also suggests ombrotrophic conditions from the abundant presence of the testate amoebae Assulina muscorum, Arcella-type and Trigonopyxis, while A. muscorum is regarded as typical of the early stages of raised bog formation (Mazei and Bubnova 2007). The pollen of calciphilous plants is recorded in low numbers, so the role of calcium rich groundwater is not clear, considering that the depression in which the sediment accumulation started is lower than the point from which spring water escapes. It has been shown that peat moss can acidify its surroundings by taking up cations, including calcium, and by releasing hydrogen ions (Clymo 1963), and this effect might have reduced the impact of calcium rich groundwater.
The loss on ignition results suggest an increasing input of mineral rich water at around 8,500 cal bp, coinciding with the lowest values of peat humification (Fig. 3). The humification index has also been used to interpret past changes in regional climate, especially changes in wetness (Chambers et al. 2011b). The increase in water content corresponds to the time of the rise in the level of the former Litorina Sea, caused by an increase in ocean water mass due to the final melting of the Laurentide Ice Sheet (Berglund et al. 2005). As the bog surface wetness is believed to be affected primarily by precipitation and temperature (Sillasoo et al. 2007), the increase in carbonate content and low humification level around 8,500 cal bp in Kanna could be attributed to an increase in groundwater discharge caused by such a rise in the level of the Litorina Sea.
Since the beginning of mire development around 9,200 cal bp and throughout the bog phase, the pollen richness is relatively low. Bogs are usually characterized by low but very distinct biodiversity (Lachance and Lavoie 2004). In addition, a potentially slow spread or delay in the spread of vegetation to land that had recently emerged from the sea could also be associated with low pollen diversity (Giesecke et al. 2012; Matthias et al. 2015).
Bog to fen transition, natural fen phase and early human impact (ca. 7,200–400 cal yrs bp)
The results of hierarchical clustering suggest changes in the vegetation as a transition, starting around 7,200 cal bp. The first 2,000 years of the second stratigraphic zone (KAN2) can be considered a transition phase between bog and fen and are characterized by the inherent properties of both phases. Declining levels of Sphagnum and ericaceous taxa, as well as an increase in various herbs such as Cyperaceae, Potentilla, Parnassia and Ranunculus suggest a change from ombrotrophic to more minerotrophic conditions that coincided with higher summer temperatures (Renssen et al. 2009) and further decreasing water levels in the Baltic Sea (Saarse et al. 2009). Although pinpointing the causes of the change is difficult, we can speculate that desiccation of the bog during a phase of warmer and drier climate led to an increasing role of mineral-rich surface water that started to flow through the mire. Hájková et al. (2012a) showed in her study that a similar change from bog to fen was related to bog surface desiccation due to a climatically-caused unstable water regime, resulting in peat surface layer decomposition and an increase in shrub and tree cover. Macrofossil analysis from Kanna shows that 30% of the peat in this phase was composed of woody material, suggesting that the surface of the site was drier and the peat was thin enough for woody plants to grow on it (Fig. 5). A decrease in water content (Fig. 3) and disappearance of most of the testate amoebae (Fig. 6) also suggest drier conditions.
Myrica gale growing in fens has been associated with an oceanic climate and open mires with slightly acidic and relatively shallow peat (Skene et al. 2000). The decrease in Myrica in the transitional phase at Kanna can be related to a decrease in Sphagnum which caused changes in pH, or to increasing woodland cover. Today Myrica still grows on the margins of Kanna and abundantly on both fen and bog margins in the western part of Estonia. The appearance of Cladium and an increasing carbonate content (Fig. 3) agree with the specific environmental conditions reported for Cladium mariscus in fens, shallow water and high calcium content (Rodwell 1995). These records of Cladium correspond to a time when it was more widespread throughout Europe (Salmina 2004; Hájková et al. 2013) and they are consistent with findings from the southeast Baltic coast (Gałka et al. 2016).
The transition phase exhibits a rapid increase in pollen richness, with the highest values around 6,000 cal bp. This features characteristics of both bog and fen phases, which probably accounts for the high local vegetation diversity. Low diversity at the beginning of the Holocene and a steady subsequent increase have been recorded in a regional study of several sites in Estonia and northern Latvia, and attributed to warmer and/or drier conditions (Fig. 7; Reitalu et al. 2015). Linear regression confirms the climatic effect on the pollen richness of Kanna, with a positive association for both modelled mean winter and summer temperatures (Table 2). The period from ca. 7,500 to 4,000 cal bp is associated on Saaremaa with rapid land emergence from the sea (Poska and Saarse 2002), resulting in the availability of new habitats and arrival of new plants, and therefore an increase in biodiversity.
Around 4,000 cal bp the site probably already had the characteristics of a fen and running surface water. A high abundance of Comarum palustre and Parnassia palustris, both typical of open seepage areas, was recorded together with the pollen of other insect-pollinated plants such as Epipactis palustris, Linum and Iris-type. An abundance of herbaceous plants has been shown to lead to increased biomass, which, in turn, leads to increased soil organic matter (Weil and Brady 1985), as demonstrated by our loss on ignition results. At the same time, the increase in biomass caused a decrease in soil minerals, which serve directly and indirectly as both sources and sinks of essential plant nutrients (Hawkesford et al. 2012). In general, from ca. 4,000 to the last 1,000 cal bp, the low variability in organic and mineral matter suggests a relatively stable fen environment.
The fen phase exhibits relatively high pollen richness and high variability. The pollen richness values from the bog and fen phases are similar to present-day diversity in bogs and mineral-rich fens. The greater richness during the fen phase is due to the increase in pH caused by an increase in calcium concentration, thereby allowing the coexistence of many calciphilous plants (Tahvanainen 2004; Rozbrojová and Hájek 2008) and relating to the large number of such taxa found in northern Europe (Zobel et al. 2011).
The first evidence of human activity here is indicated by the appearance of cultivated plants, with the first cereal grains appearing ca. 3,500 cal bp. (Figure 3) Earlier evidence of cereal cultivation in the region, from Vedruka and Pitkasoo mires, has been dated to ca. 4,200–4,000 cal bp (Poska and Saarse 2002). An abundance of Juniperus communis pollen, a light demanding shrub and indicator of more open vegetation at this time could be related to increasing pasture in the landscape. High charcoal presence in the sediments between 3,000 and 1,000 cal bp (Fig. 3) coincides with the evidence of cereal growing and suggests the use of fire in landscape management for slash and burn agriculture, rather than natural fires. Earlier studies suggest that the use of fire in landscape management on Saaremaa started ca. 4,500 cal bp (Poska and Saarse 2002). Our results indicate that the early agriculture caused no visible disturbance to fen development, probably because the fen is relatively far, > 500 m from the closest suitable agricultural areas.
Modern phase (ca. 400 cal yrs bp until present day)
The last 400 years in the development of the fen are characterized by a change in vegetation composition. This phase is dominated by Pinus pollen, with low values of pollen from thermophilous taxa, an increase in the ruderal plants Chenopodiaceae, Artemisia, and Plantago, as well as a rapid increase in Cyperaceae (Fig. 4).
The re-emergence of NPPs such as testate amoebae, Arcella-type and an increase in Centropyxis-type suggests an increase in surface wetness (Fig. 6). However, the relatively low values of carbonates and an increase in humification (Fig. 3) suggest a decrease in calcium-rich groundwater and drier conditions. Furthermore, the humification index might be misleading, as more recent environmental change can cause later-stage peat degradation. An increase in surface wetness and a rise in water level during the last 1,000 years is evident in some regional studies of fens and mires (Charman et al. 2007; Sillasoo et al. 2007; Lamentowicz et al. 2015; Gałka et al. 2016). However, the evidence is inconclusive whether there was an actual increase in surface wetness at Kanna or not.
The top part of the sediment core which covers the last 100 years is characterized by the highest variability in most of the physical properties of the peat, organic, mineral, calcium carbonate contents and humification. This high variability might reflect disturbances directly at or near the site, for example those connected with drainage systems dug in the middle of the 20th century to improve farming and forestry production or related to livestock grazing.
According to historical maps (Maa-Amet, Estonian Land Board 2018), the first improvement works close to the fen were undertaken in the 1940s to straighten the river Vedruka ca. 900 m to the west. Peat extraction from the bog at Vedruka ca. 2 km west of Kanna began in 1940 (Eesti elu 1940). A complex drainage system in the area between this bog and Kanna fen already existed in 1959. The sensitivity of peatland ecosystems and the effects of changes in environmental conditions caused by land use changes such as drainage have been studied widely (Landry and Rochefort 2012; Urbanová et al. 2012; Gałka et al. 2016; Glina et al. 2016; Stivrins et al. 2017). The profound effects of drainage on peatland biogeochemistry can cause vegetation changes that are seen in differences in organic matter quality and decomposition rate. It has been shown that a lowering of the water level increases the thickness of the aerobic surface layer and thus the peat decomposition rate (Minkkinen 1999; Landry and Rochefort 2012; Urbanová et al. 2012), and this effect might explain the increasing humification levels at the top of our core. A relatively slow groundwater flow is essential for fen systems, as it prevents erosion and stimulates iron and calcium carbonate precipitation (Middleton et al. 2006). This would account for the decrease in carbonate and the rapid decrease in organic matter, as a lowered water table exposes previously accumulated organic matter to aerobic decomposition.
A rapid decrease in pollen richness is evident during the modern phase, in contrast to both the regional richness trend noted by Reitalu et al. (Figure 7; 2015) and the landscape scale richness change in Vedruka (Poska and Saarse 2002). The changes in pollen deposition could reflect vegetation changes only within the wetland environment but not in the surrounding landscape (Waller et al. 2017) and these could be of local origin, caused by changes in the fen vegetation and environment. It bears pointing out that the present-day vegetation at Kanna is regarded as typical spring fen vegetation for western Saaremaa and is considered highly species rich. Pollen richness values from modern moss and trap samples were used to compare them with the richness from the modern phase KAN3, in order to test whether the decrease in pollen richness in sediments could be caused by decomposition. This comparison indicated no significant difference, suggesting that pollen decomposition is not the reason (Fig. 10).
Andersen-transformed pollen richness in Kanna, comparison between different stages of fen development and modern pollen samples from moss polsters and Tauber traps. Letters a and b denote a statistical significance in the difference between the groups, in which the natural fen phase is significantly different from the other phases
The decrease in groundwater flow and accompanying changes in fen vegetation might be also attributed to changes during the natural succession of the fen. The increase in peat thickness and the corresponding rise of the bog surface could have started to cover and impede groundwater escape. The groundwater flow might have decreased over higher surfaces, thereby reducing the relative abundance of calciphilous forb taxa and increasing the abundance of Cyperaceae, most probably Schoenus ferrugineus, which is dominant in the present-day vegetation. A high local abundance of Cyperaceae would decrease the probability of detecting rarer, usually insect-pollinated, forb taxa due to pollen representation bias (Odgaard 1999). However, the reasons for the increase in Cyperaceae pollen are still unclear.
Another possible factor affecting the fen vegetation is grazing by domestic livestock. The fen has probably been grazed at least since the 19th-20th centuries, but precise details and the intensity of this use as pasture are unclear. The remains of pasture fences are still visible in the landscapes of several nearby fen areas. Intense cattle grazing and trampling can cause damage to the fen system and its vegetation (Stammel et al. 2003: Bobbink et al. 2006) and can even change the ecological function of these systems (Sánchez et al. 2017). Trampling by cattle can affect peat subsidence, change bulk density, reduce the amount of biomass productivity, alter its structure and accelerate decomposition of organic matter (Sjögersten et al. 2011; Chimner et al. 2017). Grazed mires in Britain show an increase in native arboreal pollen towards the surface horizons, owing to suppression of flowering by local graminaceous plants, including Cyperaceae (Chambers et al. 2011a). These findings are inconsistent with our results. Despite the decrease in the pollen percentages of Poaceae during the last 1,000 cal yrs bp, this period featured a rapid increase in Cyperaceae and lower arboreal pollen values than in earlier zones. Certain coprophilous fungi associated with grazing, such as Podospora (Cook et al. 2011), Sordaria sp. (Krug et al. 2004) and Trichodelitschia (Ebersohn and Eicker 1992), were recorded in the samples, but their occurrence was sporadic and did not reliably indicate intense grazing activities (Fig. 6).
To better understand the effect of grazing and/or drainage on fen vegetation and diversity would require sites to be studied where these particular management methods do not overlap. Palaeoecological studies of other similar fens in the vicinity would allow evaluation of the whole pattern of decreasing diversity and provide better understanding of the reasons for these changes.
Implications for conservation
Calcareous spring fens by definition require mineral-rich springs (Rodwell 2016), which are the main feature of these biotopes and the origin of their biodiversity, and which should be the main conservation question. Although drainage and peat extraction works are several hundred metres from Kanna fen, their impact on sediment properties and vegetation composition is noticeable. This is proof that in order to conserve spring fens for the future it is essential to preserve not only their physical features within their boundaries but also their wider surrounding and their hydrological systems. Such actions would require a maintenance strategy for the whole area and efforts to stabilize the water level with the help of existing drainage systems might be needed to ensure the persistence of these diversity hotspots. Knowledge of the fen environment and the response of its taxa to past climatic changes provides information for the future that can be useful for conservation purposes.
Conclusions
Our investigation is the first highly detailed palaeoecological study of a calcareous spring fen in northern Europe. The study presents a mire development from ca. 9,200 cal bp that does not follow the typical mire succession. The ombrotrophic conditions which followed the Ancylus Lake regression favoured the development of a spring fen fed by mineral-rich calcareous groundwater around 7,200 cal bp due to a climatically and tectonically caused lowering of the overall water table. Our results suggest that the main changes in mire development, vegetation community and pollen richness in the past closely followed the changes in climatic conditions.
The establishment of a fen between ca. 7,200 and 5,000 cal bp was accompanied by exceptionally high pollen richness, which lasted through most of its history, decreasing only during the last 400 years. The higher than average local pollen richness is related to the large number of calciphilous herbaceous pollen taxa growing there as a result of the maintainance of stable conditions throughout the Holocene. The reasons for the vegetation change and decrease in pollen richness during the last 400 years are not clear from our investigation, but seem to be related partly to water level changes possibly resulting from the drainage activities 500 m down the slope below the fen. Today there are many plants growing on the fen and in its close vicinity which are considered relicts from former climatic periods or are neo-endemic; the growth of these taxa is also related to the long history of stability at the fen (Hájek et al. 2011).
Our study further affirms the high value of calcareous fens for the conservation of biodiversity in northern Europe. The fen environment and vegetation has been shown to be strongly related to past climatic changes, indicating that fens are also highly likely to be affected by ongoing climate change.
References
Alliksaar T (2000) Spatial and temporal variability of the distribution of spherical fly-ash particles in sediments in Estonia. (Dissertations on Natural Sciences 4) Tallinn Pedagogical University, Tallinn
Ammann B, Wright HE, Stefanova V, van Leeuwen JFN, van der Knaap WO, Colombaroli D, Tinner W (2013) The role of peat decomposition in patterned mires: a case study from the central Swiss Alps. Preslia 85:317–332
Andersen ST (1970) The relative pollen productivity and pollen representation of north European trees, and correction factors for tree pollen spectra. Danmarks Geologiske Undersøgelse (2. Række nr. 96) Reitzel, Kopenhagen
Berglund BE, Ralska-Jasiewiczowa M (1986) Pollen analysis and pollen diagrams. In: Berglund BE (ed) Handbook of Holocene palaeoecology and palaeohydrology. Wiley, Chichester, pp 455–484
Berglund BE, Sandgren P, Barnekow L, Hannon G, Jiang H, Skog G, Yu S-Y (2005) Early Holocene history of the Baltic Sea, as reflected in coastal sediments in Blekinge, southeastern Sweden. Quat Int 130:111–139
Beug H-J (2004) Leitfaden der Pollenbestimmung für Mitteleuropa und angrenzende Gebiete. Pfeil, München
Birks HJB, Line JM (1992) The use of rarefaction analysis for stimating palynological richness from quaternary pollen-analytical data. Holocene 2:1–10. https://doi.org/10.1177/095968369200200101
Bobbink R, Beltman B, Verhoeven JTA, Whigham TF (eds) (2006) Wetlands: functioning, biodiversity conservation, and restoration. Springer, Berlin. https://doi.org/10.1007/978-3-540-33189-6
Boyle JF (2004) A comparison of two methods for estimating the organic matter content of sediments. J Paleolimnol 31:125–127
Bragg OM, Lindsay R (eds) (2003) Strategy and action plan for mire and peatland conservation in central Europe. Wetlands International, Wageningen. https://www.wetlands.org/publications.aspx
Breitsameter L, Kayser M, Strodthoff J, Müller J, Isselstein J (2017) Performance of extensive cattle stocking on a reclaimed minerotrophic wet grassland. Mires Peat 19:1–10. https://doi.org/10.19189/MaP.2015.OMB.194
Bronk Ramsey C (2008) Deposition models for chronological records. Quat Sci Rev 27:42–60
Bronk Ramsey C (2009) Bayesian analysis of radiocarbon dates. Radiocarbon 51:337–360
Bunting MJ, Farrell M, Broström A et al (2013) Palynological perspectives on vegetation survey: a critical step for model-based reconstruction of quaternary land cover. Quat Sci Rev 82:41–55. https://doi.org/10.1016/j.quascirev.2013.10.006
Chambers FM, van Geel B, van der Linden M (2011a) Considerations for the preparation of peat samples for palynology, and for the counting of pollen and non-pollen palynomorphs. Mires Peat. https://www.mires-and-peat.net/
Chambers FM, Beilman DW, Yu Z (2011b) Methods for determining peat humification and for quantifying peat bulk density, organic matter and carbon content for palaeostudies of climate and peatland carbon dynamics. Mires Peat 7:1–10
Charman DJ, Blundell A, Members Accrotelm (2007) A new European testate amoebae transfer function for palaeohydrological reconstruction on ombrotrophic peatlands. J Quat Sci 22:209–221
Chimner RA, Cooper DJ, Wurster FC, Rochefort L (2017) An overview of peatland restoration in North America: where are we after 25 years? Rest Ecol 25:283–292. https://doi.org/10.1111/rec.12434
Clymo RS (1963) Ion exchange in Sphagnum and its relation to bog ecology. Ann Bot 27:309–324. https://doi.org/10.1093/oxfordjournals.aob.a083847
Cook EJ, van Geel B, van der Kaars S, van Arkel J (2011) A review of the use of non-pollen palynomorphs in palaeoecology with examples from Australia. Palynology 35:155–178
Council of the European Communities (1992) Council directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off J Eur Communities L206:19
Cugny C, Mazier F, Galop D (2010) Modern and fossil non-pollen palynomorphs from the Basque mountains (western Pyrenees, France): the use of coprophilous fungi to reconstruct pastoral activity. Veget Hist Archaeobot 19:391–408. https://doi.org/10.1007/s00334-010-0242-6
Dean WE (1974) Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss-on-ignition: comparison with other methods. J Sediment Petrol 44:242–248
Dombrovskaya AV, Koronieva MM, Tyuremnov SN (1959) Aтлac pacтитeльныx ocтaткoв вcтpeчaeмыx в тopфe (Atlas of plant remains found in peat, in Russian). Gosudarstvennoe Energetitcheskoe Izdateljstvo, Moscow
Ebersohn C, Eicker A (1992) Trichodelitsclzia microspora, a new coprophilous species from South Africa. S Afr J Bot 58:145–146. https://doi.org/10.1016/S0254-6299(16)30859-6
Eesti elu (1940) Nõudmine turbale suurenenud (increased demand on peat, in Estonian). Uus Eesti no 141, May 29, 1940
Fægri K, Iversen J (1989) In: Fægri K, Kaland PE, Krzywinski K (eds) Textbook of pollen analysis, 4th edn. Wiley, Chichester
Faith DP, Minchin PR, Belbin L (1987) Compositional dissimilarity as a robust measure of ecological distance. Vegetatio 69:57–68
Felde VA, Peglar SM, Bjune AE, Grytnes JA, Birks HJB (2016) Modern pollen-plant richness and diversity relationships exist along a vegetational gradient in southern Norway. Holocene 26:163–175. https://doi.org/10.1177/0959683615596843
Gałka M, Aunina L, Tobolski K, Feurdean A (2016) Development of rich fen on the SE Baltic coast, Latvia, during the Last 7500 Years, using paleoecological proxies: implications for plant community development and paleoclimatic research. Wetlands 36:689–703. https://doi.org/10.1007/s13157-016-0779-y
Gałka M, Aunina L, Feurdean A, Hutchinson S, Kołaczek P, Apolinarska K (2017) Rich fen development in CE Europe, resilience to climate change and human impact over the last ca. 3500 years. Palaeogeogr Palaeoclimatol Palaeoecol 473:57–72. https://doi.org/10.1016/j.palaeo.2017.02.030
Gałka M, Feurdean A, Hutchinson S, Milecka K, Tantau I, Apolinarska K (2018) Response of a spring-fed fen ecosystem in Central Eastern Europe (NW Romania) to climate changes during the last 4000 years: a high resolution multi-proxy reconstruction. Palaeogeogr Palaeoclimatol Palaeoecol 504:170–185. https://doi.org/10.1016/j.palaeo.2018.05.027
Giesecke T, Wolters S, Jahns S, Brande A (2012) Exploring Holocene changes in palynological richness in northern Europe—did postglacial immigration matter? PLoS ONE 7:e51624. https://doi.org/10.1371/journal.pone.0051624
Glina B, Bogacz A, Gulyás M, Zawieja B, Gajewski P, Kaczmarek Z (2016) The effect of long-term forestry drainage on the current state of peatland soils: a case study from the Central Sudetes, SW Poland. Mires Peat. https://doi.org/10.19189/MaP.2016.OMB.239
Grimm EC (1987) CONISS: a FORTRAN 77 program for stratigraphically constrained cluster analysis by the method of incremental sum of squares. Comput Geosci 13:13–35
Grimm EC (2011) TILIA 1.7.16 software. Illinois State Museum, Research and Collection Center, Springfield
Hájek M, Horsák M, Tichý L, Hájková P, Dítě D, Jamrichová E (2011) Testing a relict distributional pattern of fen plant and terrestrial snail species at the Holocene scale: a null model approach. J Biogeogr 38:742–755
Hájková P, Grootjans A, Lamentowicz M et al (2012a) How a Sphagnum fuscum-dominated bog changed into a calcareous fen: the unique Holocene history of a Slovak spring-fed mire. J Quat Sci 27:233–243. https://doi.org/10.1002/jqs.1534
Hájková P, Horsák M, Hájek M, Lacina A, Buchtová H, Pelánková B (2012b) Origin and contrasting succession pathways of the Western Carpathian calcareous fens revealed by plant and mollusk macrofossils. Boreas 41:690–706. https://doi.org/10.1111/j.1502-3885.2012.00263.x
Hájková P, Jamrichová E, Horsák M, Hájek M (2013) Holocene history of a Cladium mariscus-dominated calcareous fen in Slovakia: vegetation stability and landscape development. Preslia 85:289–315
Hájková P, Horsák M, Hájek M, Jankovská V, Jamrichová E, Moutelíková J (2015) Using multi-proxy palaeoecology to test a relict status of refugial populations of calcareous-fen species in the Western Carpathians. Holocene 25:702–715. https://doi.org/10.1177/0959683614566251
Hammarlund D, Björck S, Buchardt B, Israelson C, Thomsen C (2003) Rapid hydrological changes during the Holocene revealed by stable isotope records of lacustrine carbonates from Lake Igelsjon, southern Sweden. Quat Sci Rev 22:353–370
Hansson A-M, Hiie S, Kihno K, Masauskaite R, Moe D, Seiriene V, Torske N (1996) A vegetation historical study of Johvikasoo, an ombrotrophic mire at Tuiu, Saaremaa, Estonia. PACT 51:39–56
Hatté C, Jull AJT (2013) 14C of plant macrofossils. In: Elias S (ed) Encyclopedia of Quaternary science, 2nd edn. Elsevier, Amsterdam, pp 361–367. https://doi.org/10.1016/B978-0-444-53643-3.00049-2
Hawkesford M, Horts W, Kichey T, Lambers H, Schjoerring J, Møller IS, White P (2012) Functions of macronutrients. In: Marschner P (ed) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, London, pp 135–189. https://doi.org/10.1016/B978-0-12-384905-2.00006-6
Heinsalu A, Alliksaar T, Leeben A (2007) Sediment diatom assemblages and composition of pore-water dissolved organic matter reflect recent eutrophication history of Lake Peipsi (Estonia/Russia). Hydrobiologia 584:133–143. https://doi.org/10.1007/s10750-007-8615-2
Hicks S, Ammann B, Latałowa M, Pardoe H, Tinsley H (1996) European Pollen Monitoring Programme: project description and guidelines. Oulu University Press, Oulu
Hoogsteen MJJ, Lantinga EA, Bakker EJ, Grootaa JCJ, Tittonell PA (2015) Estimating soil organic carbon through loss on ignition: effects of ignition conditions and structural water loss. Eur J Soil Sci 66:320–328. https://doi.org/10.1111/ejss.12224
Hua Q, Barbetti M, Rakowski AZ (2013) Atmospheric radiocarbon for the period 1950–2010. Radiocarbon 55:2059–2072. https://doi.org/10.2458/azu_js_rc.v55i2.16177
Ilomets M, Truus L, Pajula R, Sepp K (2010) The species composition and structure of vascular plants and bryophytes on the water level gradient within a calcareous fen in north Estonia. Estonian J Ecol 59(1):19–38
Interpretation Manual of European Union Habitats (IMEUH) (2007) European Commission DG Environment Nature and Biodiversity. http://ec.europa.eu/environment/nature/legislation/habitatsdirective/docs/2007_07_im.pdf. Accessed 3 June 2018
Jackson DA (1993) Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology 74:2,204–2,214
Jamrichová E, Hájková P, Horsák M, Rybníčková E, Lacina A, Hájek M (2014) Landscape history, calcareous fen development and historical events in the Slovak Eastern Carpathians. Veget Hist Archaeobot 23:497–513. https://doi.org/10.1007/s00334-013-0416-0
Jänes-Kapp K, Randma E, Soosaar M (2007) Saaremaa 2: Ajalugu, majandus, kultuur (Saaremaa 2, history, economics, culture; in Estonian). Koolibri, Tallinn
Jordan S, Velty S, Zeitz J (2007) The influence of degree of peat decomposition on phosphorus binding forms in fens. Mires Peat 2. http://www.mires-and-peat.net/
Juggins S (2017) Analysis of Quaternary science data, package “rioja”. https://cran.r-project.org/web/packages/rioja/rioja.pdf
Katz NJ, Katz SV, Skobeyeva EI (1977) Atlas rastitel’nyh oostatkov v torfje (Atlas of plant remains in peats, in Russian). Nedra, Moscow
Königsson LK, Poska A (1998) Pitkasoo: a west Estonian Holocene reference site. Proc Estonian Acad Sci Geol 47:242–261
Krug JC, Benny GL, Keller HW (2004) Coprophilous fungi. In: Mueller GM, Bills GF, Foster MS (eds) Biodiversity of fungi: inventory and monitoring methods. Elsevier, Amsterdam, pp 467–499
Lachance D, Lavoie C (2004) Vegetation of Sphagnum bogs in highly disturbed landscapes: relative influence of abiotic and anthropogenic factors. Appl Veget Sci 7:183–192
Laitinen J, Kondelin H, Heikkilä R (2011) Intermediate fen patches on a sloping rock outcrop in Koitelainen, Finnish Lapland. Mires Peat. http://www.mires-and-peat.net/
Lamentowicz M, Słowiński M, Marcisz K et al (2015) Hydrological dynamics and fire history of the last 1300 years in western Siberia reconstructed from a high-resolution, ombrotrophic peat archive. Quat Res 84:312–325. https://doi.org/10.1016/j.yqres.2015.09.002
Landry J, Rochefort L (2012) The drainage of peatlands: impacts and rewetting techniques. Université Laval, Québec. http://www.gret-perg.ulaval.ca/uploads/tx_centrerecherche/Drainage_guide_Web.pdf
Legendre P, Gallagher ED (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280. https://doi.org/10.1007/s004420100716
Levesque PEM, Dinel H, Larouche A (1988) Guide to the identification of plant macrofossils in Canadian peatlands, Research Branch, Agriculture Canada. Land Resource Research Centre, Ottawa
López-Vila J, Montoya E, Cañellas-Boltà N, Rull V (2014) Modern non-pollen palynomorphs sedimentation along an elevational gradient in the south-central Pyrenees (southwestern Europe) as a tool for Holocene paleoecological reconstruction. Holocene 24:327–345. https://doi.org/10.1177/0959683613518593
Maa-amet (Estonian Land Board) (2018) Historical map collection. Topographical map 1:50000 (1943). http://xgis.maaamet.ee/xGIS/XGis?app_id=UU41&user_id=at&bbox=381923.800927423,6463758.46776826,393312.865603942,6469724.1683131&LANG=1
Mägi M, Jets I, Riiel R, Allmäe R, Limbo-Simovart J (2014) Pre-Viking and early Viking Age sacrificial place at Viidumäe, west Saaremaa. Archaeol Fieldwork Estonia 2014:153–162
Malterer TJ, Verry ES, Erjavec J (1992) Fiber content and degree of decomposition in peats: review of natural methods. Soil Sci Soc Am J 56:1,200–1,211
Matthias I, Semmler MSS, Giesecke T (2015) Pollen diversity captures landscape structure and diversity. J Ecol 103:880–890. https://doi.org/10.1111/1365-2745.12404
Matthiesen MK, Larney FJ, Selinger LB, Olson AF (2005) Influence of loss on ignition temperature and heating time on ash content of compost and manure. Commun Soil Sci Plant Anal 36:2,561–2,573
Mauquoy D, Hughes PDM, van Geel B (2010) A protocol for plant macrofossil analysis of peat deposits. Mires and Peat 06:1–5
Mazei YA, Bubnova OA (2007) Species composition and structure of testate amoebae community in a sphagnum bog at the initial stage of its formation. Biol Bull 34:619–628. https://doi.org/10.1134/S1062359007060131
Middleton BA, Holsten B, van Diggelen R (2006) Biodiversity management of fens and fen meadows by grazing, cutting and burning. Appl Veget Sci 9:307–316. https://doi.org/10.1658/1402-2001(2006)9%5b307:bmofaf%5d2.0.co;2
Minayeva TY, Bragg OM, Sirin AA (2017) Towards ecosystem-based restoration of peatland biodiversity. Mires Peat. https://doi.org/10.19189/MaP.2013.OMB.150
Minkkine K (1999) Effect of forestry drainage on the carbon balance and radiative forcing of peatlands in Finland. Dissertation, University of Helsinki, Helsinki
Miola A (2012) Tools for non-pollen palynomorphs (NPPs) analysis: a list of Quaternary NPP types and reference literature in English language (1972–2011). Rev Palaeobot Palynol 186:142–161
Nilsson K (2016) Alkaline fens: valuable wetlands but difficult to manage. TemaNord 2016:515. Nordic Council of Ministers, Copenhagen. http://dx.doi.org/10.6027/TN2016-515
Odgaard BV (1999) Fossil pollen as a record of past biodiversity. J Biogeogr 26:7–17
Ohenoja E (1995) Occurrence of Geoglossum, Trichoglossum and Microglossum (Ascomycota, Leotiales) in Finland. Doc Mycol 98–100:285–294
Oksanen J, Blanchet GF, Friendly M et al (2017) Vegan: community ecology package. R package version 2.4-3. https://CRAN.R-project.org/package=vegan
Paal J (1997) Eesti taimkatte kasvukohatüüpide klassifikatsioon (Classification of Estonian vegetation site types, in Estonian). Tartu Ülikool, Estonia
Paal J, Leibak E (2011) Estonian mires: inventory of habitats. Regio, Tartu
Pidek IA, Noryśkiewicz B, Dobrowolski R, Osadowski Z (2012) Indicative value of pollen analysis of spring-fed fens deposits. Ecológia 31:405–433. https://doi.org/10.4149/ekol_2012_04_405
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2019) nlme: Linear and nonlinear mixed effects models. R package version 3.1-139. https://CRAN.R-project.org/package=nlme
Poska A, Saarse L (2002) Vegetation development and introduction of agriculture to Saaremaa Island, Estonia: the human response to shore displacement. Holocene 12:555–568. https://doi.org/10.1191/0959683602hl567rp
Priede A, Mežaka A, Dobkeviča L, Grīnberga L (2016) Spontaneous revegetation of cutaway fens: can it result in valuable habitats? Mires Peat. https://doi.org/10.19189/MaP.2016.OMB.220
Ratas U, Kokovkin T (1989) Viidumae kompleksprofiili seletuskiri (Viidumae complex profile report (in Estonian) Tallinn, Estonia
Reille M (1992) Pollen et spores d’Europe et d’Afrique du nord. Laboratoire de Botanique historique et Palynologie, Marseille
Reimer PJ, Bard E, Bayliss A et al (2013) IntCal 13 and Marine 13 radiocarbon age calibration curves 0–50000 years cal bp. Radiocarbon 55:1,869–1,887
Reitalu T, Gerhold P, Poska A et al (2015) Novel insights into post-glacial vegetation change: functional and phylogenetic diversity in pollen records. J Veget Sci 26:911–922. https://doi.org/10.1111/jvs.12300
Reitalu T, Birks HJB, Bjune AE et al (2019) Patterns of pollen and plant richness across northern Europe. J Ecol. https://doi.org/10.1111/1365-2745.131
Renberg I, Wik M (1985) Carbonaceous particles in lake sediments—pollutants from fossil fuel combustion. Ambio 14:161–163
Renssen H, Seppä H, Heiri O, Roche DM, Goosse H, Fichefet T (2009) The spatial and temporal complexity of the Holocene thermal maximum. Nat Geosci 2:411–414
Riigi Ilmateenistus (State meteorological service) (2018) Ilmatarkus (weather accuracy). http://www.ilmateenistus.ee/kliima/climate-maps/temperature/?lang=ne. Accessed 23 Sept 2018
Rodwell J (1995) British plant communities: aquatic communities, swamps and tall-herb fens, vol 5. Cambridge University Press, Cambridge
Rodwell J (2016) European Red List of Habitats—mires habitat Group. D4.1a Small-sedge base-rich fen and calcareous spring mire. European Environment Agency (EEA). https://forum.eionet.europa.eu/european-red-list-habitats/library/terrestrial-habitats/d.-mires-and-bogs/d4.1a-small-sedge-base-rich-fen-and-calcareous-spring-mire. Accessed 13 June 2007
Rose NL (1990) A method for the selective removal of inorganic ash particles from lake sediments. J Paleolimnol 4:61–67
Rozbrojová Z, Hájek M (2008) Changes in nutrient limitation of spring fen vegetation along environmental gradients in the West Carpathians. J Veget Sci 19:613–620. https://doi.org/10.3170/2008-8-18416
Rybníček K, Rybníčková E (1987) Palaeobotanical evidence of Middle Holocene stratigraphic hiatuses in Czechoslovakia and their explanation. Folia Geobot Phytotaxon 22:313–327
Saarse L, Vassiljev J (2010) Mattunud järvesetted peegeldavad Läänemere arengulugu (Ancient lake sediments show the history of the Baltic Sea, in Estonian). Eesti Loodus 96:41–42
Saarse L, Vassiljev J, Rosentau A (2009) Ancylus Lake and Litorina Sea transition on the Island of Saaremaa, Estonia: a pilot study. Baltica 22:51–62
Salmina L (2004) Factors influencing distribution of Cladium mariscus in Latvia. Ann Bot Fenn 41:367–371
Sánchez ME, Chimner RA, Hribljan JA, Lilleskov EA, Suárez E (2017) Carbon dioxide and methane fluxes in grazed and undisturbed mountain peatlands in the Ecuadorian Andes. Mires Peat 19. https://doi.org/10.19189/MaP.2017.OMB.277
Šefferová Stanová V, Šeffer J, Janák M (2008) Management of Natura 2000 habitats—7230 Alkaline fens. The European Commission http://ec.europa.eu/environment/nature/natura2000/management/habitats/pdf/7230_Alkaline_fens.pdf. Accessed 6 Aug 2018
Shumilovskikh LS, Schlütz F, Achterberg I, Bauerochse A, Leuschner HH (2015) Non-pollen palynomorphs from mid-Holocene peat of the raised bog Borsteler Moor (Lower Saxony, Germany). Stud Quat 32:5–18. https://doi.org/10.1515/squa-2015-0001
Sillasoo Ü, Mauquoy D, Blundell A et al (2007) Peat multi-proxy data from Männikjärve bog as indicators of late Holocene climate changes in Estonia. Boreas 36:20–37. https://doi.org/10.1111/j.1502-3885.2007.tb01177.x
Sjögersten S, van der Wal R, Loonen MJJE, Woodin SJ (2011) Recovery of ecosystem carbon fluxes and storage from herbivory. Biogeochemistry 106:357–370
Skene KR, Sprent JI, Raven JA, Herdman L (2000) Myrica gale L. J Ecol 88:1,079–1,094
Stammel B, Kiehl K, Pfadenhauer J (2003) Alternative management on fens: response of vegetation to grazing and mowing. Appl Veget Sci 6:245–254
Stivrins N, Ozola I, Gałka M et al (2017) Drivers of peat accumulation rate in a raised bog: impact of drainage, climate, and local vegetation composition. Mires Peat 19. https://doi.org/10.19189/MaP.2016.OMB.262
Tahvanainen T (2004) Water chemistry of mires in relation to the poor-rich vegetation gradient and contrasting geochemical zones of the north-eastern Fennoscandian shield. Folia Geobot 39:353–369. https://doi.org/10.1007/BF02803208
Talve T, Mürk M, Lindell T, Oja T (2014) Rhinanthus plants found in calcareous fens on Gotland (Sweden): are they related to R. osiliensis from Saaremaa (Estonia)? Biochem Syst Ecol 54:113–122
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.r-project.org/
Turney CSM, Coope GR, Harkness DD, Lowe JJ, Walker MJC (2000) Implications for the dating of Wisconsinan (Weichselian) late-glacial events of systematic radiocarbon age differences between terrestrial plant macrofossils from a site in SW Ireland. Quat Res 53:114–121
Urbanová Z, Ing S, Picek T (2012) Effect of drainage and restoration on the ecology of peatlands in the Šumava Mountains. Dissertation, University of South Bohemia in České Budějovice
Väliranta M, Oinonen M, Seppä H, Korkonen S, Juutinen S, Tuittila E-S (2014) Unexpected problems in AMS 14C dating of fen peat. Radiocarbon 56:95–108. https://doi.org/10.2458/56.16917
Väliranta M, Salojärvi N, Vuorsalo A, Juutinen S, Korhola A, Luoto M, Tuittila ES (2017) Holocene fen-bog transitions, current status in Finland and future perspectives. Holocene 27:752–764. https://doi.org/10.1177/0959683616670471
Van Geel B (2001) Non-pollen palynomorphs. In: Smol JP, Birks HJB, Last WM (eds) Tracking environmental change using lake sediments, vol 3. Terrestrial, algal and siliceous indicators. Kluwer, Dordrecht, pp 99–119
Veski S (1996) A contribution to the history of vegetation and human impact in northern Saaremaa, Estonia, based on the biostratigraphy of the Surusoo mire: preliminary results. PACT 51:57–66
Völlm C, Tanneberger F (2014) Shallow inundation favours decomposition of Phragmites australis leaves in a near-natural temperate fen. Mires and Peat 14. http://www.mires-and-peat.net/pages/volumes/map14/map1406.php
Waller M, Carvalho F, Grant MJ, Bunting MJ, Brown K (2017) Disentangling the pollen signal from fen systems: modern and Holocene studies from southern and eastern England. Rev Palaeobot Palynol 238:15–33. https://doi.org/10.1016/j.revpalbo.2016.11.007
Weil RR, Brady NC (1985) The nature and properties of soil, 15th edn. Pearson Education, Columbus
Wolf EC, Cooper DJ (2015) Fens of the Sierra Nevada, California, USA: patterns of distribution and vegetation. Mires Peat 15. http://www.mires-and-peat.net/pages/volumes/map15/map1508.php
Zhang XL, Wu GJ, Yao TD, Zhang CL, Yue YH (2011) Characterization of individual fly ash particles in surface snow at Urumqi Glacier No. 1, Eastern Tianshan. Chin Sci Bull 56:3,464–3,473. https://doi.org/10.1007/s11434-011-4684-8
Zobel M, Otto R, Laanisto L, Naranjo-Cigala A, Pärtel M, Fernández-Palacios JM (2011) The formation of species pools: historical habitat abundance affects current local diversity. Glob Ecol Biogeogr 20:251–259. https://doi.org/10.1111/j.1466-8238.2010.00593.x
Acknowledgements
The authors are grateful to Mari Reitalu for comments on the recent history of the study site. We thank Hans Renssen for providing the simulated climate data. The research was carried out with the financial support of the Eesti Teadusagentuur (Estonian Research Council, PUT1173, IUT1-8, PRG323).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M.-J. Gaillard.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Blaus, A., Reitalu, T., Amon, L. et al. From bog to fen: palaeoecological reconstruction of the development of a calcareous spring fen on Saaremaa, Estonia. Veget Hist Archaeobot 29, 373–391 (2020). https://doi.org/10.1007/s00334-019-00748-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00334-019-00748-z