Abstract
Objectives
To evaluate the diagnostic performance of quantitative magnetic resonance (MR) imaging biomarkers in distinguishing between inflammatory pancreatic masses (IPM) and pancreatic cancer (PC).
Methods
A literature search was conducted using PubMed, Embase, the Cochrane Library, and Web of Science through August 2023. Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) was used to evaluate the risk of bias and applicability of the studies. The pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio were calculated using the DerSimonian-Laird method. Univariate meta-regression analysis was used to identify the potential factors of heterogeneity.
Results
Twenty-four studies were included in this meta-analysis. The two main types of IPM, mass-forming pancreatitis (MFP) and autoimmune pancreatitis (AIP), differ in their apparent diffusion coefficient (ADC) values. Compared with PC, the ADC value was higher in MFP but lower in AIP. The pooled sensitivity/specificity of ADC were 0.80/0.85 for distinguishing MFP from PC and 0.82/0.84 for distinguishing AIP from PC. The pooled sensitivity/specificity for the maximal diameter of the upstream main pancreatic duct (dMPD) was 0.86/0.74, with a cutoff of dMPD ≤ 4 mm, and 0.97/0.52, with a cutoff of dMPD ≤ 5 mm. The pooled sensitivity/specificity for perfusion fraction (f) was 0.82/0.68, and 0.82/0.77 for mass stiffness values.
Conclusions
Quantitative MR imaging biomarkers are useful in distinguishing between IPM and PC. ADC values differ between MFP and AIP, and they should be separated for consideration in future studies.
Clinical relevance statement
Quantitative MR parameters could serve as non-invasive imaging biomarkers for differentiating malignant pancreatic neoplasms from inflammatory masses of the pancreas, and hence help to avoid unnecessary surgery.
Key Points
• Several quantitative MR imaging biomarkers performed well in differential diagnosis between inflammatory pancreatic mass and pancreatic cancer.
• The ADC value could discern pancreatic cancer from mass-forming pancreatitis or autoimmune pancreatitis, if the two inflammatory mass types are not combined.
• The diameter of main pancreatic duct had the highest specificity for differentiating autoimmune pancreatitis from pancreatic cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inflammatory pancreatic masses (IPM) primarily encompass mass-forming pancreatitis (MFP) and autoimmune pancreatitis (AIP). The imaging appearance of both tumors mimics pancreatic cancer (PC) [1, 2]. However, PC is a highly fatal disease and carries an extremely poor prognosis with the lowest 5-year relative survival rate among all cancers [3]. Surgical resection remains the only curative method available. In contrast, surgery is not the optimal treatment option for patients with IPM. Given that postoperative morbidity rates remain high, it is essential to accurately differentiate between IPM and PC to avoid unnecessary surgery [4]. Magnetic resonance (MR) imaging has the advantages of no radiation exposure and high soft tissue contrast. Quantitative MR parameters can serve as non-invasive imaging biomarkers to distinguish between malignant pancreatic neoplasms from inflammatory masses.
Several quantitative MR imaging biomarkers have been widely used in clinical practice, while only a select few have been investigated in limited patient cohorts with pancreatic diseases [5,6,7,8]. Magnetic resonance cholangiopancreatography (MRCP) can evaluate the pancreatic duct system, and the maximal diameter of the upstream main pancreatic duct (dMPD) was used as an effective imaging biomarker in the diagnosis of AIP [9, 10]. Diffusion-weighted imaging (DWI) is a technique that assessed the Brownian motion of water molecules noninvasively, and the apparent diffusion coefficient (ADC) values reflected the degree of diffusion restriction [11]. Notably, the study findings regarding ADC value, which is the most used quantitative MR imaging biomarker, were controversial, and recent reviews have also pointed to this issue [12, 13]. Nevertheless, we observed that certain earlier studies conflated the concepts of the two IPM entities, namely, MFP and AIP, which exhibit distinct pathological characteristics and appear to differ in their ADC values [14,15,16,17]. Intravoxel incoherent motion (IVIM) is an extension of DWI with multiple b values that can provide quantitative information on the molecular diffusion and microcirculation perfusion effects [18, 19]. The following are the IVIM-derived parameters: slow component of diffusion (Dslow) represents the pure molecular diffusion, fast component of diffusion (Dfast) represents the incoherent microcirculation, and perfusion fraction (f) is the diffusion fraction related to microcirculation [11]. Moreover, magnetic resonance elastography (MRE) has emerged as a useful modality for quantitative assessment of mass stiffness [20].
To the best of our knowledge, the above-mentioned imaging biomarkers have not been meta-analyzed to distinguish between IPM and PC.
Therefore, this systematic review and meta-analysis aimed to determine the usefulness of quantitative MR imaging biomarkers for distinguishing between IPM and PC.
Materials and methods
This meta-analysis complied with the Preferred Reporting Items for Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies (PRISMA-DTA) guidelines [21]. Therefore, the requirement for approval from the institutional review board was waived.
Literature search
We searched the PubMed, Embase, Cochrane Library, and Web of Science databases through August 2, 2023, with an English restriction applied. The search strategy consisted of (“pancreatic” OR “pancreas”) AND (“cancer*” OR “carcinoma*” OR “malignan*” OR “neoplasm*” OR “adenocarcinoma*”) AND (“Pancreatitis”) AND (“Magnetic Resonance Imaging” OR “magnetic resonance” OR “MRI” OR “MRCP” OR “MRE” OR “diffusion-weighted” OR “DWI”) OR (“Diagnosis, Differential” OR “Sensitivity and Specificity” OR “Predictive Value of Tests” OR “diagnostic performance” OR “differentia*” OR “sensitiv*” OR “specificit*” OR “accurac*” OR (“predictive” AND “value”)). The detailed search strategy is presented in Supplementary Table 1. The reference lists of the relevant studies were also checked manually to identify additional eligible studies.
Study selection
After removing duplicates, two reviewers assessed the relevance of the studies based on the titles and abstracts to remove irrelevant studies. Next, the full-text manuscripts were retrieved. In this study, we strictly distinguished between the two concepts for clarity: MFP is a type of disease based on chronic pancreatitis (CP) and has no background of AIP, AIP is a distinct disease with no background of MFP, and IPM represents either or a combination of the two. Studies that fulfilled the predefined inclusion and exclusion criteria were ultimately assessed as eligible. The inclusion criteria were as follows: (a) patients with IPM or PC, (b) quantitative MR imaging biomarkers, (c) diagnostic performance for distinguishing between IPM and PC, and (d) both observational (retrospective or prospective) and clinical trials. The exclusion criteria were as follows: (a) insufficient data: inability to create a 2 × 2 contingency table or number of studies for the same MR imaging biomarker was less than two; (b) less than five patients; (c) mixed patient group for the diagnostic accuracy assessment of ADC, Dslow, and Dfast; (d) outlier: the direction of cutoff value of which was opposite to all the other articles of similar or larger patient numbers; (e) other types of articles, such as case reports, review articles, comments, letters, conference abstracts, editorials, or articles published in non-English languages; and (f) articles not available. Disagreements between the reviewers were resolved through discussion to reach consensus.
Data extraction
Data were extracted independently by two reviewers using a standardized form. Discrepancies were resolved by double-checking and discussion to reach consensus. The predesigned data extraction table consisted of four aspects of information: (a) study characteristics: the first author’s name, publication year, country, key conclusions, study design, reference standard, interval between imaging and reference standards, and blinding to the reference standard; (b) patient characteristics: enrollment, number of patients or lesions, age, disease type, and AIP subtype; (c) MRI parameters: MRI unit, sequence, magnetic field strength, slice thickness, field of view, region of interest (ROI) size, number of b and b values (s/mm2), repetition time (TR) (ms), and echo time (TE) (ms); (d) outcomes: true positive, false positive, false negative, and true negative numbers and the cutoff values used for differential diagnosis. If the ADC values were based on multiple b values, the raw data of the ADC value with the highest sensitivity and specificity were extracted. If there were several readers for the same MR imaging biomarker, the sensitivity and specificity obtained by the reader with the highest accuracy were extracted. In addition, the original authors were contacted in the case of insufficient information.
Quality assessment
Quality assessment was performed using Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) [22]. Two reviewers independently assessed the risk of bias and applicability concerns in the following four domains: patient selection, index test, reference standard, and flow and timing. Disagreements were resolved through discussions to reach consensus. We did not assign scores to judge the quality of the included studies because each domain of QUADAS-2 had a different impact on the study results. Quality assessment forms were plotted using RevMan version 5.4.
Statistical analysis
The DerSimonian-Laird method (random effects model) was used for the meta-analysis. In cases where the cells were devoid of data, a correction of 1/2 was added to each cell. Forest plots were developed to visualize the pooled sensitivity and specificity with a 95% CI. The statistical heterogeneity of the included studies was assessed using the Cochrane Q test and Higgins I2 test. The Cochrane Q test (p < 0.05) indicated the presence of statistical heterogeneity. An I2 value between 20 and 50% indicated moderate heterogeneity, whereas an I2 value of > 50% indicated substantial heterogeneity. Moreover, Spearman’s rank correlation test was conducted to judge the presence of threshold effects. Because the number of studies for each biomarker was less than 10, the assessment of publication bias was considered unnecessary. Univariate meta-regression analyses were performed using predesigned subgroups to determine potential factors influencing heterogeneity. All statistical analyses were conducted by a single trained researcher (Z.H.W.) using Meta-DiSc version 1.4 and Stata version 15.0.
Results
Literature search and study selection
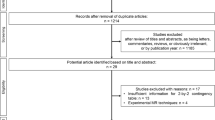
A flowchart of the literature screening process is shown in Fig. 1. A total of 1819 studies were initially retrieved. After removing 685 duplicates, a further 1030 studies were excluded because of obvious irrelevance. Next, 80 studies were excluded by the full-text review: four articles were not available; 13 articles did not involve a direct comparison of PC and any kind of IPM; eight articles did not report the diagnostic performance of quantitative MR imaging biomarkers; and 39 were other types of articles, as specified in the “Materials and methods” section. Fifteen articles were excluded due to insufficient data. Among the 15 articles, 14 were not able to construct 2 × 2 contingency tables and one reported the maximal diameter of the upstream main pancreatic duct (dMPD) with a cutoff value other than 4 mm or 5 mm for the differential diagnosis of AIP and PC [23]. Twenty-five studies were initially included for data extraction. After comparing the direction of cutoff values for MR imaging biomarkers (higher defined as IPM or lower defined as IPM), the direction of cutoff value for ADC values from one study showed obvious deviation from all the other studies which were further excluded to ensure the stability of the study results [24]. Twenty-four studies were included in the final meta-analysis [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48].
During the workflow of study selection and article review, we encountered a paradoxical aspect in the study results concerning the differentiation between IPM and PC using ADC values. Specifically, for the differential diagnosis of MFP and PC, all studies diagnosed MFP with an ADC value higher than the cutoff value, whereas for the differential diagnosis of AIP and PC, all studies diagnosed AIP with an ADC value lower than the cutoff value. Accordingly, the two IPMs were analyzed separately for the ADC values.
Data extraction and quality assessment
The characteristics of the included studies are summarized in Table 1. The study of Klauss et al [49] reported three sensitivities and specificities based on different b values, and we extracted the one with the highest sensitivity and specificity, which was b = 200 s/mm2. Figures 2 and 3 show the results of the quality assessment of the included studies using QUADAS-2. Among the included studies, 18 studies were retrospective, and six were prospective. For patient selection, eight studies were marked as having a high risk of bias because of their case-control design. Eighteen studies, reporting the diagnostic efficacy of the ADC, f, or mass stiffness, in which the cutoff values in 18 studies were all determined by the point of the largest Youden index or the point providing the balance between sensitivity and specificity on the ROC curves, were therefore marked as having an unclear risk of bias in the domain of the index test. For reference standards, 16 studies were marked as having an unclear risk of bias due to the absence of information on whether the researchers were blinded to the results of the index tests during the implementation of the reference standard. As five studies did not include all patients in the final analysis for various reasons (such as suboptimal imaging quality, imaging failure, incomplete imaging workup, and complications of other diseases), they were marked as high risk in the domain of flow and timing. Furthermore, nine studies were labeled with an unclear risk of bias concerning the unclear intervals between the index tests and the reference standards, as these details were not reported. There were no concerns regarding the applicability of the included studies.
Diagnostic performance of MR imaging biomarkers
The diagnostic performances of the quantitative MR imaging biomarkers are shown in Table 2. The study results of using ADC values to differentiate IPM from PC were controversial, but we noticed that the MFP vs. PC and AIP vs. PC groups had opposite directions concerning the cutoff value. The mean or median ADC values of the IPM and PC in individual studies are shown in Fig. 4. In the MFP vs. PC group, masses exhibiting higher ADC values were identified as MFP, while those with lower ADC values were diagnosed as AIP. This distinction underscores the importance of not conflating these two types of IPM in DWI assessment. Hence, the diagnostic efficacy of the ADC values was pooled separately for the two IPM subgroups.
In the differentiation between MFP and PC, the ADC value had a sensitivity of 0.80 (95%CI 0.61–0.92) and a specificity of 0.85 (95%CI 0.77–0.92). For distinguishing AIP and PC, the ADC value had a sensitivity of 0.82 (95%CI 0.75, 0.87) and a specificity of 0.84 (95%CI 0.81, 0.87). Additionally, for distinguishing IPM and PC, both f and mass stiffness showed relatively high sensitivities (0.82, 95%CI 0.68–0.91; 0.82, 95%CI 0.71–0.91) with moderate specificities (0.68, 95%CI 0.61–0.74; 0.77, 95%CI 0.68–0.84). To distinguish between AIP and PC, dMPD ≤ 5 mm demonstrated the highest sensitivity (0.97, 95%CI 0.93–0.99) and diagnostic odds ratio (46, 95%CI 17–126) for distinguishing AIP from PC, but the specificity was relatively low (0.52, 95%CI 0.47–0.58). Thus, dMPD > 5 mm may be considered an ideal cutoff value for excluding AIP.
Forest plots of the pooled sensitivity and specificity are shown in Supplementary Figures 1–3. For the diagnostic performance of all imaging biomarkers, the Cochrane p-values indicated the presence of heterogeneity and the I2 values showed high heterogeneity among most studies. The lowest heterogeneity was not only found in sensitivity of f and specificity of stiffness for distinguishing IPM and PC (p = 0.391, I2 = 4.1%), but also found in specificity of dMPD ≤ 4 mm and sensitivity of dMPD ≤ 5 mm for distinguishing AIP and PC. The Spearman correlation coefficient (Supplementary Table 2) revealed a considerable threshold effect for distinguishing IPM and PC using mass stiffness and differentiating MFP from PC using ADC values. No significant threshold effects were found for the diagnostic performance of other MR imaging biomarkers. Furthermore, univariate meta-regression analysis was conducted to identify other potential sources of heterogeneity.
Univariate meta-regression analysis
Univariate meta-regression analysis was performed to identify the potential factors influencing heterogeneity among the studies, and the results are shown in Table 3. For distinguishing AIP and PC, the sensitivity (0.85, 95%CI 0.77–0.93) of ADC values with high field strength (3.0T or mixed) outperformed the sensitivity (0.75, 95%CI 0.61–0.89) of ADC values with low field strength (1.5T). The ADC values using two b values showed higher sensitivity (0.86, 95%CI 0.80–0.93) than the ADC values using multiple b values (0.66, 95%CI 0.50–0.83), but no significant heterogeneity was observed for the specificity. The pooled specificity of the dMPD ≤ 5 mm was higher in studies conducted in Japan (0.86, 95%CI 0.72–1.00) than studies conducted in other countries (0.45, 95%CI 0.24–0.64), whereas the specificity did not exhibit significant heterogeneity. Owing to the limited number of studies, we did not conduct a subgroup analysis of other quantitative MR imaging biomarkers.
Discussion
In this review, relevant studies on quantitative MR imaging biomarkers for distinguishing between IPM and PC were systematically reviewed. Thereafter, we conducted an analysis to assess the combined diagnostic performance of four quantitative MR imaging biomarkers in distinguishing between IPM and PC, ADC, f, mass stiffness, and dMPD. All these biomarkers had relatively high diagnostic efficacy, among which the ADC value and dMPD represented the most commonly used MR imaging biomarkers with the best availability.
The ADC values showed distinct manifestations in MFP and AIP, which might be related to their respective pathological characteristics. Notably, although MFP and AIP are both special forms of CP that exhibit “mass-like” appearances, they are different entities with distinctive pathological features [50]. The main pathological changes in MFP include varying degrees of fibrosis and irreversible glandular atrophy, which may sometimes be accompanied by dilatation of the main pancreatic duct [51]. Conversely, AIP is histologically characterized by extensive periductal infiltration of numerous lymphocytes and IgG4-positive plasma cells, along with features such as obliterative phlebitis, storiform fibrosis, and inflammatory infiltrates [50, 52,53,54]. Because of the highly organized microstructure and high cellularity, the diffusion of water molecules may be relatively more restricted in AIP. Thus, MFP and AIP may produce different results for differential diagnosis using DWI- and IVIM-derived parameters. More studies have reported that the ADC values of MFP were significantly higher than those of PC [16, 44, 45, 55,56,57]. Meanwhile, we noticed that for the four studies that explicitly included AIP in the MFP group [14,15,16,17], the ADC values in the MFP group tended to decrease significantly as the proportion of AIP in the MFP group increased. With an increase in the proportion of AIP, the ADC values in MFP changed from significantly higher to no significant difference, and then to significantly lower than those in PC. Moreover, the meta-analytic pooled sensitivity and specificity of higher ADC values for the diagnosis of MFP were notably robust. Fortunately, the clinical features of MFP and AIP differ [10, 58]. Therefore, when using ADC values to perform the differential diagnosis of IPM and PC, it is important to first determine the subtype of IPM according to the clinical characteristics.
Different numbers and magnitudes of b values influence the calculation of ADC values. For the images obtained with low b values, the image contrast mainly depends on the T2 signal, without reducing the signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR). With the increase of b values, the detection of diffusion impediment is more sensitive [11]. Additionally, SNR is influenced by the magnitude of field strength. One study also revealed the existence of intervendor differences when different field strengths (1.5T or 3T) were applied [59]. Due to the heterogeneity of tumors, different ROI delineation methods may also affect ADC calculation. For the variation of the above-mentioned MRI parameters, there is a lack of consistency in ADC values among included studies. Future studies are needed to establish a standardize DWI acquisition method.
For IVIM-derived parameters, all included studies on the f showed that the values of f were higher in IPM than in PC. However, for other parameters, studies with different IPM types had the opposite conclusions. Kang et al used the bi-exponential model for the calculation of Dslow and Dfast, and reported that the median values of Dslow and Dfast in MFP were higher than those in PC, although there was no significant difference in Dslow values between the two groups [38]. Kim et al used a mono-exponential model for the calculation and reported that both the median Dslow and Dfast values of AIP were lower than the median values of PC, whereas no significant difference was observed [35]. In summary, considering the overall trend of previous study findings and the different pathological features of the two diseases, we suggest that MFP and AIP should not be confused when distinguishing IPM and PC with Dslow and Dfast. However, for it is more complex and time-consuming than DWI, and high degrees of variability existed due to the application of different models, and there is lack of strong evidence to confirm the clinical application value, implementing IVIM analysis remains challenging.
When applying the dMPD to distinguish between IPM and PC, it is necessary to first determine the subtype of the IPM. Differentiating between MFP and PC is likely to be difficult because the values often overlap. However, the dMPD performed well in the differential diagnosis of AIP and PC [60]. With the cutoff value of 5 mm, the dMPD has the highest sensitivity, which indicates that dMPD can help exclude patients with AIP.
For the mass stiffness, all included studies consistently showed that the values of mass stiffness were higher in the IPM than in the PC. MRE of the pancreas is a novel, non-invasive imaging technique with high reliability and reproducibility. However, MRE of the pancreas is a breath-hold sequence, and its clinical application remains limited owing to the long examination time, high cost, and lower spatial and temporal resolution than routine MRI [61, 62]. Moreover, the small number of included studies might weaken the results of the meta-analysis. Consequently, development of this technique, standardization of image acquisition, and studies with larger sample sizes are needed to better solve these clinical problems.
This meta-analysis had several limitations. First, the cases of IPM in single-center studies were limited, and a large amount of data presented a non-normal distribution; therefore, there were risks of data drift and bias. Second, some studies did not explicitly report the composition of the patient group classified as MFP. Consequently, a senior abdominal radiologist carefully read the full text and compared the clinical and laboratory findings presented in the text with the diagnostic criteria of AIP to determine whether the MFP group included AIP. Third, because the values of these MR imaging biomarkers vary significantly with different MRI scanners, field strengths, b values, or methods for ROI delineation, it remains difficult to obtain pre-specified thresholds for differential diagnosis.
In conclusion, quantitative MR imaging biomarkers performed well in the differential diagnosis of IPM and PC. Compared with PC, the ADC values were higher in MFP but lower in AIP. The ADC can distinguish PC from MFP or AIP, both with high diagnostic performance, as long as the two IPM types are not mixed together. dMPD can help exclude patients with AIP, especially those with a cutoff value of 5 mm.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AIP:
-
Autoimmune pancreatitis
- CI:
-
Confidence interval
- CP:
-
Chronic pancreatitis
- D fast :
-
Fast component of diffusion
- dMPD:
-
Maximal diameter of upstream main pancreatic duct (dMPD)
- D slow :
-
Slow component of diffusion
- DWI:
-
Diffusion-weighted imaging
- f :
-
Perfusion fraction
- IPM:
-
Inflammatory pancreatic mass
- IVIM:
-
Intravoxel incoherent motion
- MFP:
-
Mass-forming pancreatitis
- MR:
-
Magnetic resonance
- MRE:
-
Magnetic resonance elastography
- PC:
-
Pancreatic cancer
References
Bachmann K, Izbicki JR, Yekebas EF (2011) Chronic pancreatitis: modern surgical management. Langenbecks Arch Surg 396:139–149
Schima W, Bohm G, Rosch CS, Klaus A, Fugger R, Kopf H (2020) Mass-forming pancreatitis versus pancreatic ductal adenocarcinoma: CT and MR imaging for differentiation. Cancer Imaging 20:52
Siegel RA-O, Miller KA-O, Wagle NA-OX, Jemal A (2023) Cancer statistics, 2023
Gupta R, Amanam I, Chung V (2017) Current and future therapies for advanced pancreatic cancer. J Surg Oncol 116:25–34
Chouhan MD, Firmin L, Read S, Amin Z, Taylor SA (2019) Quantitative pancreatic MRI: a pathology-based review. Br J Radiol 92:20180941
Siddiqui N, Vendrami CL, Chatterjee A, Miller FH (2018) Advanced MR imaging techniques for pancreas imaging. Magn Reson Imaging Clin N Am 26:323–344
Shukla-Dave A, Obuchowski NA, Chenevert TL et al (2019) Quantitative imaging biomarkers alliance (QIBA) recommendations for improved precision of DWI and DCE-MRI derived biomarkers in multicenter oncology trials. J Magn Reson Imaging 49:e101–e121
Sullivan DC, Obuchowski NA, Kessler LG et al (2015) Metrology standards for quantitative imaging biomarkers. Radiology 277:813–825
Negrelli R, Manfredi R, Pedrinolla B et al (2015) Pancreatic duct abnormalities in focal autoimmune pancreatitis: MR/MRCP imaging findings. European Radiology 25:359–367
Shimosegawa T, Chari ST, Frulloni L et al (2011) International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas 40:352–358
Barral M, Taouli B, Guiu B et al (2015) Diffusion-weighted MR imaging of the pancreas: current status and recommendations. Radiology 274:45–63
Ha J, Choi SH, Kim KW, Kim JH, Kim HJ (2022) MRI features for differentiation of autoimmune pancreatitis from pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. Dig Liver Dis 54:849–856
Srisajjakul S, Prapaisilp P, Bangchokdee S (2020) CT and MR features that can help to differentiate between focal chronic pancreatitis and pancreatic cancer. Radiol Med 125:356–364
Kim M, Jang KM, Kim JH et al (2017) Differentiation of mass-forming focal pancreatitis from pancreatic ductal adenocarcinoma: value of characterizing dynamic enhancement patterns on contrast-enhanced MR images by adding signal intensity color mapping. Eur Radiol 27:1722–1732
Lee SS, Byun JH, Park BJ et al (2008) Quantitative analysis of diffusion-weighted magnetic resonance imaging of the pancreas: usefulness in characterizing solid pancreatic masses. J Magn Reson Imaging 28:928–936
Zhang TT, Wang L, Liu HH et al (2017) Differentiation of pancreatic carcinoma and mass-forming focal pancreatitis: qualitative and quantitative assessment by dynamic contrast-enhanced MRI combined with diffusion weighted imaging. Oncotarget 8:1744–1759
Barral M, Sebbag-Sfez D, Hoeffel C et al (2013) Characterization of focal pancreatic lesions using normalized apparent diffusion coefficient at 1.5-Tesla: preliminary experience. Diagn Interv Imaging 94:619–627
Klauss M, Lemke A, Grunberg K et al (2011) Intravoxel incoherent motion MRI for the differentiation between mass forming chronic pancreatitis and pancreatic carcinoma. Invest Radiol 46:57–63
Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M (1988) Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168:497–505
Glaser KJ, Manduca A, Ehman RL (2012) Review of MR elastography applications and recent developments. J Magn Reson Imaging 36:757–774
McInnes MDF, Moher D, Thombs BD et al (2018) Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: the PRISMA-DTA Statement. JAMA 319:388–396
Whiting PF, Rutjes AW, Westwood ME et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529–536
Sugiyama Y, Fujinaga Y, Kadoya M et al (2012) Characteristic magnetic resonance features of focal autoimmune pancreatitis useful for differentiation from pancreatic cancer. Japanese J Radio 30:296–309
Sandrasegaran K, Nutakki K, Tahir B, Dhanabal A, Tann M, Cote GA (2013) Use of diffusion-weighted MRI to differentiate chronic pancreatitis from pancreatic cancer. AJR Am J Roentgenol 201:1002–1008
Malagi AV, Shivaji S, Kandasamy D et al (2023) Pancreatic mass characterization using IVIM-DKI MRI and machine learning-based multi-parametric texture analysis. Bioengineering (Basel) 10:83. https://doi.org/10.3390/bioengineering10010083
Shiraishi M, Igarashi T, Hiroaki F, Oe R, Ohki K, Ojiri H (2022) Radiomics based on diffusion-weighted imaging for differentiation between focal-type autoimmune pancreatitis and pancreatic carcinoma. British J Radiology 95:20210456. https://doi.org/10.1259/bjr.20210456
Sekito T, Ishii Y, Serikawa M et al (2021) The role of apparent diffusion coefficient value in the diagnosis of localized type 1 autoimmune pancreatitis: differentiation from pancreatic ductal adenocarcinoma and evaluation of response to steroids. Abdom Radiol (NY) 46:2014–2024
Jia H, Li J, Huang W, Lin G (2021) Multimodel magnetic resonance imaging of mass-forming autoimmune pancreatitis: differential diagnosis with pancreatic ductal adenocarcinoma. BMC Med Imaging 21:149
Kwon JH, Kim JH, Kim SY et al (2019) Differentiating focal autoimmune pancreatitis and pancreatic ductal adenocarcinoma: contrast-enhanced MRI with special emphasis on the arterial phase. Eur Radiol 29:5763–5771
De Robertis R, Cardobi N, Ortolani S et al (2019) Intravoxel incoherent motion diffusion-weighted MR imaging of solid pancreatic masses: reliability and usefulness for characterization. Abdominal Radio 44:131–139
Shi Y, Gao F, Li Y et al (2018) Differentiation of benign and malignant solid pancreatic masses using magnetic resonance elastography with spin-echo echo planar imaging and three-dimensional inversion reconstruction: a prospective study. SAGE Open Med Case Rep 28:936–945
Shi Y, Cang L, Zhang X et al (2018) The use of magnetic resonance elastography in differentiating autoimmune pancreatitis from pancreatic ductal adenocarcinoma: a preliminary study. Eur J Radiol 108:13–20
Liu Y, Wang M, Ji R, Cang L, Gao F, Shi Y (2018) Differentiation of pancreatic ductal adenocarcinoma from inflammatory mass: added value of magnetic resonance elastography. Clin Radiol 73:865–872
Lee S, Kim JH, Kim SY et al (2018) Comparison of diagnostic performance between CT and MRI in differentiating non-diffuse-type autoimmune pancreatitis from pancreatic ductal adenocarcinoma. Eur Radiol 28:5267–5274
Kim B, Lee SS, Sung YS et al (2017) Intravoxel incoherent motion diffusion-weighted imaging of the pancreas: characterization of benign and malignant pancreatic pathologies. J Magn Reson Imaging 45:260–269
Klauß M, Maier-Hein K, Tjaden C, Hackert T, Grenacher L, Stieltjes B (2016) IVIM DW-MRI of autoimmune pancreatitis: therapy monitoring and differentiation from pancreatic cancer. Eur Radiol 26:2099–2106
Choi SY, Kim SH, Kang TW, Song KD, Park HJ, Choi YH (2016) Differentiating mass-forming autoimmune pancreatitis from pancreatic ductal adenocarcinoma on the basis of contrast-enhanced MRI and DWI findings. AJR Am J Roentgenol 206:291–300
Kang KM, Lee JM, Yoon JH, Kiefer B, Han JK, Choi BI (2014) Intravoxel incoherent motion diffusion-weighted MR imaging for characterization of focal pancreatic lesions. Radiology 270:444–453
Sun GF, Zuo CJ, Shao CW, Wang JH, Zhang J (2013) Focal autoimmune pancreatitis: radiological characteristics help to distinguish from pancreatic cancer. World J Gastroenterol 19:3634–3641
Naitoh I, Nakazawa T, Hayashi K et al (2012) Clinical differences between mass-forming autoimmune pancreatitis and pancreatic cancer. Scand J Gastroenterol 47:607–613
Muhi A, Ichikawa T, Motosugi U et al (2012) Mass-forming autoimmune pancreatitis and pancreatic carcinoma: differential diagnosis on the basis of computed tomography and magnetic resonance cholangiopancreatography, and diffusion-weighted imaging findings. J Magn Reson Imaging 35:827–836
Hur BY, Lee JM, Lee JE et al (2012) Magnetic resonance imaging findings of the mass-forming type of autoimmune pancreatitis: comparison with pancreatic adenocarcinoma. J Magn Reson Imaging 36:188–197
Takuma K, Kamisawa T, Tabata T, Inaba Y, Egawa N, Igarashi Y (2011) Utility of pancreatography for diagnosing autoimmune pancreatitis. World J Gastroenterol 17:2332–2337
Klauss M, Lemke A, Grünberg K et al (2011) Intravoxel incoherent motion MRI for the differentiation between mass forming chronic pancreatitis and pancreatic carcinoma. Invest Radiol 46:57–63
Huang WC, Sheng J, Chen SY, Lu JP (2011) Differentiation between pancreatic carcinoma and mass-forming chronic pancreatitis: usefulness of high b value diffusion-weighted imaging. J Dig Dis 12:401–408
Park SH, Kim MH, Kim SY et al (2010) Magnetic resonance cholangiopancreatography for the diagnostic evaluation of autoimmune pancreatitis. Pancreas 39:1191–1198
Kamisawa T, Takuma K, Anjiki H et al (2010) Differentiation of autoimmune pancreatitis from pancreatic cancer by diffusion-weighted MRI. Am J Gastroenterol 105:1870–1875
Ren H, Mori N, Hamada S et al (2021) Effective apparent diffusion coefficient parameters for differentiation between mass-forming autoimmune pancreatitis and pancreatic ductal adenocarcinoma. Abdom Radiol (NY) 46:1640–1647
Klau M, Lemke A, Grünberg K et al (2011) Intravoxel incoherent motion MRI for the differentiation between mass forming chronic pancreatitis and pancreatic carcinoma. Invest Radio 46:57–63
Suda K, Takase M, Fukumura Y, Kashiwagi S (2007) Pathology of autoimmune pancreatitis and tumor-forming pancreatitis. J Gastroenterol 42(Suppl 18):22–27
Liu Y, Wang M, Ji R, Cang L, Gao F, Shi Y (2018) Differentiation of pancreatic ductal adenocarcinoma from inflammatory mass: added value of magnetic resonance elastography. Clinical Radio 73:865–872
Kamisawa T, Zen Y, Nakazawa T, Okazaki K (2018) Advances in IgG4-related pancreatobiliary diseases. Lancet Gastroenterol Hepatol 3:575–585
Kloppel G, Luttges J, Lohr M, Zamboni G, Longnecker D (2003) Autoimmune pancreatitis: pathological, clinical, and immunological features. Pancreas 27:14–19
Zamboni G, Luttges J, Capelli P et al (2004) Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch 445:552–563
Ruan Z, Jiao J, Min D et al (2018) Multi-modality imaging features distinguish pancreatic carcinoma from mass-forming chronic pancreatitis of the pancreatic head. Oncol Lett 15:9735–9744
El-Shinnawy MA, Zidan DZ, Maarouf RA (2013) Can high-b-value diffusion weighted imaging differentiate between pancreatic cancer, mass forming focal pancreatitis and normal pancreas? Egyptian J Radio Nuclear Med 44:687–695
Fattahi R, Balci NC, Perman WH et al (2009) Pancreatic diffusion-weighted imaging (DWI): comparison between mass-forming focal pancreatitis (FP), pancreatic cancer (PC), and normal pancreas. J Magn Reson Imaging 29:350–356
Lee H, Lee JK, Kang SS et al (2007) Is there any clinical or radiologic feature as a preoperative marker for differentiating mass-forming pancreatitis from early-stage pancreatic adenocarcinoma? Hepatogastroenterology 54:2134–2140
Donati OF, Chong D, Nanz D et al (2014) Diffusion-weighted MR imaging of upper abdominal organs: field strength and intervendor variability of apparent diffusion coefficients. Radiology 270:454–463
Ichikawa T, Sou H, Araki T et al (2001) Duct-penetrating sign at MRCP: usefulness for differentiating inflammatory pancreatic mass from pancreatic carcinomas. Radiology 221:107–116
Qi YM, Xiao EH (2023) Advances in application of novel magnetic resonance imaging technologies in liver disease diagnosis. World J Gastroenterol 29:4384–4396
Steinkohl E, Bertoli D, Hansen TM, Olesen SS, Drewes AM, Frokjaer JB (2021) Practical and clinical applications of pancreatic magnetic resonance elastography: a systematic review. Abdom Radiol (NY) 46:4744–4764
Funding
This study was funded by the National Natural Science Foundation of China (82371950 to L. Zhu), and National High Level Hospital Clinical Research Funding (2022-PUMCH-B-069 to H. Xue).
National Natural Science Foundation of China,82371950,Liang Zhu ,National High Level Hospital Clinical Research Funding ,2022-PUMCH-B-069,Hua-dan Xue
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Professor Hua-dan Xue from Department of Radiology, Peking Union Medical College Hospital, Beijing, China.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required because this study was a systematic and meta-analysis.
Ethical approval
Institutional Review Board approval was not required because this study was a systematic and meta-analysis.
Methodology
-
retrospective
-
diagnostic study
-
performed at one institution
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hua-dan Xue and Liang Zhu are co-corresponding authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Zh., Zhu, L., Xue, Hd. et al. Quantitative MR imaging biomarkers for distinguishing inflammatory pancreatic mass and pancreatic cancer—a systematic review and meta-analysis. Eur Radiol 34, 6738–6750 (2024). https://doi.org/10.1007/s00330-024-10720-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-024-10720-9