Abstract
Pollen influx (number of pollen grains cm−2 year−1) can objectively reflect the dispersal and deposition features of pollen within a certain time and space, and is often used as a basis for the quantitative reconstruction of palaeovegetation; however, little is known about the features and mechanisms of vertical dispersal of pollen. Here we present the results from a 5 year (2006–2010) monitoring program using pollen traps placed at different heights from ground level up to 60 m and surface soil samples in a mixed coniferous and deciduous broad-leaved woodland in the Changbai mountains, northeastern China. The pollen percentages and pollen influx from the traps have very similar characteristics to the highest values for Betula, Fraxinus, Quercus and Pinus, among the tree taxa and Artemisia, Chenopodiaceae and Asteraceae among the herb taxa. Pollen influx values vary significantly with height and show major differences between three distinct layers, above-canopy (≥32 m), within the trunk layer (8 ≤ 32 m) and on the ground (0 m). These differences in pollen influx are explained by differences in (i) the air flows in each of these layers and (ii) the fall speed of pollen of the various taxa. We found that the pollen recorded on the ground surface is a good representation of the major part of the pollen transported in the trunk space of the woodland. Comparison of the pollen influx values with the theoretical, calculated “characteristic pollen source area” (CPSA) of 12 selected taxa indicates that the pollen deposited on the ground surface of the woodland is a fair representation with 85–90 % of the total pollen deposited at a wind speed of 2.4 m s−1 coming from within ca. 1–5 km for Pinus and Quercus, ca. 5–10 km for Ulmus, Tilia, Oleaceae and Betula, ca. 20–40 km for Fraxinus, Poaceae, Chenopodiaceae, Populus and Salix, and ca. 30–60 km for Artemisia; it is also a good representation with 90–98 % of the total pollen deposited coming from within 60 km at a wind speed of 2.4 m s−1, or 100 km at a wind speed: 6 m s−1, for the 12 selected taxa used in the CPSA calculation. Furthermore, comparison with the vegetation map of the area around the sampling site shows that the pollen deposited on the ground represents all plant communities which grow in the study area within 70 km radius of the sampling site. In this study, the pollen percentages obtained from the soil surface samples are significantly biased towards pollen taxa with good preservation due to thick and robust pollen walls. Therefore, if mosses are available instead, soil samples should be avoided for pollen studies, in particular for the study of pollen-vegetation relationships, the estimation of pollen productivities and quantitative reconstruction of past vegetation. The results also indicate that the existing model of pollen dispersal and deposition, Prentice’s model, provides a fair description of the actual pollen dispersal and deposition in this kind of woodland, which suggests that the application of the landscape reconstruction algorithm would be relevant for reconstruction of this type of woodland in the past.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the development of modern palynology, quantitative reconstruction of palaeovegetation and palaeoclimate has become one of the main goals of Quaternary pollen analysis (Vincens et al. 2003; Xu et al. 2010). However, establishing the quantitative relationship between pollen and vegetation remains a fundamental task (Davis 1963; Jackson et al. 1995; Gaillard et al. 1994; Xu et al. 2006).
Pollen influx, the number of pollen grains deposited per square cm and year, can objectively reflect dispersal and deposition features of pollen in time and space (Hicks 1996) and provide a measure of the pollen productivity of plant taxa (Sugita et al. 2010a; Mazier et al. 2012). Hereafter, we use the term “pollen influx” instead of “pollen accumulation rate” (PAR) for the pollen collected in pollen traps, PAR being more adequately used for the absolute pollen values calculated from pollen data in lake sediments, peat and soils, given that these deposits are dated and a chronology established (Sugita et al. 2010a). Within the pollen monitoring programme (PMP), pollen influx was measured in a large number of European regions, in particular in high altitudinal and latitudinal areas, using pollen traps (Hicks 1996, 2001; Koff 2001). These studies have laid a foundation for the reconstruction of local vegetation (Hicks 2001; Mazier et al. 2012). Since the 1980s, many Chinese researchers have published pollen influx data (Sun and Wu 1988; Xu et al. 2007a; Wang et al. 2008; Li et al. 2009; Tian et al. 2010; Xu et al. 2012); however, few studies have investigated features and mechanisms of vertical pollen dispersal (Xu et al. 2007a; Zhang et al. 2009; Li et al. 2011).
Another important factor in pollen-based quantitative reconstruction of vegetation is the pollen source area. It has been discussed and defined in many different ways through the years (Jacobson and Bradshaw 1981; Prentice 1985; Sugita 1994; Xu et al. 2012; Xu and Zhang 2013). The pollen source area is dependant on many factors of which the most important are the pollen productivity and fall speed of pollen (FSP) of plant taxa within the regional vegetation, the type and size of the sedimentary basin and the wind speed (Prentice 1985; Jacobson and Bradshaw 1981; Sugita 1994; Calcote 1995; Wang and Herzschuh 2011). Nevertheless, little research on the pollen source area has so far been conducted in China (Wang and Herzschuh 2011; Xu et al. 2012).
Woodland is an important vegetation type and well suited for the study of the relationships between pollen, vegetation and climate (Zhang et al. 2003; Li et al. 2009). Therefore, the Changbai mountains were chosen for this study, as they are characterised by well preserved, natural woodland ecosystems. For the reasons given above, the aims of the study were (i) to study the vertical dispersal of pollen and its relationship to the pollen assemblages deposited on the woodland floor with the purpose to understand processes of dispersal and deposition of pollen in woodlands, and (ii) to calculate the characteristic pollen source area (CPSA) of major woodland taxa to provide useful insights for the interpretation of fossil pollen assemblages in terms of the vegetation spatial scale represented by the pollen record.
Study area
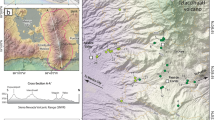
The study site (42°24′9″N, 128°05′45″E, 738 m a.s.l.) is located in the Plot No. 1 of Pinus koraiensis (Korean pine) broad-leaved mixed woodland in the inventory of the forest ecosystem station in the Changbai mountains, Chinese Academy of Sciences (Fig. 1a). The area is characterised by a temperate, continental monsoon climate with arid and windy springs, hot and rainy summers and autumns, and cold and dry winters. Mean annual precipitation varies between 500 and 900 mm, with 60 % of the annual precipitation occurring between June and August. In addition, the mean annual temperature is 3.6 °C, with averages of 20.5 °C in August and −16.5 °C in January (Zhao et al. 2005; Song et al. 2009).
Location of the study area and pollen sampling. a topography of the study area, location of the sampling site and the Songjiang meteorological station, b the weather tower located in Plot No. 1 of Pinus koraiensis broad-leaved mixed woodland in the inventory of the Forest Ecosystem Station in the Changbai mountains, Chinese Academy of Sciences, c a plastic container was used as pollen trap (diameter 10 cm, height 30 cm, aperture 5.2 cm in diameter) with a covering screen of netting (Li et al. 2009)
The area is affected by strong winds in winter and spring because of its location in a mountainous area (Li et al. 2001). The wind speed data are from the Songjiang meteorological station (42.32°N, 128.15°E, 591 m a.s.l.; China’s Meteorological Data Sharing Service System, Station No. 54285, data from http://cdc.cma.gov.cn/home.do) which is located approximately 10 km southeast of the sampling site (Fig. 1a). For the years 1976–2005, the mean annual wind speed was 2.4 m s−1, and relatively high wind speeds were recorded each year, so there were 70 days with wind speeds >10 m s−1, 130 days with wind speeds >8 m s−1 and 210 days with wind speeds of >6 m s−1.
In the study area, the density of the tree canopy is generally greater than 0.7, and the soil pH is between 5.5 and 6.0 (Wu 1980). Pinus koraiensis, Quercus mongolica, Fraxinus mandshurica and Acer pictum are the dominant tree species there (Zhao et al. 2005), while Juglans mandshurica, Cortex phellodendri and Populus ussuriensis are the major components of the woodlands around the sampling site (Xing 1988; ESM Appendix 1). At a greater distance from the sampling site, Betulaceae, Ulmus propinqua, Abies spp., Picea spp., Larix spp. and Q. mongolica grow in large numbers (Wu 1980). In the shrub layer, the dominant species include Syringa reticulata, Euonymus verrucosides, Sambucus williamsii and Corylus mandshurica. The dominant herbs include Maianthemum bifolium, Thalictrum aquilegifolium, Humulus scandens and Carex tristachya, and the dominant lianas are Schisandra chinensis and Vitis amurensis (Wu 1980).
Methods
Field work
A ca. 61.8 m high weather tower is located in Plot No. 1 of P. koraiensis broad-leaved mixed woodland (Fig. 1b). In this study, plastic containers with a diameter of 10 cm, height 30 cm, aperture 5.2 cm in diameter and with a covering net were used as pollen traps for the collection of pollen at different heights and on the ground (Fig. 1c; Li et al. 2009). In September 2005, one pollen trap was placed on the tower at each of the six heights of 8, 16, 22, 26, 32 and 40 m. Moreover, two traps were placed on the ground close to the tower (8 trap samples in total for the years 2005–2006) and two 1 cm surface soil samples were collected beside the ground traps. The trap samples were collected in September 2006. In the years 2006–2009, three traps were placed at each of the same heights as above with the addition of the two heights 50 and 60 m. Three traps were placed on the ground close to the tower and three 1 cm surface soil samples were collected beside the ground traps. The traps on the tower and on the ground were sampled each year 2007–2010, so three traps at eight heights and over 4 years, and also three traps on the ground over 4 years, that is 108 trap samples in total for the years 2007–2010. Overall, 116 trap samples were collected in 2006–2010 and 14 surface soil samples (two samples in 2005 and three samples each year 2006–2009).
Pollen analysis
The trap and soil samples (each 10 g dry weight) were extracted with a modified HCl-NaOH method (Li et al. 2009). One tablet of Lycopodium spores with ca. 11,670 ± 110 spores (samples from 2006) or ca. 27,637 ± 563 spores (samples from 2007 to 2010) was added to each trap sample to calculate the pollen influx as follows (Sun and Wu 1988):
where P ij is the number of pollen type i at sample j, N is the added number of Lycopodium spores, C j is the recorded numbers of Lycopodium spores in sample j, S is the opening area (cm2) of the Tauber trap.
Ca. 600 pollen grains per sample from a minimum of 3–4 slides were identified and counted using Olympus BX-51 and Carl Zeiss AX10 light microscopes at a 400 × magnification. The identification of pollen grains was done using the Pollen Flora of China (Wang 1995) and the modern reference collection of the Environmental Archaeology Laboratory of Hebei Normal University.
Pollen data handling
Both pollen percentages and pollen influxes were very similar between the traps located at the same height. Therefore, we calculated the average annual values per height (2005–2006, six heights + ground level for 1 year; 2007–2010, eight heights + ground level for 4 years, so 43 values). The 43 annual values for pollen percentages and influxes were divided into three groups based on the heights and taxa composition (ESM Appendix 1; Sun and Zhao 1995; Xia et al. 2010). The first group includes the values from the traps above the tree canopy (≥32 m, so the heights 32, 40, 50 and 60 m), making 18 values. The second group includes the values from the traps in the trunk layer (8 ≤ 32 m, so the heights 8, 16, 22 and 26 m), making 20 values. The third group includes the values from the ground traps (0 m), making 5 values. Similarly, the pollen percentages from the 14 surface soil samples were averaged per year (average of 2 (2005) or 3 (2006–2009) soil samples per year), making five soil annual pollen influxes in total.
In addition, a few pollen taxa are summed into “ecological groups” representing plant taxa that are characteristic of similar environments, such as Abies + Picea, Alnus + Carpinus, Celtis + Ulmus and Asteraceae + Chenopodiaceae (Figs. 2, 3).
Percentages of major pollen taxa from pollen traps at different heights on a meteorological tower in Pinus koraiensis broad-leaved mixed woodland in the Changbai mountains, a yearly pollen deposition in percentages of major pollen taxa at different heights, b average % deposition of major pollen taxa over 2007–2010 (heights 50 m, 60 m) and over 2006–2010 (all other heights)
Statistical analysis
Discriminant analysis (DA) is an effective numerical method for evaluating how significant are the differences between pollen values (pollen percentages or pollen influxes) (Lynch 1996; Reese and Liu 2005; Tian et al. 2010). In this study, DA was used to verify the correctness of the grouping based on height and pollen composition (see Methods section, above). DA implements stepwise regression of pollen percentages and pollen influxes of the trap samples. We used the data for 21 selected pollen taxa (minimum percentage >1 %, number of occurrences >5 times, total percentage of the 21 taxa > 90 %). Discriminant Function is then used to crosscheck the stepwise regression.
Analysis of variance (ANOVA) is a powerful statistical technique that looks for significant differences between the means of the groups. In order to further evaluate the differences between surface soil and trap samples on the ground, ANOVA was done using MS Excel.
Characteristic pollen source area
In order to calculate the CPSA of 12 major pollen taxa (Pinus, Quercus, Salix, Fraxinus, Betula, Ulmus, Tilia, Populus, Oleaceae, Chenopodiaceae, Artemisia and Poaceae), values of FSP for those taxa were needed. These were calculated using the methods described in Gregory (1973), using Stokes’ law for spheroidal pollen grains and Falck’s assumption for ellipsoidal pollen grains (ESM Appendix 2).
The CPSA was calculated using the bog model of pollen dispersal and deposition of Prentice (1985), in which the weather tower was assumed to be comparable to a bog with a radius of 5 m where the pollen is deposited at a single point of the “deposition basin” rather than on its entire surface, as on a lake bottom (Sugita 1993). Most published values of CPSA were calculated using a single wind speed (Prentice 1985; Sugita 1994; Sugita et al. 1999; Broström et al. 2004; Bunting et al. 2004). However, some studies have showed that the wind speed significantly influences the CPSA (Xu et al. 2012; Xu and Zhang 2013). As the sampling site in this study is located in a mountainous area with a large wind speed variability (Li et al. 2001), the CPSA was calculated for different wind speeds. The maximum area of regional vegetation from which pollen can come was set to a radius of 200 km.
Results
Discriminant analysis
Discriminant analysis of the pollen data from all trap samples expressed in pollen percentages works by grouping the samples into the same clusters as in the grouping by woodland layers (Sun and Zhao 1995; Xia et al. 2010), which are the above-canopy, trunk and ground layers. Except for one sample (height 26 m, year 2006–2007), the result does not change after cross checking (Discriminant Function), and the rate of correct classification is 76.7 % (Table 1a). Discriminant analysis of the pollen data from all trap samples expressed in pollen influx also groups the samples according to the woodland layers, except for one sample (height 22 m, year 2005–2006) included in Group 1 instead of Group 2. The result does not change after cross checking, and the rate of correct classification is greater than 75 % (Table 1b).
The sample scores (for pollen percentages and influx) in the DA plots are spread out around the group centroids for Groups 1 and 2 (heights ≥32 m and 8 ≤ 32 m), whereas they are close to the group centroid for Group 3 (0 m) (Fig. 2).
Pollen data
In all, 61 pollen taxa were identified from the 116 pollen traps and 14 surface soil pollen samples (ESM Appendix 3). We found 19 tree pollen types, with a dominance of Pinus, Betula, Quercus, Fraxinus and Tilia, nine shrub pollen types, mainly Araliaceae, Celastraceae and Rosaceae, and 33 herb pollen types, with a dominance of Artemisia, Asteraceae + Chenopodiaceae, Scrophulariaceae and Poaceae. The most frequent spores were trilete and monolete spores from ferns and Selaginella sinensis.
Pollen percentages
Overall, the pollen assemblages in percentages are dominated by trees (with an average of 88.3 %), and the differences between the pollen assemblages are mainly reflected in differences of tree composition. Herb pollen accounts for 9.3 % of the total pollen. Shrub pollen and fern spores are much less common (Fig. 3).
Betula accounts for the largest portion of the assemblage (28.2 %) in the first group of traps (above-canopy layer, ≥32 m), followed by Fraxinus and Quercus (both 16 %), and Pinus (11 %). Alnus + Carpinus, Juglans and Ulmus pollen have lower values of 3.7, 5 and 3.1 %, respectively. The 9 % herb pollen is dominated by Artemisia and Asteraceae + Chenopodiaceae. The shrub pollen is 3 % and fern spores are low (Fig. 3).
In the trunk layer (8 ≤ 32 m), Betula (19.8 %), Alnus + Carpinus, Juglans and Celtis + Ulmus have generally lower percentages than in the canopy layer, while Fraxinus (23.5 %) and Tilia (except a high percentage at 40 m) have higher percentages. All herbs have lower percentages in the trunk layer than in the canopy layer, except Scrophulariaceae (16 m) and Ranunculaceae (8 m) (Fig. 3).
Near the ground (0 m) pollen percentages in the traps are comparable to those at 8 m except for Quercus (28.6 %), Urticaceae and Brassicaceae, which have higher percentages near the ground. Pollen percentages in the soil samples are significantly different, with a dominance of Pinus (54.9 %) and Abies + Picea (6.2 %), both of which have higher percentages than in the traps, Tilia, Artemisia (2.4 %), Asteraceae + Chenopodiaceae (1.8 %) and Brassicaceae pollen with similar percentages to the ones in the traps, while all other trees and herbs have lower percentages. The spores of ferns (with an average of 3.1 %, mainly trilete spores and Selaginella sinensis) have higher percentages than in the traps (Fig. 3).
Pollen influx
The average pollen influx from 2006 to 2010 is 17,305 grains cm−2 yr−1, the maximum average pollen influx is 37,042 grains cm−2 yr−1 at 16 m height, and the minimum pollen influx is 7,399 grains cm−2 yr−1 at 60 m height (Fig. 4b).
The tree pollen influx is highest (average 14,685 grains cm−2 yr−1) with a maximum of 26,392 grains cm−2 yr−1 at 16 m. The dominant tree pollen taxa include Betula (3,922 grains cm−2 yr−1), Fraxinus (3,600 grains cm−2 yr−1), Quercus (2,944 grains cm−2 yr−1) and Pinus (1,856 grains cm−2 yr−1). In contrast, the shrub (Rosaceae and others) influx (average 328 grains cm−2 yr−1) is low. The herbs have an average and maximum pollen influx of 2,265 grains cm−2 yr−1 and 10,742 grains cm−2 yr−1 (at 16 m) respectively. The highest values of pollen influx are found for Artemisia, Asteraceae + Chenopodiaceae, Ranunculaceae and Fabaceae. The ferns with an average of 14 spores cm−2 yr−1 show a maximum of 54 spores cm−2 yr−1 on the ground and fewer than 30 spores cm−2 yr−1 at other heights (Fig. 4b).
Pollen influx also varies between years (Fig. 4a; Table 3). Total pollen influx was highest at 21,390 grains cm−2 yr−1 in 2007 and lowest with 11,010 grains cm−2 yr−1 in 2010. Among the main types of tree pollen, Fraxinus (6,770 grains cm−2 yr−1), Quercus (3,700 grains cm−2 yr−1) and Populus (620 grains cm−2 yr−1) had the highest pollen influx values in 2007 and the lowest values in 2008 (Fraxinus and Quercus) and 2010 (Populus). Pinus pollen influx was the highest in 2010 with 2,960 grains cm−2 yr−1 and the lowest in 2009 with 1,080 grains cm−2 yr−1. In addition, pollen influx of Betula (10,010 grains cm−2 yr−1), Alnus + Carpinus (1,090 grains cm−2 yr−1) and Ulmus (1,520 grains cm−2 yr−1) were the highest in 2008 and the lowest in 2010 (Betula and Alnus + Carpinus) and 2009 (Ulmus) respectively. The variations observed for major herb pollen taxa such as Artemisia and Chenopodiaceae + Asteraceae follow the same trends. The pollen influx of Artemisia and Chenopodiaceae + Asteraceae was highest in 2009 with 980 and 300 grains cm−2 yr−1 respectively and lowest in 2010 with 220 and 90 grains cm−2 yr−1 respectively.
Surface soil samples and trap samples on the ground
Standard deviations for the major tree pollen taxa indicate that there are significant differences between the pollen assemblages from surface soil samples and those from trap samples on the ground (Fig. 5; ESM Appendix 4). Pollen percentages of Pinus and Picea + Abies in the surface soil samples are higher than in trap samples by 40 and 6 %, respectively. In contrast, the percentages of Quercus, Fraxinus, Salix, Populus, Ulmus, and Juglans are lower in the surface soil samples than in the trap samples on the ground. Moreover, a high proportion of the Quercus, Fraxinus and Populus pollen in the soil samples was seen to be damaged during identification.
Characteristic pollen source area
The FSP for 12 selected major pollen taxa is given in Table 2. The effect of different wind speeds on the CPSA is shown in Fig. 6. If the wind speed is constant, the lower the values of FSP are, the larger is the CPSA.
The characteristic pollen source area (CPSA) of 12 pollen taxa for different wind speeds in the Changbai mountains. a mean annual wind speed 2.4 m s−1, b maximum mean annual wind speed 6 m s−1, and c CPSA for 90–98 % of the 12 taxa (% depending on the taxa) for wind speeds of 2.4 m s−1 (60 km), or 6 m s−1 (100 km) indicated on the vegetation map, and CPSA for 90 % of Pinus and Quercus (5 or 45 km), Ulmus, Oleaceae, Tilia, Betula (10 or 45 km), Fraxinus, Poaceae, Chenopodiaceae, Populus and Salix (45 or 85 km, also for Artemisia) for wind speeds of 2.4 and 6 m s−1, and Artemisia (60 km) for a wind speed of 2.4 m s−1
The CPSA for 90 % of the Pinus and Quercus pollen at the sampling point, for example, is 5 or 45 km for wind speeds of 2.4 and 6 m s−1, while it is 60 or 85 km for 90 % of Artemisia. Other examples are provided in Fig. 6c. 75–95 % of the whole pollen assemblage with 12 pollen taxa, the percentage depending on the taxon, is coming from an area of 10 km radius when the wind speed is 2.4 m s−1. However 65–75 %, less pollen, out of the whole pollen assemblage is coming from 10 km radius when the wind speed is 6 m s−1. Furthermore, the CPSA for 90–98 % of the whole pollen assemblage is 60 or 100 km with a wind speed of 2.4 or 6 m s−1 (Fig. 6b). The values of 60 or 100 km correspond approximately to the maximum pollen source areas of the 12 pollen taxa, although 2–10 % of the pollen at the sampling point is coming from distances greater than 60 or 100 km. The calculated values of CPSA also imply that the higher the wind speed, the smaller the amount of pollen from a given distance, and the larger the distance from which a given percentage of the pollen is coming.
Discussion
Vertical pollen dispersion, pollen influx and pollen percentages
The discriminant analysis of both pollen percentages and influx values (Fig. 2) demonstrates that the grouping of pollen samples according to the various woodland layers is appropriate for discussing the results from the pollen traps in relation to the plant composition of various layers in the woodland. It also indicates that the differences in pollen composition between the woodland layers are related both to the plant composition in these layers and to the processes of pollen dispersal within the layers. The dominant pollen types in both pollen influx and pollen percentages are Betula, Fraxinus, Quercus and Pinus among trees and Artemisia, and Asteraceae + Chenopodiaceae among herbs. There are, however, some differences in ranking of the pollen taxa between percentages and pollen influx that are explained by the variation of pollen influx with height (Fig. 4).
The values of pollen influx are relatively low above 32 m and decrease with elevation up to 60 m. They are highest for most taxa from the ground to 26 m, but decrease from 16 m with decreased elevation for several taxa. However, Tilia, Quercus, Asteraceae, Urticaceae and Brassicaceae have higher influx on the ground than at 8 m, suggesting that these pollen grains either fall more rapidly from their point of dispersal to the ground in the case of Tilia and Quercus, or they have their flowers, which are the point of dispersal, closer to the ground, as in the case of herbs. These results also indicate that a small part of the pollen dispersed by the woodland is transported above the canopy. Although the pollen influx is generally higher at 16 m than at 8 m and on the ground, the overall pollen rain collected on the woodland floor is more similar to the pollen rain in the trunk layer than to that above the canopy layer, in terms of both amount of pollen deposited and taxa composition, which is in accordance with earlier studies in China (Liu et al. 1997; Ma et al. 1993; Zhang et al. 2009; Li et al. 2011), the conceptual model and observations of Tauber (1965), and the recent study of Matthias and Giesecke (2014) in northern Germany. This phenomenon is important to understand, because the pollen data used for reconstruction of past vegetation may either be from small or large sites within woodlands, which will have implications for the amount of pollen coming from the trunk space or from above the tree canopy. For instance, pollen influx of pine is much lower than that of birch in the above-canopy layer, while the two taxa have similar pollen influx in the trunk and ground layers (Fig. 4). Therefore, a pollen sample from a small peat hollow in a woodland, given adequate pollen preservation for all plant taxa, should be representative of the pollen dispersed by the trees, shrubs and herbs within the woodland.
Gravity and variations in airflow are the main factors influencing pollen influx. The highest values of pollen influx at a height of 16 m may be explained by the large gaps and stronger airflows between the trunks (Zhang et al. 2009). Liu et al. (1997) suggested that the effects of turbulence in the trunk layer are greater than the effects of turbulence in the canopy or near the ground; in addition, down draughts carry the upper pollen and up draughts carry the lower pollen to that layer (Zhang et al. 2009). The decrease in pollen influx with heights from 32 m might be due to the great air turbulence above the canopy layer, where most pollen is transported downwards (Ma et al. 1993). In contrast, the pollen influx increases with decreasing height from 26 m downwards due to the influence of gravity on pollen grains (Li et al. 2011).
There are clear cycles of low and high pollen influx years over the 4 to 5 years of pollen monitoring (Table 3). These cycles differ between taxa and are therefore probably caused by the natural physiological properties of the plant taxa. However, there are similarities in the cycles of Betula and Alnus + Carpinus, and those of Pinus and Quercus. The latter indicate that pollen influx might also be related to climate factors, however different ones depending on the taxa. It is known from studies in Europe, for example, that some trees react positively with high pollen influx to the summer temperature of the year before dispersal of the pollen, while others may react to the winter temperatures prior to pollen dispersal, because a long period of winter dormancy due to low temperatures can cause high pollen influx the next year (Autio and Hicks 2004; Barnekow et al. 2007; Kuoppamaa et al. 2009). More detailed conclusions for the results from our study would require a correlation analysis of the pollen influx data and meteorological data for the years 2006–2010, which was not done as part of this project.
The differences in pollen percentages between the three layers (Fig. 3) are easily explained by the differences in the values of pollen influx (Fig. 4). For instance, the decrease in the percentages of birch between the above-canopy layer and the trunk layer is explained by the significantly higher pollen influx of pine in the trunk layer compared to the above-canopy layer, while the influx of birch is not much higher in the trunk layer. Betula, Alnus + Carpinus and Juglans are the tree taxa that show the least differences in pollen influx between the layers, suggesting that a larger part of the pollen dispersed by them compared to other taxa is staying and transported above the canopy, and/or that most of the pollen is coming from the regional rather than the local vegetation. Betula is indeed mainly represented in the Betulaceae woodlands situated ca. 30 km from the sampling site, while it is not a major taxon in the local broad-leaved and pine mixed woodland.
Differences in pollen assemblages between soil and trap samples—implications
Generally, pollen in surface soils represents what has been deposited there in the past decades and the pollen assemblages are dominated by the pollen types with the most decay-resistant pollen walls (Wilmshurst and McGlone 2005; Xu et al. 2009). In contrast, traps can provide accurate quantitative information on pollen deposition in a given spatio-temporal framework, and they are not affected by factors of pollen preservation (Hicks 1996; Li et al. 2009). In this study the pollen assemblages (in percentages) from the soil samples, when compared to those from the traps, are clearly biased towards pollen types that are easily preserved due to their thick and robust walls, such as Pinus, Abies, Picea, Tilia, Artemisia, Chenopodiaceae, Asteraceae, Brassicaceae and spores of Selaginella.
Based on data from pollen traps and surface samples from forest and steppe communities in northern China, Xu et al. (2009) and Li et al. (2009) suggested that the pollen assemblages from both traps and soil surface samples can reflect the basic characteristics of a plant community. However, the pollen assemblages in soil surface samples reflect the most dominant plant taxa and often also the most decay-resistant pollen grains, which may bias the relationship between pollen and vegetation. In contrast, pollen assemblages from traps are a better representation of the vegetation than those from surface soil samples and they provide better pollen-vegetation and pollen-climate relationships (Li et al. 2009). Therefore, the authors suggested that soil samples should be avoided in modern pollen training datasets for the development of transfer functions and their application in the quantitative reconstruction of past climate using fossil pollen records from lake sediment cores in arid and semi-arid China (Zhao et al. 2009).
Our results also suggest that pollen records from soils in the study region are biased and might provide erroneous pollen-vegetation relationships, which in turn will imply unreliable reconstruction of past vegetation. For such investigations, pollen should be extracted from pollen traps for annual data over many years, or moss polsters (for further discussion on the pollen content of pollen traps versus moss polsters, peat and lake sediments, see Räsänen et al. 2004; Lisitsyna et al. 2012). However, soil samples will have to be used in situations where moss polsters are not available, which is often the case in arid and semi-arid regions, and/or pollen trapping over many years is not possible because of the time and cost aspects of such studies. In such cases, pollen data from soils are valuable, but should be interpreted with caution.
Characteristic pollen source area (CPSA) and pollen-vegetation relationships
The fall speed of pollen (FSP) has a major effect on the calculated CPSA, so the lower the values of FSP, the larger the CPSA with a given wind speed. It implies that many herb pollen types such as Artemisia, Poaceae and Chenopodiaceae, which often have a lower FSP than tree pollen types such as Pinus, Quercus, Ulmus, Tilia, Betula and Oleaceae, and also have a much larger CPSA than tree pollen. Examples of tree pollen with low FSP and CPSAs comparable to herb pollen are Fraxinus, Populus and Salix. The equation used for the calculation of the CPSA does not take into account the effect of the morphology of many conifer pollen types, such as Pinus, Abies and Picea, which have two “air sacs” on each side of the “body” (central cell) of the pollen grain. These air sacs increase the ability of these grains to fly over long distances. Therefore, the values of calculated CPSA for these tree taxa may be misleading. It is indeed known that pollen from Pinus is transported over very long distances (Ertl et al. 2012), although it might be only a relatively small portion of the total pollen which it disperses (Fig. 6b). Distances of 60 and 100 km correspond approximately to the maximum radius of the pollen source area of the 12 studied pollen taxa (90–98 % of the pollen) when the wind speed is 2.4 or 6 m s−1. It implies that the pollen assemblages collected in the pollen traps on the ground give a fair representation of the vegetation around the traps for those areas (60 and 100 km radius), given these wind speeds (2.4 and 6 m s−1). Moreover, at least 80 % of the pollen of all 12 taxa is coming from an area of 20 or 40 km, when the wind speeds are 2.4 or 6 m s−1, which suggests that the pollen assemblages on the ground also give a fairly good representation of the vegetation within those shorter distances.
Theoretically, pollen taxa with large CPSAs will have a tendency to represent more strongly the regional vegetation, while the pollen taxa with small CPSAs will better represent the local vegetation. In this case, according to the calculated CPSAs, the pollen taxa that best represent the local vegetation in an area of a few km to 5 km radius are the trees Pinus, Quercus, Ulmus, Tilia, Oleaceae and Betula (Fig. 6a, b). Therefore, the mixed woodland around the pollen sampling site of deciduous broad-leaved trees and P. koraiensis is theoretically well represented by the pollen assemblages recorded in the traps; only the pollen records of Fraxinus, Populus and Salix would match better with a larger area of woodland around the sampling point (Figs. 6a, b). Most herb taxa, such as Artemisia, Poaceae and Chenopodiaceae, would also match better the vegetation in a larger area, and most of their pollen, in the case of this study, probably comes from the farmlands situated at 80–100 km from the sampling site (Fig. 6c).
According to the vegetation map (Fig. 6c), the minimum distances between the parent plant populations and the sampling sites are ca. 8 km for hazel scrub, ca. 25 km for spruce and fir woodland (Picea + Abies), 30 km for Betulaceae woodland (Betula, Alnus) and Larix woodland and Chrysanthemum-Pinus woodland, ca. 65 km for deciduous oak woodland and ca. 70 km for farmland. The theoretical, calculated CPSAs of the 12 selected taxa indicate that all the vegetation units in the study area should be well represented in the pollen records collected in this woodland landscape, except for the hazel scrub and Larix woodland, from which no pollen grains were found. This may be due to the low pollen productivity and bad preservation ability of Larix (Li et al. 2009) and to poor pollen dispersal from Corylus scrub within the woodland (Xu et al. 2007b). The large amounts of Betulaceae pollen (Betula and Alnus + Carpinus) in the trap samples suggest that this pollen is coming from at least 30 km. Although the Abies-Picea woodland is situated at a minimum distance of 25 km, the pollen values of Abies + Picea are relatively low compared to those of Pinus and Betulaceae. This might be due to i) their higher FSP and smaller theoretical CPSAs (not shown), ii) the smaller surface covered by the Abies-Picea woodland compared to the broad-leaved and pine mixed woodland (see vegetation map), and iii) their lower pollen productivity compared to Betula. By comparison with the vegetation map, we know that Betula and herb pollen, for example, are regional components in the pollen records from the traps, as these taxa are located at a considerable distance from the sampling point, 30 km for Betula and 70 km for herbs. But this does not mean that pollen from Betula and herbs can, in general, be considered as principally representative of regional vegetation. The theoretical CPSA of Betula is smaller than that of herbs. Moreover, CPSA values do not necessarily imply that, for example, pollen from Betula and herbs are primarily representative of regional vegetation because their CPSAs are large relative to other taxa. The theoretical CPSAs of pollen taxa only indicate that, theoretically, the proportion of the pollen coming from a long distance is larger for taxa such as Betula and herbs than for Abies and Picea. Single pollen taxa cannot be characterised as “regional” vs. “local” taxa. Distinguishing between the local and regional part of a pollen assemblage at a sampling site can only be achieved with modelling approaches such as that used in the Landscape Reconstruction Algorithm (LRA; Sugita 2007a, b), which requires that estimates of pollen productivity are available for the taxa occurring in the sample.
The soundness of Prentice’s pollen dispersal models: evidence from pollen influx data
In a recent study of modern pollen influx in European lake sediments (Matthias and Giesecke 2014), the authors argue that the models of pollen dispersal and deposition as used in the LRA (Sugita 2007a, b) are not adequate for small sites, because they use a pollen source at ground level and assume that all pollen is dispersed over the tree canopy. However, one should remember that the use of a pollen source at ground level does not imply that the pollen in the trunk layer is not taken into account in the models (as stated by Matthias and Giesecke 2014, p. 21). It is correct that the models assume that horizontal pollen transport by wind occurs above the tree canopy, while vertical pollen transport within the woodland occurs by gravity. However, the assumption of a pollen source at ground level in fact implies that the model considers the woodland as one layer without any significant height, as if it were grassland. Although pollen dispersal processes are greatly simplified, these models have been shown to describe pollen dispersal adequately, so (i) simulated pollen influx values using models assuming a pollen source at ground level were closer to empirical pollen influx values from lakes and bogs than those using models assuming an elevated pollen source (Prentice 1985), and (ii) the LRA approach using the Prentice-Sugita model (Sugita 1993) was successful in translating pollen percentages into absolute cover of plant taxa in several regions of the world (Hellman et al. 2008; Sugita et al. 2010b).
Our results show that (i) the pollen influx values of trees, shrubs and herbs are very similar, both in the lower part of the trunk layer (8–26 m) and on the ground. They suggest that the pollen values recorded on the ground are a fair representation of the major part of the pollen transported in trunk space of the woodland. They also show that pollen deposition at various heights above the ground, up to 26 m in this study, does not differ significantly from that on the ground. Therefore, simulated CPSA values for pollen deposition in the traps at various heights on the weather tower using Prentice’s model should be relevant approximations. Furthermore, all major plants in the woodland and vegetation types occurring in an area within 70 km radius of our study site are present in the ground surface pollen samples. Thus, pollen records from small basins in the woodland, as in bog deposits or lake sediments with good pollen preservation, should also be representative of the vegetation around the study site within a radius of several kilometres. The application of Prentice’s model (Prentice 1985) for bogs provides values of CPSAs of up to 60 km, which again indicates that models assuming a pollen source at ground level are describing pollen dispersal and deposition reasonably well, even though the pollen sources in woodland are located at various heights above the ground.
Conclusions
-
(1)
Pollen percentages and pollen influx collected in traps at various heights in the P. koraiensis broad-leaved mixed woodlands of the Changbai mountains, northeastern China, have very similar characteristics to pollen assemblages, with the largest values of tree pollen coming from Betula, Fraxinus, Quercus and Pinus, and herb pollen from Artemisia, Chenopodiaceae and Asteraceae.
-
(2)
Pollen influx values vary significantly with height and show major differences between three distinct layers, above the canopy, within the trunk layer and on the ground. These differences in pollen influx are explained by differences in (i) the air flows in each of these layers and (ii) the fall speeds of pollen of the various taxa.
-
(3)
The differences in occurrences of pollen taxa and pollen percentage values between soil samples and pollen traps on the ground suggest that pollen records from soils are biased and should be avoided for studies of pollen-vegetation relationships for the interpretation of pollen records from lakes and bogs and for the calculation of pollen productivity estimates. However, pollen data from soils may be valuable when no other sources of pollen data are available, although they should be interpreted with caution.
-
(4)
Comparison of the pollen influx values with (i) the theoretical, calculated CPSA of 12 selected taxa and (ii) with the vegetation map of the area around the sampling site, indicates that the pollen deposited on the ground surface of the woodland (i) is a fair representation, in which 85–90 % of the total pollen deposited, with a wind speed of 2.4 m s−1, comes from within ca. 1–5 km of the sampling site for Pinus and Quercus, ca. 5–10 km for Ulmus, Tilia, Oleaceae and Betula, 20–40 km for Fraxinus, Poaceae, Chenopodiaceae, Populus and Salix, and 30–60 km for Artemisia; (ii) is a good representation in which 90–98 % of the total pollen deposited comes from within 60 km, at a wind speed of 2.4 m s−1, or 100 km at a wind speed of 6 m s−1, for the 12 selected taxa used in the CPSA calculation; and (iii) represents all plant communities growing in the study area within 70 km radius, except Corylus scrub and Larix woodland.
-
(5)
Our results confirm that although the pollen dispersal processes are strongly simplified in Prentice’s model, it does describe pollen dispersal and deposition reasonably well in woodlands of the type that we have studied, which also implies that application of the LRA approach using pollen records from small sites in these woodlands would be relevant.
References
Autio J, Hicks S (2004) Annual variations in pollen deposition and meteorological conditions on the fell Aakenustunturi in northern Finland: potential for using fossil pollen as a climate proxy. Grana 43:31–47
Barnekow L, Loader NJ, Hicks S, Froyd CA, Goslar T (2007) Strong correlation between summer temperature and pollen accumulation rates for Pinus sylvestris, Picea abies and Betula spp. in a high-resolution record from northern Sweden. J Quat Sci 22:653–658
Broström A, Sugita S, Gaillard MJ (2004) Pollen productivity estimates for the reconstruction of past vegetation cover in the cultural landscape of southern Sweden. Holocene 14:368–381
Bunting MJ, Gaillard MJ, Sugita S, Middleton R, Broström A (2004) Vegetation structure and pollen source area. Holocene 14:651–660
Calcote R (1995) Pollen source area and pollen productivity: evidence from forest hollows. J Ecol 83:591–602
Davis MB (1963) On the theory of pollen analysis. Am J Sci 261:897–912
Ertl C, Pessi AM, Huusko A, Hicks S, Kubin E, Heino S (2012) Assessing the proportion of “extra-local” pollen by means of modern aerobiological and phenological records—an example from Scots pine (Pinus sylvestris L.) in northern Finland. Rev Palaeobot Palynol 185:1–12
Gaillard MJ, Birks HJB, Emanuelsson U, Karlsson S, Lagerås P, Olausson D (1994) Application of modern pollen/land-use relationships to the interpretation of pollen diagrams—reconstructions of land-use history in south Sweden 3000-0 BP. Rev Palaeobot Palynol 82:47–73
Gregory PH (1973) The microbiology of the atmosphere, 2nd edn. Wiley, New York
Hellman S, Gaillard MJ, Broström A, Sugita S (2008) The REVEALS model, a new tool to estimate past regional plant abundance from pollen data in large lakes: validation in southern Sweden. J Quat Sci 23:21–42
Hicks S (1996) The feasibility of using pollen deposition data as climatic indices. Paläoklimaforschung 20:173–187
Hicks S (2001) The use of annual arboreal pollen deposition values for delimiting tree-lines in the landscape and exploring models of pollen dispersal. Rev Palaeobot Palynol 117:1–29
Jackson ST, Webb T III, Prentice IC, Hansen JE (1995) Exploration and calibration of pollen/vegetation relationships: a PC program for the extended R-value models. Rev Palaeobot Palynol 84:365–374
Jacobson GL, Bradshaw RHW (1981) The selection of sites for paleovegetational studies. Quat Res 16:80–96
Koff T (2001) Pollen influx into Tauber traps in Estonia in 1997–1998. Rev Palaeobot Palynol 117:53–62
Kuoppamaa M, Huusko A, Hicks S (2009) Pinus and Betula pollen accumulation rates from the northern boreal forest as a record of interannual variation in July temperature. J Quat Sci 24:513–521
Li JD, Wu BH, Sheng LX (2001) Jilin Vegetation. Jilin Science and Technology Press, Changchun (in Chinese)
Li YC, Xu QH, Zhang LY, Wang XL, Cao XY, Yang XL (2009) Modern pollen assemblages of the forest communities and their relationships with vegetation and climate in northern China. J Geogr Sci 19:643–659
Li JY, Xu QH, Cao XY, Pang RM, Ding W, Lin FY (2011) Two year’s pollen influx and pollen vertical dispersion in the coniferous and deciduous broad-leaved mixed forest of Changbai Mountains. Quat Sci 31:171–179 (in Chinese, with English abstract)
Lisitsyna OV, Hicks S, Huusko A (2012) Do moss samples, pollen traps and modern lake sediments all collect pollen in the same way? A comparison from the forest limit area of northernmost Europe. Veget Hist Archaeobot 21:187–199
Liu HP, Liu SH, Zhu TY, Jin CJ, Kong FZ (1997) Turbulence structure characteristics within and above Changbai Mountain Forest. Acta Sci Nat Univ Pekin 33:246–253 (in Chinese, with English abstract)
Lynch EA (1996) The ability of pollen from small lakes and ponds to sense fine-scale vegetation patterns in the Central Rocky Mountains, USA. Rev Palaeobot Palynol 94:197–210
Ma YM, Gao YQ, Li DX, Hu JL (1993) Observational study of atmospheric turbulence above and within a pine forest. Meteorol Mon 19:13–18 (in Chinese, with English abstract)
Matthias I, Giesecke T (2014) Insights into pollen source area, transport and deposition from modern pollen accumulation rates in lake sediments. Quat Sci Rev 87:12–23
Mazier F, Nielsen AB, Broström A, Sugita S, Hicks S (2012) Signals of tree volume and temperature in a high-resolution record of pollen accumulation rates in northern Finland. J Quat Sci 27:564–574
Prentice IC (1985) Pollen representation, source area, and basin size: toward a unified theory of pollen analysis. Quat Res 23:76–86
Räsänen S, Hicks S, Odgaard BV (2004) Pollen deposition in mosses and in a modified ‘Tauber trap’ from Hailuoto, Finland: what exactly do the mosses record? Rev Palaeobot Palynol 129:103–116
Reese CA, Liu K (2005) A modern pollen rain study from the central Andes region of South America. J Biogeogr 32:709–718
Song YM, Guo WD, Zhang YC, Cheng YL (2009) Performances of CoLM and NCAR CLM3.0 in simulating land-atmosphere interactions over typical forest ecosystems in China. Part I: preliminary analysis of the simulations based on different models. Clim Environ Res 14:229–242 (in Chinese, with English abstract)
Sugita S (1993) A model of pollen source area for an entire lake surface. Quat Res 39:239–244
Sugita S (1994) Pollen representation of vegetation in Quaternary sediments: theory and method in patchy vegetation. J Ecol 82:881–897
Sugita S (2007a) Theory of quantitative reconstruction of vegetation I: pollen from large sites REVEALS regional vegetation composition. Holocene 17:229–241
Sugita S (2007b) Theory of quantitative reconstruction of vegetation II: all you need is LOVE. Holocene 17:243–257
Sugita S, Gaillard MJ, Broström A (1999) Landscape openness and pollen records: a simulation approach. Holocene 9:409–421
Sugita S, Hicks S, Sormunen H (2010a) Absolute pollen productivity and pollen-vegetation relationships in northern Finland. J Quat Sci 25:724–736
Sugita S, Parshall T, Calcote R, Walker K (2010b) Testing the landscape reconstruction algorithm for spatially explicit reconstruction of vegetation in northern Michigan and Wisconsin. Quat Res 74:289–300
Sun XJ, Wu YS (1988) Modern pollen rain of needle and broadleaved mixed forest in Changbai Mountains. Acta Botanica Sinica 30:549–557 (in Chinese, with English abstract)
Sun ZW, Zhao SD (1995) Community features of Tilia-Korean Pine Forest on Northern Slope of Changbai Mountains. Chin J Ecol 14:26–30 (in Chinese, with English abstract)
Tauber H (1965) Differential pollen dispersion and the interpretation of pollen diagrams. Dan Geol Unders II Række 89:1–69
Tian F, Xu QH, Li YC, Cao XY (2010) Characteristics of pollen influx and pollen source dynamics of Baiyang Lake. Sci Geogr Sin 30:955–961 (in Chinese, with English abstract)
Vincens A, Williamson D, Thevenon F, Taieb M, Buchet G, Decobert M, Thouveny N (2003) Pollen-based vegetation changes in southern Tanzania during the last 4200 years: climate change and/or human impact. Palaeogeogr Palaeoclimatol Palaeoecol 198:321–334
Wang FX (1995) Pollen flora of China. Science Press, Beijing (in Chinese)
Wang YB, Herzschuh U (2011) Reassessment of Holocene vegetation change on the upper Tibetan Plateau using the pollen-based REVEALS model. Rev Palaeobot Palynol 168:31–40
Wang XL, Li YC, Xu QH, Yang XL, Zhang ZQ, Jia HJ, Cao XY, Zhang LY (2008) Modern pollen distribution in the middle and north of Luliang Mountains, Shanxi Province. Acta Ecol Sin 28:3,682–3,690 (in Chinese, with English abstract)
Wilmshurst JM, McGlone MS (2005) Origin of pollen and spores in surface lake sediments: comparison of modern palynomorph assemblages in moss cushions, surface soils and surface lake sediments. Rev Palaeobot Palynol 136:1–15
Wu ZY (1980) Vegetation of China. Science Press, Beijing (in Chinese)
Xia FC, Zhao XH, Pan CF, Jia YZ, Wang JS (2010) Stand structure of broad-leaved and Korean pine (Pinus koraiensis) mixed forest in the Changbai Mountains, China. Chin J Appl Environ Biol 16:529–534 (in Chinese, with English abstract)
Xing SP (1988) Jilin Forest. Jilin Science and Technology Press, Changchun (in Chinese)
Xu QH, Zhang SR (2013) Advance in pollen source area. Adv Earth Sci 28:968–975 (in Chinese, with English abstract)
Xu QH, Li YC, Li Y, Yang XL, Zhang ZQ, Jia HJ (2006) Modern pollen process and several issues concerning the study of Quaternary environment. Prog Nat Sci 16:647–656 (in Chinese)
Xu QH, Li YC, Zhou LP, Li YY, Zhang ZQ, Lin FY (2007a) Pollen flux and vertical dispersion in coniferous and deciduous broadleaved mixed forest in the Changbai Mountains. Chin Sci Bull 52:1,540–1,544
Xu QH, Li YC, Yang XL, Zheng ZH (2007b) Quantitative relationship between pollen and vegetation in northern China. Sci China, Ser D Earth Sci 50:582–599
Xu QH, Li YC, Tian F, Cao XY, Yang XL (2009) Pollen assemblages of tauber traps and surface soil samples in steppe areas of China and their relationships with vegetation and climate. Rev Palaeobot Palynol 153:86–101
Xu QH, Li YC, Bunting MJ, Tian F, Liu JS (2010) The effects of training set selection on the relationship between pollen assemblages and climate parameters: implications for reconstructing past climate. Palaeogeogr Palaeoclimatol Palaeoecol 289:123–133
Xu QH, Tian F, Bunting MJ, Li YC, Ding W, Cao XY, He ZG (2012) Pollen source areas of lakes with inflowing rivers: modern pollen influx data from Lake Baiyangdian, China. Quat Sci Rev 37:81–91
Zhang JY, Dong WJ, Ye DZ, Fu CB (2003) New evidence for effects of land cover in China on summer climate. Chin Sci Bull 48:401–405
Zhang LY, Xu QH, Li YC, Wang XL, Cao XY, Tian F (2009) Comparison of two year’s pollen influx and vertical dispersion in the mixed conifer and broad-leaved forest of the Changbai Mountain. Acta Palaeontol Sin 48:222–227 (in Chinese, with English abstract)
Zhao XS, Guan DX, Wu JB, Jin CJ, Han SJ (2005) Distribution of footprint and flux source area of the mixed forest of broad-leaved and Korean pine in Changbai Mountains. J Beijing For Univ 27:17–23 (in Chinese, with English abstract)
Zhao Y, Xu QH, Huang XZ, Guo XL, Tao SC (2009) Differences of modern pollen assemblages from lake sediments and surface soils in arid and semi-arid China and their significance for pollen-based quantitative climate reconstruction. Rev Palaeobot Palynology 156:519–524
Acknowledgments
We sincerely thank three anonymous reviewers and the editor for their constructive suggestions and comments. This study was supported by the National Science Foundation of China (Grant No.: 41371215), the Key Technology R&D Program of Hebei Province (Grant No.: 13277611D), the Foundation of Key Discipline of Hebei Province and Hebei Key Laboratory of Environmental Change and Ecological Construction, the Recruitment Program of High-end Foreign Experts (Grant No.: GDW20151300002), the Faculty of Health and Life Sciences of Linnaeus University, and the Swedish Strategic Research Area ‘Modelling the Regional and Global Earth system-MERGE’ (http://www.merge.lu.se).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Y. Zhao.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, S., Xu, Q., Gaillard, MJ. et al. Characteristic pollen source area and vertical pollen dispersal and deposition in a mixed coniferous and deciduous broad-leaved woodland in the Changbai mountains, northeast China. Veget Hist Archaeobot 25, 29–43 (2016). https://doi.org/10.1007/s00334-015-0532-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00334-015-0532-0