Abstract
Objective
The study aims at comparing the diagnostic accuracy of qualitative and quantitative assessment of the susceptibility in the precentral gyrus in detecting amyotrophic lateral sclerosis (ALS) with predominance of upper motor neuron (UMN) impairment.
Methods
We retrospectively collected clinical and 3T MRI data of 47 ALS patients, of whom 12 with UMN predominance (UMN-ALS). We further enrolled 23 healthy controls (HC) and 15 ALS Mimics (ALS-Mim). The Motor Cortex Susceptibility (MCS) score was qualitatively assessed on the susceptibility-weighted images (SWI) and automatic metrics were extracted from the quantitative susceptibility mapping (QSM) in the precentral gyrus. MCS scores and QSM-based metrics were tested for correlation, and ROC analyses.
Results
The correlation of MCS score and susceptibility skewness was significant (Rho = 0.55, p < 0.001). The susceptibility SD showed an AUC of 0.809 with a specificity and positive predictive value of 100% in differentiating ALS and ALS Mim versus HC, significantly higher than MCS (Z = −3.384, p-value = 0.00071). The susceptibility skewness value of −0.017 showed specificity of 92.3% and predictive positive value of 91.7% in differentiating UMN-ALS versus ALS mimics, even if the performance was not significantly better than MCS (Z = 0.81, p = 0.21).
Conclusion
The MCS and susceptibility skewness of the precentral gyrus show high diagnostic accuracy in differentiating UMN-ALS from ALS-mimics subjects. The quantitative assessment might be preferred being an automatic measure unbiased by the reader.
Clinical relevance statement
The clinical diagnostic evaluation of ALS patients might benefit from the qualitative and/or quantitative assessment of the susceptibility in the precentral gyrus as imaging marker of upper motor neuron predominance.
Key Points
• Amyotrophic lateral sclerosis diagnostic work-up lacks biomarkers able to identify upper motor neuron involvement.
• Susceptibility-weighted imaging/quantitative susceptibility mapping–based measures showed good diagnostic accuracy in discriminating amyotrophic lateral sclerosis with predominant upper motor neuron impairment from patients with suspected motor neuron disorder.
• Susceptibility-weighted imaging/quantitative susceptibility mapping–based assessment of the magnetic susceptibility provides a diagnostic marker for amyotrophic lateral sclerosis with upper motor neuron predominance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amyotrophic lateral sclerosis (ALS) is a highly lethal progressive neurodegenerative disorder of unknown etiology, characterized by degeneration of both upper (UMN) and lower motor neurons (LMN) [1]. The discovery of clinical, biological, genetic, radiological, and neurophysiological biomarkers of ALS is currently one of the main challenges [2]. The ideal biomarker should shorten the diagnostic delay and improve the accuracy, thus reducing the number of misdiagnosed cases [3].
Currently, the diagnosis is based on the latest revision of the El Escorial criteria, relying on clinical examination to assess signs of UMN impairment during a long period of time to confirm the progress of the disease, while relying on electromyography (EMG) and muscle biopsy to objectively detect signs of LMN impairment [4]. Neuroimaging and laboratory tests allow to exclude ALS-mimicking diseases (ALS-mim); in particular, brain and/or spine MRI are usually performed to exclude tumors, ischemic or demyelinating lesions, spine compression, and other neurodegenerative diseases [5].
Recent neuroimaging research has been focused on the analysis of the iron deposition in the precentral cortex, which is thought to be a marker of upper motor neuron degeneration in ALS patients [6,7,8,9,10]. Since iron has high magnetic susceptibility, it appears as a low signal cortical rim on susceptibility-weighted image (SWI) sequence [11,12,13]. The diagnostic value of SWI-based visual assessment using qualitative rating scales of the hyperintense cortical rim of the precentral cortex for the diagnosis of UMN involvement has been investigated [3, 13,14,15]. The main limitation of SWI is that it does not provide quantitative measurements. This issue has been addressed with the development of a quantitative susceptibility mapping (QSM) algorithm that allows in vivo quantification of the magnetic susceptibility [16]. A fully automatic QSM measure assessing the iron-related alterations of the precentral cortex was used to predict the UMN impairment in a cohort of ALS subjects [8].
The aim of this study is to compare the SWI-based visual qualitative assessment and the QSM-based automatic quantitative measures and evaluate the diagnostic accuracy in suspected motor neuron disease and ALS phenotypes.
Materials and methods
Subject groups
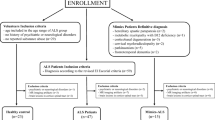
We retrospectively enrolled 54 ALS patients who had consecutively undergone a comprehensive evaluation, including neurological history, neurophysiological assessment, and MRI the IRCCS Istituto Auxologico Italiano in Milan (Italy), between January 2016 and December 2017. Diagnosis of ALS was made according to the revised El Escorial criteria [4].
Neurological examination was performed by two neurologists with more than 15 years of experience in the assessment of neuromuscular disorders.
According to our clinical protocol, the patients underwent MRI within 1 day from the clinical assessment. In addition to the MR images, in all subjects we collected the following clinical data: predominance of UMN or LMN impairment signs, site of onset (spinal or bulbar), and disease duration. The severity of UMN impairment was quantified using the Penn UMN score [17], and the grade of disability was measured using the ALS Functional Rating Scale-Revised (ALSFRS-R) [18]. The exclusion criteria for patient selection were (a) concomitant psychiatric or neurological disorders; (b) MR imaging artifacts; or (c) brain lesions shown on MRI images involving the motor cortex and corticospinal tracts not related to ALS.
Patients were divided into three subgroups according to the predominance of UMN and LMN impairment: 12 UMN-predominant ALS (UMN-ALS), 16 LMN-predominant ALS (LMN-ALS), and 19 ALS patients with no clinically defined predominance (c-ALS).
We recruited 23 healthy controls (HC) including volunteers and non-blood relatives of the patients between January 2018 and September 2018. HC enrollment was performed according to the following criteria: (a) in the age range of the ALS group; (b) no history of psychiatric or neurological disorders; and (c) no reported substance abuse. The exclusion criteria were as follows: (a) image artifacts on the T1-weighted and/or gradient-echo images, or (b) brain MRI showing abnormal findings, except for sporadic small gliotic lesions in the white matter. Study participants were selected from the cohort of previous studies on QSM assessment in ALS [7, 8, 19].
Additionally, we retrospectively collected images of 15 patients defined as mimics and chameleons (ALS-Mim), as defined by Turner and Talbot [5], who had undergone the same MRI protocol as ALS and HC patients, between January 2016 and September 2018. The definite diagnoses of ALS-Mim were extracted from clinical records as follows: two hereditary spastic paraparesis, one metabolic myelopathy with vitamin B12 deficiency, three corticobasal degeneration, two cervical myeloradiculopathy, five parkinsonism, and two frontotemporal dementia.
For statistical analysis purposes, we also considered the group of subjects with suspected motor neuron disease (SMD) composed of ALS and ALS-mim.
The research protocol was reviewed and approved by the local ethics committee, in accordance with the Declaration of Helsinki. All participants gave written, informed consent for the protocol.
Image acquisition and processing
All subjects underwent a brain MRI on a 3T SIGNA General Electric (GE Healthcare Medical Systems) unit at the Istituto Auxologico Italiano, IRCCS, Milan (Italy). The protocol included the following: whole-brain sagittal three-dimensional FSPGR BRAVO T1-weighted sequence (TR = 8.7 ms, TE = 3.2 ms, inversion time = 450 ms, voxel size = 1 × 1 × 1 mm3, flip angle = 12°, acquisition matrix 256 × 256); sagittal 3D fluid-attenuated inversion recovery (FLAIR) (TR = 6000 ms, TE = 108 ms, inversion time = 1824 ms, voxel size = 1 × 1 × 1.4 mm3, flip angle = 90°, acquisition matrix 224 × 224); axial T2-weighted fast spin-echo (FSE) (TR= 3000 ms, TE = 82 ms, pixel size = 0.234×0.234 mm2, slice thickness = 2 mm, slice gap = 0.2 mm, flip angle = 111°, acquisition matrix 320 × 320); three-dimensional spoiled gradient-echo multi- echo (GRE) pulse sequences (TR = 39 ms, TE = 24 ms, delta TE = 3.3 ms, number of echoes = 7, voxel size = 0.468 × 0.468 × 1.4 mm3, flip angle = 20°, acquisition matrix 416 × 320). The images collected from the different echoes of the spoiled GRE were averaged and the phase images were high-pass filtered by the scanner.
The brain MRIs were assessed by two neuroradiologists (with 4 and 30 years of experience) to define motion artifacts in the T1 or GRE sequences, arriving at a consensus. The brain MRI assessments identified severe motion artifacts in seven ALS patients, who were excluded from further analysis.
Visual qualitative assessment
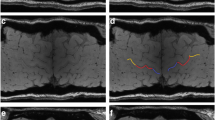
The Motor Cortex Susceptibility (MCS) score described in detail in [13] was used for the visual qualitative assessment of the motor cortex susceptibility. Briefly, for each of the six subregions of the primary motor cortex (upper limb, lower limb, and bulbar musculature in each hemisphere) 0 to 2 points on a discrete scale were allotted, according to the level of hypointensity of the primary motor cortex in a curvilinear multi-planar reconstruction of the cortical surface in magnitude images of the SWI (Fig. 1A).
A SWI-based MCS score: hypointensities in the primary motor cortex are visually evaluated in the subregions of lower limbs (in blue), upper limbs (in red), and bulbar musculature (in yellow) on both hemispheres as described in [13]; B QSM-based metrics: QSM values in the precentral gyrus (in red) are automatically extracted by image segmentation and coregistration to compute SuscMean, SuscMedian, SuscSD, SuscSkew, and SuscKurt as described in Contarino et al [8]
Automatic quantitative assessment
The primary motor cortex QSM was performed as described in Contarino et al [8]. Briefly, the whole-brain QSM was calculated from the 3D spoiled gradient-echo pulse sequence by using STI Suite tool (https://people.eecs.berkeley.edu/~chunlei.liu/software.html) and coregistered to the 3D T1-weighted image. The 3D T1- weighted images were segmented by using FreeSurfer [20, 21] and the Desikan-Killiany atlas [22] (Fig. 1B). Then, the susceptibility mean (SuscMean), susceptibility median (SuscMedian), susceptibility standard deviation (SuscSD), susceptibility skewness (SuscSkew), and susceptibility kurtosis (SuscKurt) neuroimaging metrics were calculated from the susceptibility values distribution in the automatically segmented precentral cortex.
Statistical analysis
The statistical analysis was performed using R 4.1.2 [R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing. URL https://www.R-project.org/]. A p-value smaller than 0.05 was considered significant.
Shapiro-Wilk tests were performed to test the distribution normality of the continuous variables.
The Mann-Whitney/Kruskal-Wallis tests were used to compare the continuous and ordinal variables between/among groups, and a chi-square test was used to compare nominal variables. Dunn-Holm’s tests were carried out for post hoc pairwise comparisons. In light of the distribution of the values of the qualitative and quantitative variables into the groups, we defined the following group-comparison and accuracy assessment: (1) SMD vs HC and (2) UMN-ALS vs ALS-mim.
Spearman’s test was used to assess the correlation between SuscSkew and MCS.
The area under the curve (AUC) value of the receiver operating characteristic (ROC) curve was used to evaluate the accuracy of MCS and QSM metrics in differentiating SMD and HC, and UMN-ALS and ALS-Mim. Sensitivity (SE), specificity (SP), positive predictive value (PPV), and negative predictive value (NPV) were then calculated for the MCS, SuscSD, SuscSkew, using the value that maximizes SP and PPV as a cut-off. Delong’s test was used to compare AUCs.
Results
Group-comparison in demographic, clinical, and neuroimaging data
The cohort of ALS subjects (male: 20/47) had a median age of 62 years (IQR: 54.5–68.5 years) and a median duration of illness of 18 months (IQR 11.5–25.5 months). The ALS-mim subjects (male: 7/15) had a median age of 70 years (IQR: 57–74.5 years) and the HC (male: 8/23) had a median age of 57 years (IQR: 53–62.5 years). Table 1 summarizes the demographic, clinical, and neuroimaging data in the subgroups.
No statistically significant difference was found among c-ALS, UMN-ALS, LMN-ALS, ALS-mim, and HC in terms of gender (χ2(4) = 1.24, p = 1) and age (H(4) = 10.6, p = 0.371). c-ALS, UMN-ALS, and LMN-ALS did not statistically differ in terms of ALSFRS-R (H(2) = 0.00617, p = 1), and disease duration (H(2) = 2.72, p = 1). UMN-ALS showed significantly higher Penn UMN scores than LMN-ALS and c-ALS (H(2) = 34.5, p < 0.0001).
MSC score of 1 and 2 was more frequent in UMN-ALS than LMN-ALS, c-ALS, ALS-mim and HC (H(4) = 33.4, p = 0.00114, Table 1 and Supplementary Table 1).
SuscSD was significantly higher in all ALS phenotype groups than in HC (H(4) = 20.1, p = 0.00563) (Table 1 and Supplementary Table 1).
SuscSkew was significantly higher in UMN-ALS compared to other c-ALS, LMN-ALS, ALS-mim and HC (H(4) = 23.0, p = 0.00154, Table 1 and Supplementary Table 1).
Figure 2 shows the distribution of the neuroimaging metrics in the subjects’ groups.
For SMD subjects, no statistically significant differences were found in terms of gender from HC (χ2(1) = 0.232, p = 1) and age (U = 502, p = 0.23).
MCS score of 1 and 2 was more frequent in SMD than in HC (SMD MCS 0 = 45; 1 = 4; 2 = 13; HC MCS score 0 = 23; 1 = 0; 2 = 0; U = 518; p = 0.048). SuscSD was significantly higher in SMD than HC (respectively median = 21.8 ppb, IQR 20.3–24.3, and median = 19.9, IQR = 18.8–20.7; U = 264, p = 0.0001). No other susceptibility metrics significantly differ between groups.
Correlation between qualitative and quantitative assessment and stratification according to MCS score
The MCS score showed a significant correlation with SuscMean (Rho = 0.27, p = 0.01), SuscSD (Rho = 0.45, p < 0.001), and SuscSkew (Rho = 0.55, p < 0.001) (Fig. 3). The correlations are shown in Supplementary Table 2.
Subjects grouped according to the MCS score showed statistically significant differences in QSM metrics (Table 2). In particular, subjects with MCS score of 0 had lower SuscSkew values (H(2) = 25, p < 0.0001) and lower SuscSD (H(2) = 18, p = 0.000122) compared to subjects with MCS score 2, and lower SuscSkew (H(2) = 25.1, p < 0.0001) than subjects with MCS score 1 as shown in supplementary Table 3. No statistical differences were found in SuscMean, SuscMedian, and SuscKurt.
Diagnostic accuracy of qualitative and quantitative measures
The ROC analysis demonstrated that SuscSD (AUC = 0.809, p < 0.0001) and MCS (AUC = 0.64, p = 0.003) (Fig. 4A) are able to differentiate SMD from HC with specificity and a positive predictive value of 100% considering a cut-off of 21.26 ppb and a cut-off of 1 for SuscSD and MCS, respectively. SuscSD was a significantly better discriminative metric than MCS (Delong’s test Z = −3.384, p-value = 0.00071).
The ROC analysis demonstrated that SuscSkew (AUC: 0.936, p < 0.0001) and MCS (AUC: 0.861, p < 0.0001) are able to differentiate UMN-ALS versus ALS-Mim (Fig. 4B). SuscSkew did not have a significantly higher AUC than MCS (Delong’s test Z = 0.81; p = 0.21). SuscSkew showed the highest specificity (92.3%) and positive predictive value (91.7%) considering a cut-off of −0.017.
Discussion
Our study shows a good concordance between the SWI-based visual qualitative and QSM-based automatic quantitative measures in assessing the magnetic susceptibility alteration in the motor cortex. We demonstrated sufficient and very good diagnostic accuracy to differentiate subjects with SMD from HC respectively for MCS and SuscSD and very good and excellent accuracy to differentiate ALS-UMN from ALS-Mim respectively for MCS and SuscSkew.
Indeed, the concordance between visual qualitative and automatic quantitative assessment was highlighted by significant positive correlations between MCS and QSM metrics and the strongest correlations were demonstrated for SuscSD and SuscSkew. Since the two quantitative and qualitative measures that are based on different images and different imaging methods provide similar results, that reinforces their role in representing the alterations in the motor cortex.
SMD showed altered MCS and SuscSD values compared to HC. In the ROC analysis, MCS and SuscSD showed high accuracy, SP and PPV in differentiating the two groups, suggesting two promising methods to detect non-specific cortical iron-related alterations. Moreover, the automatic quantitative SuscSD showed better performances than the qualitative visual MSC with the advantage being unbiased by the reader.
Iron-related alteration in the cortex is one of the etiopathogenetic mechanisms suggested for pathologies other than ALS that involve neurodegeneration and can mimic the clinical pictures of motor neuron disease [5, 10, 23]. Thus, alteration of iron homeostasis in the cortex measured by QSM could be of help in the discrimination of subjects with a SMD from HC, ruling in pathological subjects with involvement of the motor cortex. We might speculate that the SuscSD might also be linked to a less clearly defined boundary between gray matter and white matter due to the degeneration of the motor cortex and the white matter, a process that has been recognized for ALS and ALS mimics subjects [10, 24,25,26].
UMN-ALS demonstrated altered MCS and SusckSkew values compared to other ALS phenotypes, HC and, more interestingly, to ALS-Mim. In literature, iron-related hypointensity in the motor cortex can be influenced by disease phenotype, in particular by the degree of UMN impairment [27]. Studies combining imaging with anatomopathological assessment showed that MRI signal changes of the motor cortex correspond to intracellular ferritin inclusion in microglial cells [10, 11]. We support the thesis that iron accumulation may be one of the mechanisms of degeneration in UMN-ALS subjects, while in LMN-ALS subjects the pathological mechanism is likely to be TDP-43 proteinopathy such as aggregates of cleaved and hyperphosphorylated TDP-43 and FUS-positive basophilic inclusion bodies [28, 29]. TDP-43 proteinopathy that starts in the LMNs and eventually spreads from the spinal cord and brainstem to the primary motor cortex was demonstrated in the LMN-ALS subgroup of progressive muscular atrophy [29].
A high SusckSkew means that the distribution of the QSM values is asymmetrical due to a larger right tail representing high susceptibility values. Interesting, previously published anatomopathological analysis of the precentral cortex of subjects affected by ALS demonstrated sparsely and intensely Perls-stained for ferric iron astrocytes and macrophages [10, 30]. A more recent study by [10] demonstrated how the microglia of the middle and deep layer of the motor cortex of subjects affected by ALS are selectively involved and the pattern of involvement is different from other pathologies that can affect the motor cortex. Indeed, the motor cortex of subjects affected by Alzheimer’s and Parkinson’s disease, is more diffusively involved and iron accumulation is also present in the superficial white matter of the gyrus. Thus, we postulate that the increase in the SuscSD and SuscSkew could reflect the non-uniform and selective increase in ferric iron of the microglia of the precentral cortex of subjects affected by UMN-ALS. In our cohort, UMN-ALS were discriminated from ALS-Mim with high accuracy by using MSC, and SuscSkew. A previous publication [13] demonstrated how visual assessment of susceptibility of the motor cortex can be helpful in differentiating the UMN-ALS subjects from HC and ALS-Mim, being able to rule in ALS in subjects without LMN impairment using a MCS score ≥ 2 as a cut-off. Another study, using manually drawn ROIs [9], demonstrated how the relative mean and relative maximum QSM value of the cortex of the hand knob can differentiate ALS subjects from ALS-Mim with a specificity of 100%. In our study, SuscSkew is the most accurate metric to differentiate UMN-ALS from ALS-Mim, with sensibility, specificity, negative predictive value, and positive predictive value above 90% considering a cutoff of −0.017. The accuracy of SuscSkew is greater than MCS score, though not significantly. We can conclude that both SucSkew and MCS are reliable metrics to rule in subjects affected by ALS with prevalence of upper motor neuron signs. We provided a highly specific user independent cut-off that might ease the diagnosis of UMN-ALS and the enrollment in future clinical trials that would benefit from quantification of iron content alteration before and after treatment, such as the iron-chelation trials.
However, some limitations of our study need to be acknowledged. First, the sample size of our cohort is small and needs to be enlarged to validate the promising results of the present study.
Tissue susceptibility measured by QSM in the precentral cortex is determined by concentration of paramagnetic (iron) and diamagnetic (cortical myelin) species in a voxel. Combined MRI-histological studies or advanced processing and modelling of QSM data (source separation) could be useful to discriminate the contribution of each component to the susceptibility properties of the tissue [31].
Further prospects might be the validation of the SWI-based qualitative visual and the QSM-based quantitative automatic measures in multicentric studies and the assessment of their accuracy in the early stages of the disease and their predicting roles in subjects at risk.
In conclusion, the SWI-based visual qualitative and the QSM-based automatic quantitative measures were concordant in assessing iron-related alterations in the primary motor cortex and might be evaluated in larger multicentric studies to validate their role in the identification of UMN-ALS among patients with suspected motor neuron disease.
Abbreviations
- ALS:
-
Amyotrophic lateral sclerosis
- ALSFRS-R:
-
ALS Functional Rating Scale-Revised
- ALS-mim:
-
ALS-mimicking diseases
- c-ALS:
-
ALS patients with no clinically defined predominance
- HC:
-
Healthy controls
- LMN:
-
Lower motor neurons
- LMN-ALS:
-
LMN-predominant ALS
- MCS:
-
Motor Cortex Susceptibility
- QSM:
-
Quantitative susceptibility mapping
- SMD:
-
Suspected motor neuron disease
- SuscKurt:
-
Susceptibility kurtosis
- SuscMean:
-
Susceptibility mean
- SuscMedian:
-
Susceptibility median
- SuscSD:
-
Susceptibility standard deviation
- SuscSkew:
-
Susceptibility skewness
- SWI:
-
Susceptibility-weighted images
- UMN:
-
Upper motor neuron
- UMN-ALS:
-
UMN-predominant ALS
References
Brown RH Jr, Al-Chalabi A (2017) Amyotrophic lateral sclerosis. N Engl J Med 377:1602
Lenglet T, Camdessanché J-P (2017) Amyotrophic lateral sclerosis or not: keys for the diagnosis. Rev Neurol 173:280–287
Mazón M, Vázquez Costa JF, Ten-Esteve A, Martí-Bonmatí L (2018) Imaging biomarkers for the diagnosis and prognosis of neurodegenerative diseases. The Example of Amyotrophic Lateral Sclerosis. Front Neurosci 12:784. https://doi.org/10.3389/fnins.2018.00784
Ludolph A, Drory V, Hardiman O et al (2015) A revision of the El Escorial criteria - 2015. Amyotroph Lateral Scler Front Degener 16:291–292
Turner MR, Talbot K (2013) Mimics and chameleons in motor neurone disease. Pract Neurol 13:153–164
Acosta-Cabronero J, Machts J, Schreiber S et al (2018) Quantitative susceptibility MRI to detect brain iron in amyotrophic lateral sclerosis. Radiology 289:195–203
Conte G, Contarino VE, Casale S et al (2021) Amyotrophic lateral sclerosis phenotypes significantly differ in terms of magnetic susceptibility properties of the precentral cortex. Eur Radiol 31:5272–5280
Contarino VE, Conte G, Morelli C et al (2020) Toward a marker of upper motor neuron impairment in amyotrophic lateral sclerosis: a fully automatic investigation of the magnetic susceptibility in the precentral cortex. Eur J Radiol 124:108815
Dean KE, Shen B, Askin G et al (2021) A specific biomarker for amyotrophic lateral sclerosis: quantitative susceptibility mapping. Clin Imaging 75:125–130
Kwan JY, Jeong SY, Van Gelderen P et al (2012) Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: correlating 7 tesla MRI and pathology. PLoS One 7:e35241
Adachi Y, Sato N, Saito Y et al (2015) Usefulness of SWI for the detection of iron in the motor cortex in amyotrophic lateral sclerosis. J Neuroimaging 25:443–451
Sheelakumari R, Madhusoodanan M, Radhakrishnan A et al (2016) A potential biomarker in amyotrophic lateral sclerosis: can assessment of brain iron deposition with SWI and corticospinal tract degeneration with DTI help? AJNR Am J Neuroradiol 37:252–258
Conte G, Sbaraini S, Morelli C et al (2021) A susceptibility-weighted imaging qualitative score of the motor cortex may be a useful tool for distinguishing clinical phenotypes in amyotrophic lateral sclerosis. Eur Radiol 31:1281–1289
Cosottini M, Donatelli G, Costagli M et al (2016) High-resolution 7T MR imaging of the motor cortex in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol 37:455–461
Rizzo G, Marliani AF, Battaglia S, et al (2020) Diagnostic and prognostic value of conventional brain MRI in the clinical work-up of patients with amyotrophic lateral sclerosis. J Clin Med Res 9. https://doi.org/10.3390/jcm9082538
Eskreis-Winkler S, Zhang Y, Zhang J, et al (2017) The clinical utility of QSM: disease diagnosis, medical management, and surgical planning. NMR Biomed 30. https://doi.org/10.1002/nbm.3668
Quinn C, Edmundson C, Dahodwala N, Elman L (2020) Reliable and efficient scale to assess upper motor neuron disease burden in amyotrophic lateral sclerosis. Muscle Nerve 61:508–511
Cedarbaum JM, Stambler N, Malta E et al (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 169:13–21
Conte G, Sbaraini S, Morelli C et al (2021) Correction to: A susceptibility-weighted imaging qualitative score of the motor cortex may be a useful tool for distinguishing clinical phenotypes in amyotrophic lateral sclerosis. Eur Radiol 31:4404
Liu C, Li W, Tong KA et al (2015) Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. J Magn Reson Imaging 42:23–41
Fischl B (2012) FreeSurfer. Neuroimage 62:774–781
Desikan RS, Ségonne F, Fischl B et al (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980
Sheelakumari R, Kesavadas C, Varghese T et al (2017) Assessment of iron deposition in the brain in frontotemporal dementia and its correlation with behavioral traits. AJNR Am J Neuroradiol 38:1953–1958
Yoshida M (2004) Amyotrophic lateral sclerosis with dementia: the clinicopathological spectrum. Neuropathology 24:87–102
Ferraro PM, Agosta F, Riva N et al (2017) Multimodal structural MRI in the diagnosis of motor neuron diseases. Neuroimage Clin 16:240–247
Kocar TD, Müller H-P, Ludolph AC, Kassubek J (2021) Feature selection from magnetic resonance imaging data in ALS: a systematic review. Ther Adv Chronic Dis 12:20406223211051000
Vázquez-Costa JF, Mazón M, Carreres-Polo J et al (2018) Brain signal intensity changes as biomarkers in amyotrophic lateral sclerosis. Acta Neurol Scand 137:262–271
Riku Y, Atsuta N, Yoshida M et al (2014) Differential motor neuron involvement in progressive muscular atrophy: a comparative study with amyotrophic lateral sclerosis. BMJ Open 4:e005213
Geser F, Stein B, Partain M et al (2011) Motor neuron disease clinically limited to the lower motor neuron is a diffuse TDP-43 proteinopathy. Acta Neuropathologica 121:509–517
Oba H, Araki T, Ohtomo K et al (1993) Amyotrophic lateral sclerosis: T2 shortening in motor cortex at MR imaging. Radiology 189:843–846
Dimov AV, Gillen KM, Nguyen TD et al (2022) Magnetic susceptibility source separation solely from gradient echo data: histological validation. Tomography 8:1544–1551
Acknowledgements
Authors would like to thank all the subjects that participated to the study.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Claudia Morelli.
Conflict of interest
Fabio Maria Triulzi is a member of the European Radiology Scientific Editorial Board. The remaining authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
The statistics were defined by Conte and Lo Russo
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
There exists a partial overlap with:
• Contarino VE, Conte G, Morelli C, et al (2020): Development of the QSM method, no SWI assessments were performed
• Conte G, Contarino VE, Casale S, et al (2021): Evaluation of the QSM measures accross the ALS clinical phenotypes, no SWI assessments were performed
• Conte G, Sbaraini S, Morelli C, et al (2021): Development of the SWI method, no QSM assessments were
Methodology
• Retrospective
• observational
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lo Russo , F., Contarino, V.E., Conte, G. et al. Amyotrophic lateral sclerosis with upper motor neuron predominance: diagnostic accuracy of qualitative and quantitative susceptibility metrics in the precentral gyrus. Eur Radiol 33, 7677–7685 (2023). https://doi.org/10.1007/s00330-023-10070-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10070-y