Abstract
Cardiovascular MR imaging has become an indispensable noninvasive tool in diagnosing and monitoring a broad range of cardiovascular diseases. Key to its clinical success and efficiency are appropriate clinical indication triage, technical expertise, patient safety, standardized preparation and execution, quality assurance, efficient post-processing, structured reporting, and communication and clinical integration of findings. Technological advancements are driving faster, more accessible, and cost-effective approaches. This ESR Essentials article presents a ten-step guide for implementing a cardiovascular MR program, covering indication assessments, optimized imaging, post-processing, and detailed reporting. Future goals include streamlined protocols, improved tissue characterization, and automation for greater standardization and efficiency.
Clinical relevance statement
The growing clinical role of cardiovascular MR in risk assessment, diagnosis, and treatment planning highlights the necessity for radiologists to achieve expertise in this modality, advancing precision medicine and healthcare efficiency.
Key Points
• Cardiovascular MR is essential in diagnosing and monitoring many acute and chronic cardiovascular pathologies.
• Features such as technical expertise, quality assurance, patient safety, and optimized tailored imaging protocols, among others, are essential for a successful cardiovascular MR program.
• Ongoing technological advances will push rapid multi-parametric cardiovascular MR, thus improving accessibility, patient comfort, and cost-effectiveness.
Key Recommendations
• Cardiovascular MR is essential in diagnosing and monitoring a wide array of cardiovascular pathologies (Level of Evidence: High).
• A successful cardiovascular MR program depends on standardization (Level of Evidence: Low).
• Future developments will increase the efficiency and accessibility of cardiovascular MR (Level of Evidence: Low).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
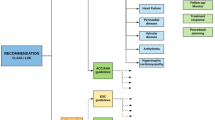
This ESR Essentials article aims for comprehensive step-by-step guidance to establish/execute a program from clinical indication assessment, optimized image acquisition, and post-processing to sophisticated reporting and communication of findings (Fig. 1).
Step 1: Start with appropriate cardiovascular MR indications selection
Cardiovascular MR allows comprehensive, noninvasive evaluation of cardiac/vascular function, flow, and myocardial tissue characteristics. Hence, it has increasingly been incorporated into clinical guidelines over recent years [1] (Table 1). Cardiovascular MR is superior to other imaging modalities, such as echocardiography and computed tomography, in the comprehensive assessment of global/regional cardiac function and tissue characterization. These benefits can be used to assess the extent and severity of myocardial fibrosis/scaring in ischemic and non-ischemic cardiomyopathies, to differentiate hypertrophic and dilated phenotypes, and to identify inflammatory diseases such as myocarditis and infiltrative diseases such as sarcoidosis and amyloidosis [2, 4, 5].
Cardiovascular MR has proven its ability to evaluate morphology and function comprehensively in patients with congenital heart disease. It represents the well-established standard of reference for assessing cardiac size and function. Therefore, it enables critical insights, especially in complex anatomical situations required for interventional planning [6].
Moreover, the exam is highly valuable in patients with valvular heart disease, providing direct visualization of valves and quantifying the severity of valvular defects and remodeling effects on the ventricles. In addition, cardiovascular MR is also of primary importance in the differential diagnosis of cardiac masses, tumors, and thrombi based on their specific tissue characteristics [3].
In patients with aortic diseases such as vasculitis, aneurysm, or syndromic/genetic aortopathies, the integration of high-resolution imaging for both vessel wall and lumen, along with its precision in measuring aortic dimensions and the absence of ionizing radiation, plays a crucial role in the frequently necessary lifelong monitoring of the disease [9].
In summary, cardiovascular MR provides a highly versatile tool based on its unparalleled tissue characterization, detailed morphological display, and functional accuracy. It has become essential to contemporary noninvasive cardiovascular diagnostics and treatment planning. Hence, its widespread clinical use continues to grow, supported by increased inclusion in various clinical guidelines.
Step 2: Exam/patient preparation and positioning
MR technicians and physicians are essential in the exam/patient preparation and positioning. Examinations are commonly performed at 1.5 and 3 T, involving standardized patient preparation, including screening questionnaires to identify potential risks/contraindications to gadolinium-based contrast agents (GBCAs) and potential safety hazards (e.g., passive/active implants, foreign bodies, metal-containing fabrics).
Applying GBCA or performing MR with metallic/conductive implants in specific situations requires individual risk–benefit analysis as highlighted by international guidance on contrast use in MR and MR safety [10,11,12].
While fasting is not mandatory, only light meals are advised due to the potential risk of nausea and vomiting related to applied drugs (GBCA and pharmacologic stress agents) [13]. Specific instructions to the patients are required before stress perfusion imaging due to possible counteracting effects of pharmacologic stressors (see step 4). In addition, stress perfusion imaging requires specific patient monitoring (e.g., blood pressure) and the availability of dedicated equipment in case of adverse events.
Explanation of the scanning procedure and rehearsing required breathing instructions before the examination are highly beneficial for a successful and high-quality exam. While expiratory breath-holding allows for better reproducibility of the cardiac position and thus improved image quality, adjusting to the patient’s abilities and comfort remains critical, which may require inspiratory breath-hold maneuvers.
Most commonly, patients are positioned supine and head-first on the scanner table with arms to the side (with rare exceptions). In any case, direct skin-to-skin contact between the arms (specifically hands) and the body must be avoided to prevent thermal/electrical burns (if the patient’s surface is in contact with the bore wall, additional padding is required) [14]. For physiological synchronization, electrocardiogram (ECG) electrodes are attached to the chest (positioning of the electrodes varies with the system used) after appropriate skin preparation, and a multielement phased-array coil is placed on the chest for improved signal reception. Changing with systems, specific steps may be required to optimize ECG signal tracing, and re-verification of the ECG trace after centering the patient in the bore is recommended for reliable synchronization and optimized image quality. Mandatory noise protection (most commonly headphones) must also allow for appropriate bi-directional communication during scanning.
Step 3: How to select an appropriate exam protocol
Protocols for any MR exam (including cardiovascular MR) should be kept as short as possible, enabling short examination times with improved patient comfort, reduced motion artifacts, and increased patient throughput, resulting in improved cardiovascular MR resource access [15]. Appropriate clinical patient information, interaction with referring physicians regarding the specific questions to be answered, and personnel competency are paramount to ensure proper protocol selection and optimized diagnostic image quality to help guide diagnostic, prognostic, or therapeutic purposes [16]. This holds explicitly in complex morphologies or challenging patient populations (e.g., IC/CCU patients, children).
Despite the required tailoring of protocols to the specific clinical indication, most exams include modules for anatomical and functional assessment in various cardiac axes adjusted orientations using bSSFP sequences (see step 5). This is commonly supplemented by late gadolinium enhancement (LGE) imaging for assessing myocardial pathologies. However, the cardiovascular MR Toolbox provides a magnitude of additional techniques that allow for tissue characterization (e.g., T2-weighted imaging, quantitative myocardial biomarkers such as parametric mapping), flow imaging (2D or 4D) phase-contrast techniques, perfusion imaging (e.g., first pass perfusion), or 3D MR angiography (contrast-enhanced/non-contrast-enhanced) whose application is depending on the specific clinical indication (Fig. 2). Hence, appropriate indication assessment is pivotal for efficient and effective cardiovascular MR.
Step 4: Stressing the patient
Stress cardiovascular MR is commonly performed using first-pass perfusion techniques with pharmacological vasodilators (e.g., adenosine, regadenoson, dipyridamol). Concerning specific agents and doses, it remains essential to consider that availability and approval may vary by jurisdiction, potentially limiting their use for particular applications in certain countries or regions.
The main contraindications for vasodilator stress include 2nd-/3rd-degree atrioventricular block and active bronchospastic disease, but in detail depends on the specific vasodilator in use. To ensure appropriate vasodilatation, patients should be instructed to refrain from xanthine-containing substances (e.g., coffee, cacao, dark chocolate) and drugs such as theophylline or dipyridamole 12–24 h before the examination [13]. Appropriate “stress” response is defined by a ≥ 10-bpm heart rate increase and/or > 10-mmHg blood pressure drop. Patients may experience side effects (flushing, chest pain, palpitations, breathlessness).
First-pass perfusion imaging should provide short-axis coverage (at least 3 slices) following the contrast bolus for 40–60 s after 0.05–0.1 mmol/kg GBCA (rate 3–7 mL/s), followed by a saline flush. While “stress” perfusion imaging should be performed first, assessment of the rest perfusion can be achieved by repeating the identical protocol after subsidence of vasodilator effects. While adenosine effects decay within < 1 min of infusion stop, regadenoson effects last for at least 10 min but may be reversed with aminophylline similar to dipyridamol. Results of first-pass perfusion imaging should always be interpreted together with LGE imaging for the best possible interpretation.
Step 5: How to sequentially acquire dedicated cardiac chambers and valve planes
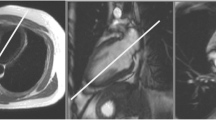
Graphical guidance to standardized cardiac axes planning is illustrated in Fig. 3.
The first step is to acquire axial, coronal, and sagittal localizers. Next, localizers roughly depicting the long axes of the left ventricle (LV) are obtained by positioning planes through the center of the mitral valve mid-point LV apex:
-
1.
Obtain a vertical long-axis (pseudo-2Ch) view on the axial localizer.
-
2.
On the pseudo-2Ch view, acquire a horizontal long-axis (pseudo-4Ch) view.
Next, capture a series of short-axis images parallel to the mitral valve on both long-axis planes, covering both ventricles. This establishes standardized LV and right ventricle (RV) views, including the 2Ch, three-chamber (3Ch), and 4Ch views. These standardized views are formed by placing planes from the mitral valve center to the LV apex, and on the short axis:
-
A plane parallel to the interventricular septum and perpendicular to the anterior and inferior LV walls for the 2Ch view.
-
A plane perpendicular to the center of the aortic valve for the 3Ch view.
-
A plane from the widest RV point to the anterior LV papillary muscle for the 4Ch view.
Additionally, standardized RV views, RV 2Ch, and RV 3Ch can be generated as follows:
-
RV 2Ch is obtained by placing a plane from the tricuspid valve center to the RV apex on a 4Ch view and should be verified in the short axis and/or axial scout images.
-
RV 3Ch can be acquired by positioning three points on the tricuspid valve, the RV apex from the 4Ch view, and the pulmonary valve from a sagittal RVOT view.
Alternatively, the RV can be imaged using a stack of axial planes covering the RV.
For assessing the aortic valve, create an orthogonal plane through the valve’s center on the LV 3Ch view (LVOT-plane). This allows the aortic valve plane to be planned parallel to the valve based on 3Ch and LVOT views. Similarly, the pulmonary valve plane can be planned parallel to the valve using the RV 3Ch and sagittal RVOT plane.
Obtain the aortic arch by planning three points on the ascending and descending aorta and the aortic arch from axial views. Outside of cine and valvular phase-contrast imaging, acquire long- and a subset of short-axis views with identical orientations for tissue characterization.
Step 6: How to manage artifacts and motion
Cardiovascular MR may be affected by inherent MR physics-related artifacts and artifacts related to patient motion or cyclic respiratory or cardiac motion. The most common artifact causes and potential solutions are highlighted in Table 2.
Most common MR physics-related artifacts are off-resonance artifacts (e.g., dark bands) in bSSFP techniques. While more commonly seen at higher field strength (e.g., 3 T), they may rarely also be on display at 1.5 T. Subendocardial “dark rim” artifacts may affect the assessment of perfusion deficits, possibly mimicking ischemia. However, combining strategies can help reduce such artifacts or exclude ischemia. While not part of all exams, spin-echo techniques may provide important information. The blood contrast mechanisms of these techniques are complex, and to optimize image quality, several steps are required, as outlined in Table 2. Further information can be found in other detailed publications and is outside of the scope of this Essentials article [17].
Dedicated data sampling and physiologic triggering approaches are required for high-quality cardiovascular MR without motion artifacts. Sampling strategies include data acquisition across multiple heartbeats (k-space segmentation) and lower-resolution single-shot approaches (data acquisition within a single cardiac cycle).
Respiratory motion artifacts can be avoided by following approaches:
-
Breath-holding during data acquisition
-
Single-shot acquisition with or without motion correction/registration
-
Navigator techniques with data acquisition windows determined by the diaphragmatic position
Acquiring/reconstructing images during specific phases of the cardiac cycle requires ECG synchronization. While in the setting of occasional arrhythmia, retrospective ECG gating with arrhythmia rejection techniques or prospective ECG triggering may help diagnostic image quality, in complex arrhythmia, ECG synchronization with k-space segmentation will likely fail, and single-shot techniques are beneficial. The latter similarly applies to patients who cannot hold their breath reliably. It is worth mentioning that any approach to shorten the data acquisition (e.g., parallel imaging, compressed sensing) will reduce the likelihood of image quality deterring artifacts from arrhythmia or breathing. Data binning techniques and/or motion correction/registration techniques improve diagnostic image quality and are also highly important for quantitative myocardial biomarker imaging (e.g., T1-/T2-mapping) [18, 19].
Step 7: Image analysis and post-processing
Post-processing in cardiovascular MR primarily focuses on accurately and precisely assessing ventricular size, function, and valvular flow. In addition, further analysis of quantitative myocardial imaging biomarkers such as T1 and T2 relaxation times has become clinically relevant. Other quantitative post-processing parameters may have a case for research-oriented examinations. Basic requirements include regulatory-approved software with DICOM functionality, integration with local PACS, and secure patient data management. Furthermore, appropriate computer hardware (including monitor) meeting the vendor’s specifications and requirements are mandatory. Applied post-processing and analysis software should enable cine viewing with zoom, pan, and contrast adjustment and support (semi-)automated endocardial and epicardial contour tracings [20]. Cross-referencing functionality aids in confirming slice position and relationships (Fig. 4).
Biventricular functional evaluation with a dedicated artificial intelligence-based post-processing platform. For left ventricular quantification, the endocardial (red) and epicardial (green) contours are automatically delineated in a stack of short-axis slices covering the whole left ventricle; right ventricular endocardial contouring (in yellow) is traced in the same stack of images. Displayed results include biventricular volumes and masses and added functionality for cross-referencing to verify slice positions in the upper right and bottom right, respectively
Specific requirements depend on the application. For example, phase-contrast flow analysis requires additional tools for anti-aliasing and background flow correction, while MR angiography analysis benefits from multiplanar reformatting, curved planar (centerline), and maximum intensity projection (MIP) reconstructions able to measure distance and area in 3D imaging [20].
Analysis and standardization should be aided by simultaneous visualization of various contrasts/techniques (e.g., Cine, LGE, T1/T2 mapping) and direct side-by-side comparability with prior studies for serial analysis. Segmental analysis of quantitative parameters requires software-based standardized American Heart Association (AHA) model segmentation of the myocardium with additional region-of-interest capability.
The integration of artificial intelligence (AI) in post-processing in recent years has helped improve standardization, efficiency, and workflow and reduce the complexity and learning curve associated with the required manual interaction [21]. As such, AI may also empower less-experienced readers to analyze cardiovascular MR analysis and improve patient care [22].
Step 8: How to report and communicate results
Structured reporting is crucial as it reduces variability and improves quality. This facilitates the clinician to extract information efficiently, enhances communication, and provides guidance on patient management. It is therefore supported by multiple societies, including European Society of Radiology (ESR), European Society of Cardiovascular Radiology (ESCR), and European Society of Medical Imaging Informatics (EuSoMII) [16, 23, 24]. Reporting templates and software solutions have become commercially available, although the design of generic templates remains an important focus [7, 25].
To effectively report cardiovascular MR, it is advised to address, at a minimum, the following components:
-
(1)
Clinical information and indication.
-
(2)
Description of the protocol.
-
(3)
Information on study quality (e.g., by the image quality, artifacts, contrast administration, and success of stressing).
-
(4)
Comparison to previous tests.
-
(5)
Structured summary of quantitative results related to function and dimensions (absolute and body surface area indexed values), tissue characterization, or flow results (if available).
-
(6)
Structured description of relevant findings.
-
(7)
Clinical interpretation.
-
(8)
Urgent and unexpected (extra-cardiac) findings.
-
(9)
Concise conclusion addressing the clinical indication and request.
-
(10)
If: –to whom and when findings were communicated.
It is advised to use standardized terminology. This includes a description of pathology, such as LGE distribution and pattern (e.g., subepicardial) or disease extent (e.g., % transmural LGE). It is crucial to consider reference ranges normalized for body size, gender, age, and physical activity [26, 27]. Communication with the clinician is essential to ensure up-to-date reporting, providing the information needed for current patient management. Multi-disciplinary conferences are highly valuable for best patient management practice and therapy decisions.
Using a structured report template might ensure that the report will contain all relevant information in a standardized way and guide less-experienced readers with an itemized list of topics to cover. The ESCR provides free structured report templates for cardiac imaging examinations (https://www.escr.org/smart-reporting/).
Step 9: Understanding the prognostic value of imaging-derived functional and tissue biomarkers
Cardiovascular MR’s impact on patient management depends on the availability of appropriate therapeutic approaches. The multitude of available MR information may also provide prognostic implications in general or specific to certain diseases. In general, the results of the cardiovascular MR examination should be effectively discussed with the referring physician (e.g., in multi-disciplinary conferences) to ensure adequate changes in patient management.
The ventricular ejection fraction is known for its predictive value. It can not only guide therapeutic management but also guide primary prevention of sudden cardiac death. Across various ischemic and non-ischemic diseases, the presence and the amount of myocardial replacement fibrosis/scar identified by LGE have shown additional prognostic implications [28]. This is now reflected in various guidelines but typically embedded in multiple risk criteria [29].
The potential of quantitative myocardial imaging biomarkers (e.g., T1-/T2-mapping) is currently being explored. While results are promising, technical aspects such as inter-scan variability, potential physiologic influences, and variations depending on the software and hardware used require further developments and considerations [30].
The prognostic use of cardiovascular MR, as opposed to a diagnostic approach that typically provides a diagnosis at the individual patient level, is based on cohort statistics. As such, the actual prognosis may vary substantially for the individual patient.
Step 10: Shaping the future of cardiovascular MR
In the dynamic landscape of cardiac imaging, it is crucial to identify critical directions for advancing cardiovascular MR, optimizing its impact, and enhancing its accessibility.
Short protocols for enhanced efficiency and cost-effectiveness
Cardiovascular MR’s limited accessibility hinders its widespread utilization. Lengthy examinations impact patients, facilities, and providers as reimbursement rates in many regions may impede financial sustainability [15]. The array of techniques limits routine clinical implementation when added to protocols without sufficient consideration for scan duration. Common clinical questions can be addressed effectively using core protocols applicable to most scanners, enabling time-efficient exams and increasing accessibility and cost-effectiveness [15]. Accelerated imaging sequences and AI-assisted reconstruction techniques may further support this.
Tissue characterization and insights into novel therapies
Integrating quantitative myocardial biomarkers has shown potential in enabling accurate tissue characterization, offering new avenues for diagnosing and monitoring cardiovascular diseases. Expanding our understanding of myocardial fibrosis, edema, and perfusion provides valuable insights into disease progression and guides tailored treatment strategies. An opportunity to unravel mechanistic insights into novel therapies is also available. For instance, in oncology, cardiovascular MR can help assess anticancer treatment-associated cardio-toxicity, shedding light on the underlying pathophysiology and aiding in developing cardio-protective strategies [31]. Therefore, cardiovascular MR can actively contribute to optimizing therapeutic interventions and promoting personalized patient care.
Perspective: automated acquisition for standardization and efficiency
The future holds promise regarding automated acquisition, allowing for improved standardization and efficiency. Automated acquisition protocols, guided by AI algorithms, may optimize scan parameters and improve the reproducibility of examinations by consistent and high-quality image acquisition. This, coupled with AI-based image analysis, holds great potential to optimize cardiovascular MR workflows, reducing scan times and optimizing cost-effectiveness while maintaining diagnostic accuracy.
Summary statement
In summary, cardiovascular MR is a crucial clinical tool well-represented in guidelines. It should follow standardized preparation, acquisition, and interpretation protocols, with structured reporting and interdisciplinary communication. The future aims to optimize protocols, improve cost-effectiveness, and leverage multi-parametric imaging for tissue characterization, ensuring its continued importance in modern clinical cardiology.
Patient summary
Cardiovascular MR is a noninvasive imaging method that allows the reliable identification of cardiac and vascular diseases and is, therefore, integrated into several clinical guidelines. Crucial for executing a successful cardiovascular MR examination is the standardization of all significant examination steps, e.g., identification of the clinical question, preparation and examination planning, execution, and analysis of results and their communication to the referring physician. In the future, technical developments may improve and simplify these steps to allow wider accessibility of this imaging method outside of specialized centers. This article provides comprehensive step-by-step guidance to establish a cardiovascular MR program.
Change history
05 May 2024
Correction of minor typographical error in guest editor's name.
Abbreviations
- AI :
-
Artificial intelligence
- ECG :
-
Electrocardiogram
- GBCA:
-
Gadolinium-based contrast agents
- LGE :
-
Late gadolinium enhancement
- LV :
-
Left ventricle
- RV :
-
Right ventricle
References
Esposito A, Gallone G, Palmisano A et al (2020) The current landscape of imaging recommendations in cardiovascular clinical guidelines: toward an imaging-guided precision medicine. Radiol Med 125:1013–1023. https://doi.org/10.1007/s11547-020-01286-9
Emrich T, Halfmann M, Schoepf UJ, Kreitner K-F (2021) CMR for myocardial characterization in ischemic heart disease: state-of-the-art and future developments. Eur Radiol Exp 5:14. https://doi.org/10.1186/s41747-021-00208-2
Leiner T, Bogaert J, Friedrich MG et al (2020) SCMR Position Paper (2020) on clinical indications for cardiovascular magnetic resonance. J Cardiovasc Magn Reson 22:76. https://doi.org/10.1186/s12968-020-00682-4
Ferreira VM, Schulz-Menger J, Holmvang G et al (2018) Cardiovascular Magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 72:3158–3176. https://doi.org/10.1016/j.jacc.2018.09.072
Caobelli F, Cabrero JB, Galea N et al (2023) Cardiovascular magnetic resonance (CMR) and positron emission tomography (PET) imaging in the diagnosis and follow-up of patients with acute myocarditis and chronic inflammatory cardiomyopathy : a review paper with practical recommendations on behalf of the European Society of Cardiovascular Radiology (ESCR). Int J Cardiovasc Imaging. https://doi.org/10.1007/s10554-023-02927-6
Fogel MA, Anwar S, Broberg C et al (2022) Society for Cardiovascular Magnetic Resonance/European Society of Cardiovascular Imaging/American Society of Echocardiography/Society for Pediatric Radiology/North American Society for Cardiovascular Imaging Guidelines for the use of cardiovascular magnetic resonance in pediatric congenital and acquired heart disease: Endorsed by The American Heart Association. J Cardiovasc Magn Reson 24:1–78. https://doi.org/10.1186/s12968-022-00843-7
Francone M, Budde RPJ, Bremerich J et al (2020) CT and MR imaging prior to transcatheter aortic valve implantation: standardisation of scanning protocols, measurements and reporting-a consensus document by the European Society of Cardiovascular Radiology (ESCR). Eur Radiol 30:2627–2650. https://doi.org/10.1007/s00330-019-06357-8
Esposito A, Gatti M, Trivieri MG et al (2023) Imaging for the assessment of the arrhythmogenic potential of mitral valve prolapse. Eur Radiol. https://doi.org/10.1007/s00330-023-10413-9
Isselbacher EM, Preventza O, Hamilton Black J 3rd et al (2023) 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J Thorac Cardiovasc Surg 166:e182–e331. https://doi.org/10.1016/j.jtcvs.2023.04.023
Uhlig J, Al-Bourini O, Salgado R et al (2020) Gadolinium-based contrast agents for cardiac MRI: use of linear and macrocyclic agents with associated safety profile from 154 779 European Patients. Radiol Cardiothorac Imaging 2:e200102. https://doi.org/10.1148/ryct.2020200102
Kodzwa R (2019) ACR manual on contrast media: 2018 updates. Radiol Technol 91:97–100
MacIntyre S (2021) Regulating MR safety standards. Radiol Technol 93:75–89
Kramer CM, Barkhausen J, Bucciarelli-Ducci C et al (2020) Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson 22:17. https://doi.org/10.1186/s12968-020-00607-1
Tsai LL, Grant AK, Mortele KJ et al (2015) A practical guide to MR Imaging safety: what radiologists need to know. Radiographics 35:1722–1737. https://doi.org/10.1148/rg.2015150108
Raman SV, Markl M, Patel AR et al (2022) 30-minute CMR for common clinical indications: a Society for Cardiovascular Magnetic Resonance white paper. J Cardiovasc Magn Reson 24:13. https://doi.org/10.1186/s12968-022-00844-6
Natale L, Vliegenthart R, Salgado R et al (2023) Cardiac radiology in Europe: status and vision by the European Society of Cardiovascular Radiology (ESCR) and the European Society of Radiology (ESR). Eur Radiol 33:5489–5497. https://doi.org/10.1007/s00330-023-09533-z
Ismail TF, Strugnell W, Coletti C et al (2022) Cardiac MR: from theory to practice. Front Cardiovasc Med 9:826283. https://doi.org/10.3389/fcvm.2022.826283
Kellman P, Larson AC, Hsu L-Y et al (2005) Motion-corrected free-breathing delayed enhancement imaging of myocardial infarction. Magn Reson Med 53:194–200. https://doi.org/10.1002/mrm.20333
Cross R, Olivieri L, O’Brien K et al (2016) Improved workflow for quantification of left ventricular volumes and mass using free-breathing motion corrected cine imaging. J Cardiovasc Magn Reson Off J Soc Cardiovasc Magn Reson 18:10. https://doi.org/10.1186/s12968-016-0231-8
Schulz-Menger J, Bluemke DA, Bremerich J et al (2020) Standardized image interpretation and post-processing in cardiovascular magnetic resonance - 2020 update. J Cardiovasc Magn Reson 22:19. https://doi.org/10.1186/s12968-020-00610-6
Weikert T, Francone M, Abbara S et al (2021) Machine learning in cardiovascular radiology: ESCR position statement on design requirements, quality assessment, current applications, opportunities, and challenges. Eur Radiol 31:3909–3922. https://doi.org/10.1007/s00330-020-07417-0
Leiner T, Rueckert D, Suinesiaputra A et al (2019) Machine learning in cardiovascular magnetic resonance: basic concepts and applications. J Cardiovasc Magn Reson Off J Soc Cardiovasc Magn Reson 21:61. https://doi.org/10.1186/s12968-019-0575-y
(2018) ESR paper on structured reporting in radiology. Insights Imaging 9:1–7. https://doi.org/10.1007/s13244-017-0588-8
Kotter E, Pinto Dos Santos D (2021) Structured reporting in radiology : German and European radiology societies’ point of view. Radiologe 61:979–985. https://doi.org/10.1007/s00117-021-00921-4
Saba L, Loewe C, Weikert T et al (2023) State-of-the-art CT and MR imaging and assessment of atherosclerotic carotid artery disease: standardization of scanning protocols and measurements-a consensus document by the European Society of Cardiovascular Radiology (ESCR). Eur Radiol 33:1063–1087. https://doi.org/10.1007/s00330-022-09024-7
Kawel-Boehm N, Hetzel SJ, Ambale-Venkatesh B et al (2020) Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J Cardiovasc Magn Reson 22:87. https://doi.org/10.1186/s12968-020-00683-3
Prakken NH, Velthuis BK, Teske AJ et al (2010) Cardiac MRI reference values for athletes and nonathletes corrected for body surface area, training hours/week and sex. Eur J Cardiovasc Prev Rehabil 17:198–203. https://doi.org/10.1097/HJR.0b013e3283347fdb
Di Marco A, Brown PF, Bradley J et al (2021) Improved risk stratification for ventricular arrhythmias and sudden death in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol 77:2890–2905. https://doi.org/10.1016/j.jacc.2021.04.030
Ommen SR, Mital S, Burke MA et al (2021) 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Thorac Cardiovasc Surg 162:e23–e106
Thavendiranathan P, Zhang L, Zafar A et al (2021) Myocardial T1 and T2 mapping by magnetic resonance in patients with immune checkpoint inhibitor-associated myocarditis. J Am Coll Cardiol 77:1503–1516. https://doi.org/10.1016/j.jacc.2021.01.050
Francone M, Figliozzi S, Monti L et al (2023) Multiparametric cardiac magnetic resonance unveiling the mechanisms and early manifestations of anticancer drug cardiotoxicity. Eur Radiol. https://doi.org/10.1007/s00330-023-09948-8
Acknowledgements
The authors thank Dr. Moritz Halfmann for his support on the generation of the Central Illustration.
This paper was endorsed by the Executive Council of the European Society of Radiology (ESR) and the Executive Board of the European Society of Cardiovascular Radiology (ESCR) in January 2024.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Marco Francone.
Conflict of interest
The authors of this manuscript declare relationships with the following companies:
Dr. Emrich has received speaker fees, travel, and institutional research support and is a member of the advisory board “Photon-counting Detector CT” from Siemens Healthineers.
Dr. Wintersperger has received research support and speaker’s honorarium from Siemens Healthineers and has provided consultation to Bayer AG. Dr. Wintersperger is an inventor of the IG fitting method for T1 mapping owned by UHN (US10314548B2/ EP15844590.8) and receives non-financial research support by Siemens Healthineers. University Health Network has a Master Research Agreement with Siemens Healthineers.
Prof. M. Francone has received speaker’s honorarium from Bayer AG, Canon and Bracco Suisse. Prof. M. Francone is Section Editor for Cardiac in the European Radiology Scientific Editorial Board. He has not taken part in the review or selection process of this article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required.
Ethical approval
Institutional Review Board approval was not required.
Study subjects or cohorts overlap
Not applicable.
Methodology
• Expert Consensus document
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to ESR Essentials series guest edited by Marc Dewey (Berlin/Germany).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Emrich, T., Wintersperger, B.J., Greco, F.D. et al. ESR Essentials: ten steps to cardiac MR—practice recommendations by ESCR. Eur Radiol 34, 2140–2151 (2024). https://doi.org/10.1007/s00330-024-10605-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-024-10605-x