Abstract
Objective
To compare tumor therapy response assessments with whole-body diffusion-weighted imaging (WB-DWI) and 18F-fluorodeoxyglucose ([18F]FDG) PET/MRI in pediatric patients with Hodgkin lymphoma and non-Hodgkin lymphoma.
Materials and methods
In a retrospective, non-randomized single-center study, we reviewed serial simultaneous WB-DWI and [18F]FDG PET/MRI scans of 45 children and young adults (27 males; mean age, 13 years ± 5 [standard deviation]; age range, 1–21 years) with Hodgkin lymphoma (n = 20) and non-Hodgkin lymphoma (n = 25) between February 2018 and October 2022. We measured minimum tumor apparent diffusion coefficient (ADCmin) and maximum standardized uptake value (SUVmax) of up to six target lesions and assessed therapy response according to Lugano criteria and modified criteria for WB-DWI. We evaluated the agreement between WB-DWI- and [18F]FDG PET/MRI–based response classifications with Gwet’s agreement coefficient (AC).
Results
After induction chemotherapy, 95% (19 of 20) of patients with Hodgkin lymphoma and 72% (18 of 25) of patients with non-Hodgkin lymphoma showed concordant response in tumor metabolism and proton diffusion. We found a high agreement between treatment response assessments on WB-DWI and [18F]FDG PET/MRI (Gwet’s AC = 0.94; 95% confidence interval [CI]: 0.82, 1.00) in patients with Hodgkin lymphoma, and a lower agreement for patients with non-Hodgkin lymphoma (Gwet’s AC = 0.66; 95% CI: 0.43, 0.90). After completion of therapy, there was an excellent agreement between WB-DWI and [18F]FDG PET/MRI response assessments (Gwet’s AC = 0.97; 95% CI: 0.91, 1).
Conclusion
Therapy response of Hodgkin lymphoma can be evaluated with either [18F]FDG PET or WB-DWI, whereas patients with non-Hodgkin lymphoma may benefit from a combined approach.
Clinical relevance statement
Hodgkin lymphoma and non-Hodgkin lymphoma exhibit different patterns of tumor response to induction chemotherapy on diffusion-weighted MRI and PET/MRI.
Key Points
• Diffusion-weighted imaging has been proposed as an alternative imaging to assess tumor response without ionizing radiation.
• After induction therapy, whole-body diffusion-weighted imaging and PET/MRI revealed a higher agreement in patients with Hodgkin lymphoma than in those with non-Hodgkin lymphoma.
• At the end of therapy, whole-body diffusion-weighted imaging and PET/MRI revealed an excellent agreement for overall tumor therapy responses for all lymphoma types.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluorine-18 fluorodeoxyglucose ([18F]FDG) PET is the imaging modality of choice for monitoring treatment response in pediatric patients with lymphoma [1, 2]. [18F]FDG PET measures the glucose metabolism of tumor cells, which can be quantified using standardized uptake values (SUVs) to provide an estimate of tumor metabolic activity [3]. Chemotherapy damages tumor cells, resulting in reducing the cellular [18F]FDG uptake and lowering the SUV of lymphoma on [18F]FDG PET scans [4]. [18F]FDG PET/MRI is a reliable alternative to [18F]FDG PET/CT for pediatric patients with lymphoma. Because both technologies rely on [18F]FDG PET imaging, they provide a similar diagnostic accuracy [5]. Replacing CT with MRI for anatomical colocalization of [18F]FDG PET data significantly reduces ionizing radiation exposure in children with cancer [6,7,8].
However, the availability of [18F]FDG PET/MRI is limited to large tertiary medical centers in world-leading countries, in contrast to the worldwide availability of conventional MRI. Whole-body (WB) MRI is another feasible alternative method that provides both anatomical information and diffusion-weighted imaging (DWI) for functional information without ionizing radiation [9]. DWI measures the mobility of water molecules in the tumor microenvironment and can be quantified as the apparent diffusion coefficient (ADC) to provide an indirect estimate of tumor cell density [10]. At the time of diagnosis, most lymphomas have low ADC values due to high cell density and high nuclear-to-cytoplasmic ratios [11]. Chemotherapy induces cell death and necrosis. The breakdown of cellular barriers in necrotic tissue leads to increased diffusion of water molecules and increased ADC values of lymphoma on DWI scans [12].
Nevertheless, it is unclear whether DWI is comparable to [18F]FDG PET for treatment monitoring in lymphoma patients. The results in the literature to date are inconsistent regarding the information provided by [18F]FDG PET and DWI [13,14,15,16]. SUVs and ADCs are considered independent biomarkers for lymphomas [14]. Some authors reported concordant therapy response of lymphomas on [18F]FDG PET or DWI [17,18,19], while others reported discordant therapy response [20,21,22]. Therapy-induced changes in pediatric lymphomas on DWI and [18F]FDG PET may depend on underlying tumor histology. Few studies have assessed how different types of lymphoma respond to chemotherapy in terms of changes in tumor metabolism and diffusion using [18F]FDG PET and DWI, respectively [18, 23]. Mayerhoefer et al reported different patterns of metabolic activity and ADC changes in specific lymphoma subtypes after treatment initiation in adult patients [23]. However, WB-DWI and [18F]FDG PET response assessments have not been compared according to tumor type in the pediatric population.
Therefore, the purpose of our study was to compare tumor therapy response assessments using WB-DWI and [18F]FDG PET/MRI in pediatric patients with Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL).
Materials and methods
Study participants
Our study was approved by the institutional review board (IRB) of our institution. We collected de-identified imaging studies and relevant clinical information (patient age, sex, tumor type, serum lactate dehydrogenase, stage, bone marrow, and central nervous system involvement) in a centralized image registry. Parents provided written informed consent and pediatric patients provided assent to donate their medical images and related clinical data to the registry for research analyses. All patients younger than 25 years with histologically confirmed lymphoma who underwent combined [18F]FDG PET/MRI and WB-DWI in the same examinations between February 2018 and October 2022 were included. The exclusion criteria were incomplete imaging data and the absence of measurable lesions.
Imaging
All patients with blood glucose levels < 140 mg/dL were injected with [18F]FDG (3–5 megabecquerel per kg body weight) and scanned 60 min later, on a 3-T Signa PET/MRI scanner (GE Healthcare), using appropriate surface coils. The imaging protocol consisted of axial DWI (\(b\) values = 50 and 600 or 800 s/mm2), Dixon sequences, and breath-hold fat-saturated T1-weighted gradient-echo sequences after gadolinium-chelate administration. PET data were reconstructed using scanner-specific algorithms. ADC maps were generated from DWI data using the OsiriX MD software (version 13.0.0; Pixmeo).
Assessments
One radiologist (W.M.) and one nuclear medicine physician (L.B.) evaluated the original and fused [18F]FDG PET/MRI and WB-DWI side by side to identify and select the target lesions for quantitative analysis in consensus. Fourteen nodal regions according to anatomical regions for the staging of lymphoma and other organs as extranodal regions were evaluated [24]. According to the Lugano criteria, we identified up to six largest measurable target lesions in different body regions per patient. Tumor lesions were considered measurable if they were larger than 15 mm in the longest diameter for nodal lesions and 10 mm in the longest diameter for extranodal lesions [25]. The lesion also must be visible on both [18F]FDG PET images and DWI. Imaging characteristics on T2-weighted and post-contrast T1-weighted images, including enhancement pattern, tumoral necrosis, effusion, ascites, region of disease involvement, and number of extranodal involvement, were recorded. The investigators were blinded to tumor histopathology, clinical data, and treatment outcome.
The nuclear medicine physician (L.B.) measured the maximum standardized [18F]FDG uptake values (SUVmax) of the target lesions on [18F]FDG PET/MR scans using the MIM software (version 7.2.8; MIM Software Inc.) based on isocontour volumes of interest (VOIs) constructed using a 41% SUVmax threshold. The radiologist (W.M.) assessed target lesions on WB-DWI and measured the minimum apparent diffusion coefficient (ADCmin) on ADC maps based on manually defined regions of interest (ROIs).
To determine reproducibility, a radiologist (W.M.) measured the SUV and a nuclear medicine physician (L.B.) measured the ADC of random 20 tumors (10 HL and 10 NHL) at least 2 weeks apart. The concordance correlation between the first and second measurements was calculated.
Interim [18F]FDG PET/MRI scans were performed after 1–3 cycles of chemotherapy. End-of-therapy [18F]FDG PET/MRI scans were defined as the first scan after completion of chemotherapy. The Lugano classification was used to evaluate the tumor therapeutic response for [18F]FDG PET/MRI [25]. The modified tumor response assessment for WB-DWI was adapted from prior studies (Table 1) [18, 19]. Lesions with restricted diffusion were defined as lesions that demonstrated high signal on high \(b\) value DWI and low signal on ADC map as compared to the muscle for nodal lesions or compared to surrounding tissue for extranodal lesions [18, 26]. Baseline scans were available to compare with interim and end-of-therapy scans to ensure that the same target lesions were measured. The treatment response was evaluated and assigned to four categories based on SUV and ADC measurements: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The least response target lesion represented disease response of each patient on each modality.
All clinical data at the end of therapy, including histology and imaging, served as the reference standard for tumor therapy response. Based on their response to therapy, we divided patients into two groups, responders (CR and PR) and non-responders (SD and PD).
The amount of ionizing radiation that patients received during the [18F]FDG PET/MRI scan was calculated using the RADAR Medical Procedure Radiation Dose Calculator [27].
Statistical analysis
An expert statistician (T.L.) performed all analyses. The agreement between WB-DWI- and [18F]FDG PET/MRI–based therapy response assessments (CR, PR, SD, PD) after chemotherapy induction and at the end of therapy was determined using Gwet’s agreement coefficient (AC) based on per-patient basis. In addition, a correlation of SUVmax and ADCmin values of the lesions was tested using the Spearman rank correlation. The agreement between WB-DWI- and [18F]FDG PET/MRI–based therapy response assessments after chemotherapy induction and clinical response assessments at the end of therapy was determined using Gwet’s AC. The Kaplan–Meier survival analysis was performed to assess the progression-free survival (PFS). The log-rank test was used to test equality of survival functions between responders and non-responders based on interim [18F]FDG PET/MRI and WB-DWI. Statistical analyses were performed with the STATA software (version 17.0; StataCorp), assuming significant differences for p < 0.05.
Results
Patient demographics
The participant flowchart is shown in Fig. 1. Based on our registry, we retrospectively identified 85 pediatric patients with lymphoma. Forty patients were excluded because of incomplete imaging data (n = 36) and no measurable lesions (n = 4). We analyzed imaging data of 45 patients with HL (n = 20) and NHL (n = 25), including 27 males and 18 females with a mean age of 13 years ± 5 (range 1–21 years). The demographic and clinical information of the patients is shown in Table 2. The histological subtypes are provided in Table S1.
Mean and median ionizing radiation exposure from [18F]FDG PET were 2.81 mSv ± 1.02, and 2.94 mSv (interquartile range 2.10–3.53), respectively.
Evaluation of the baseline scans
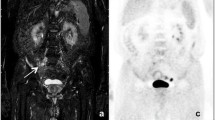
Qualitative imaging characteristics were not significantly different between patients with HL and NHL. A total of 65% of HL and 60% of NHL cases demonstrated homogeneous contrast enhancement on gadolinium-chelate-enhanced T1-weighted MRI scans. NHL demonstrated a tendency towards a higher incidence of tumoral necrosis (36% vs. 15%), extranodal involvement (68% vs. 40%), and presence of pleural effusion or ascites (36% vs. 15%) compared to HL (Table 3). Among seven patients with diffuse large B cell lymphoma (DLBCL), three patients with Epstein-Barr virus (EBV)–positive DLBCL showed a large central necrosis with a thin rim of contrast enhancement (Fig. 2). The remaining four patients with DLBCL, which were not otherwise specified, presented as predominantly solid masses.
A 7-year-old male with Epstein-Barr virus (EBV)–positive diffuse large B cell lymphoma. A Axial post-gadolinium T1-weighted MRI (post-Gd T1WI), (B) fluorine-18 fluorodeoxyglucose ([18F]FDG) PET, (C) color-coded fused [18F]FDG/MRI, (D) whole-body diffusion-weighted imaging (DWI), (E) apparent diffusion coefficient (ADC) map of the brain, (F–G) the neck, and (K–O) the liver demonstrating multiple rim-enhancing lesions with peripheral FDG uptake in the right temporal lobe, right internal jugular chain, bilateral parapharyngeal spaces, and right lobe of the liver (arrows). These lesions show peripheral restricted diffusion on whole-body DWI and low signal intensity on the ADC map (arrows)
At baseline, all HL showed a strong metabolic activity on [18F]FDG PET (mean SUVmax 10.98 ± 5.78 g/mL) and restricted diffusion on WB-DWI (mean ADCmin 0.72 ± 0.31 × 10−3 mm2/s). Similarly, all NHL showed a strong and inhomogeneous metabolic activity on [18F]FDG PET (mean SUVmax 11.90 ± 7.51 g/mL) and restricted diffusion on WB-DWI (mean ADCmin 0.73 ± 0.33 × 10−3 mm2/s). NHL that subsequently responded to chemotherapy (CR and PR) showed a mean baseline SUVmax of 10.68 ± 6.14 g/mL and a mean baseline ADCmin of 0.71 ± 0.33 × 10−3 mm2/s. NHL that did not respond to therapy (SD and PD) showed a mean baseline SUVmax of 21.21 ± 10.45 g/mL and a mean ADCmin of 0.86 ± 0.28 × 10−3 mm2/s.
To assess reproducibility, the SUVmax and ADCmin of 10 HL and 10 NHL tumor foci were measured by two different readers. Concordance correlation was calculated, and no significant difference between the measurements of the two readers was observed. Bland–Altman plots are available in supplementary materials (Fig. S1).
Therapy response assessment on interim scans
Hodgkin lymphoma
After induction chemotherapy, 19 of 20 patients with HL (95%) showed a decreased tumor metabolic activity on [18F]FDG PET and increased ADC values on WB-DWI, consistent with a concordant complete (n = 18) (Fig. 3) or partial response to therapy (n = 1). One patient (5%) showed a complete response on [18F]FDG PET/MRI but only a partial response on WB-DWI. HL responders demonstrated a relative change in SUVmax between baseline and interim scans of − 79.52% ± 14.22 and a relative change in ADCmin of 161.14% ± 126.78. Interim therapy response assessments with WB-DWI and [18F]FDG PET/MRI demonstrated a very good agreement in patients with HL (Gwet’s AC = 0.94; Table 4). Compared to the clinical end of therapy as the reference standard, both interim [18F]FDG PET/MRI and WB-DWI showed 100% agreement with the end-of-therapy response (Gwet’s AC = 1; Table S2).
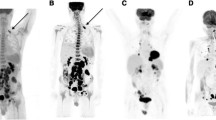
A 16-year-old female with Hodgkin lymphoma and a concordant complete response on fluorine-18 fluorodeoxyglucose ([18F]FDG) PET/MRI and whole-body diffusion-weighted imaging (DWI). A–D Before therapy, (A) [18F]FDG PET maximum intensity projection (MIP) image and (B) coronal color-coded fused [18F]FDG PET/MRI demonstrating an anterior mediastinal mass with increased FDG uptake. The mass shows restricted diffusion on (C) coronal reformatted whole-body DWI, and a low signal on (D) the apparent diffusion coefficient (ADC) map (arrows). E–H After induction therapy, the mass decreased in size and showed no abnormal FDG uptake on (E, F) [18F]FDG PET/MRI, no restricted diffusion on (G) whole-body DWI, and a high signal on (H) the ADC map (arrowheads)
Non-Hodgkin lymphoma
After induction chemotherapy, 15 of the 25 patients with NHL (60%) showed concordant complete (n = 14) or partial response (n = 1) on [18F]FDG PET/MRI and WB-DWI. Two patients (8%) showed an increased tumor SUV and decreased ADC values, consistent with progressive disease. One patient (4%) showed no significant changes in SUV and ADC values, consistent with stable disease.
Seven patients (28%) showed discordant therapy response. Three patients (one with extranodal NK/T cell lymphoma, one with ALK-positive anaplastic large cell lymphoma, and one with primary mediastinal large B cell lymphoma) showed a complete response on [18F]FDG PET/MRI and a partial response on WB-DWI. One patient with EBV-positive DLBCL showed a partial response on [18F]FDG PET/MRI and stable disease on WB-DWI. Two patients (one with Burkitt lymphoma and one with primary mediastinal large B cell lymphoma) showed a partial response on [18F]FDG PET/MRI and a complete response on WB-DWI (Fig. 4). One patient with T-lymphoblastic lymphoma had stable disease on [18F]FDG PET/MRI, but a complete response on WB-DWI (Table 5). WB-DWI and [18F]FDG PET/MRI interim assessments of therapy response for NHL patients showed a moderate agreement (Gwet’s AC = 0.66; Table 4).
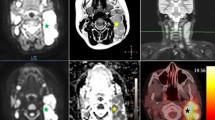
A 17-year-old female with primary mediastinal large B cell lymphoma and a discordant response on fluorine-18 fluorodeoxyglucose ([18F]FDG) PET/MRI and whole-body diffusion-weighted imaging (DWI). A–D Before therapy, (A) coronal [18F]FDG PET/MRI shows a heterogeneous anterior mediastinal mass with high metabolic activity (arrows). The lesion shows a low signal on (B) the apparent diffusion coefficient (ADC) map with a hyperintense signal on (C) axial DWI and (D) T2-weighted MRI (T2WI) (arrows). E–H After induction therapy, the mass decreased in size but demonstrated a remaining high metabolic activity on (E).[18F]FDG PET/MRI, consistent with a partial metabolic response (arrowhead). However, the lesion shows a high signal on (F) the ADC map, which is higher than that of the muscle as an internal reference standard, compatible with a complete response according to the modified criteria for DWI (arrowhead). The mild hyperintense signal of this lesion on (G) DWI is likely due to the T2-shine-through effect since it demonstrates a markedly hyperintense signal on (H) T2WI (arrowheads)
From baseline to interim scans, NHL responders (CR and PR) showed a mean relative change in SUVmax of − 73.36% ± 16.73 and a mean relative change in ADCmin of 183.63% ± 197.72. In contrast, NHL non-responders (SD and PD) showed a mean relative change in SUVmax and a mean relative change in ADCmin of 21.22% ± 59.63 and − 7.71% ± 66.56, respectively.
Eighty percent of interim [18F]FDG PET/MRI (20 of 25) and 88% of interim WB-DWI (22 of 25) agreed with the clinical response at the end of therapy in NHL patients (Gwet’s AC = 0.72; 95% confidence interval [CI] 0.44, 0.996; and Gwet’s AC = 0.83; 95% CI 0.62, 1, respectively; Table S2).
Therapy response assessment on end-of-therapy scans
[18F]FDG PET/MRI and WB-DWI scans at the end of therapy were not available for nine patients due to an adequate clinical response to induction therapy (n = 8) and death (n = 1). Of the remaining 36 patients (15 with HL and 21 with NHL), 97% (35 of 36) showed concordant responses between [18F]FDG PET/MRI and WB-DWI at the end of therapy (Gwet’s AC = 0.97; Table 4). One patient with NHL (Fig. 4) was assigned a partial response on [18F]FDG PET/MRI and a complete response on WB-DWI on the first imaging investigation after completion of chemotherapy, followed by complete remission on subsequent 4-month off-therapy imaging.
The ADCmin and SUVmax of all lymphomas showed a strong inverse correlation (Spearman’s rho = − 0.71, p < 0.001, n = 309; Fig. S2A). There was a moderate inverse correlation between the relative degree of changes in SUVmax and ADCmin (Spearman’s rho = − 0.45, p < 0.001, n = 200; Fig. S2B). Therapy-induced changes in tumor ADCmin were more variable than those in SUVmax (Fig. S3).
The log-rank test demonstrated different progression-free survivals between responders and non-responders, classified based on either [18F]FDG PET/MRI or WB-DWI (p = 0.009 and p = 0.01, respectively; Fig. 5).
Discussion
Our data show that the therapeutic response of Hodgkin lymphoma can be assessed by either [18F]FDG PET/MRI or WB-DWI after induction therapy, whereas patients with non-Hodgkin lymphoma may benefit from a combined approach.
Several studies have compared WB-DWI and [18F]FDG PET for treatment monitoring of lymphoma [17,18,19, 21, 26, 28,29,30]. However, most previous studies were conducted in adults [17, 18, 26, 28, 29]. Lin et al reported that the ADC analysis reduced false-positive findings in adult patients with NHL on WB-DWI [26]. Mayerhoefer et al found a 97% agreement between [18F]FDG PET/CT and WB-DWI on follow-up examinations in 64 adults with HL and NHL [18]. Theruvath et al reported an agreement between [18F]FDG PET/MRI and WB-DWI at interim follow-up in 30 of 37 (81%) pediatric patients with lymphoma [19]. This is in accordance with our study, which found 82% agreement for interim scans and 97% agreement for end-of-therapy examinations.
Theruvath et al observed that the tumor metabolic activity declined first and the tumor cell density decreased afterwards in lymphoma patients who showed a mismatch in tumor response between [18F]FDG PET/MRI and WB-DWI [19]. In the present study, we examined the type of tumor and found a higher agreement between interim [18F]FDG PET/MRI and WB-DWI in patients with HL than in those with NHL. In a few patients, we noted a complete response on interim WB-DWI and partial response on interim [18F]FDG PET/MRI. We hypothesized that these tumors might have shown an inflammatory response rather than a residual tumor metabolic activity. This is compounded by the fact that follow-up scans of all tumors with this pattern demonstrated a complete response at the end of the therapy scans. An experimental study also reported that the ADC analysis helped distinguish between inflammatory and malignant lymph nodes in a rabbit model of VX2 carcinoma [31]. An inflammatory reaction in a residual mass causing an increased metabolic activity in the absence of histologically viable tumor cells has been described in patients with NHL in both interim and end-of-therapy assessments [32,33,34]. In these cases, adding information from DWI scans may increase the predictive accuracy of interim scans.
Few studies investigated the ability of WB-DWI to predict the long-term survival of patients with lymphoma [28, 35, 36], and none was conducted in pediatric patients. Tsuji et al reported that the absence of restricted diffusion predicted recurrence-free survival in adults [36]. In our cohort of pediatric NHL patients, interim WB-DWI provided a superior agreement with end-of-therapy outcomes compared with interim [18F]FDG PET (88% vs. 80%). De Paepe et al reported 86.7% agreement between interim DWI and clinical outcome and 71.4% agreement between interim [18F]FDG PET and clinical outcome in adult patients with NHL. Therefore, they suggested using an increase in ADC ratio greater than − 0.23% for nodal lesions, less than 67.8% for bone lesions, and greater than 36.1% for extranodal lesions to predict a longer progression-free survival [28]. We found a significantly longer progression-free survival in patients whose tumors demonstrated increased ADC values compared to those with stable or decreased ADC values on interim WB-DWI. Thus, the ADC semiquantitative analysis could be considered an independent criterion for the prediction of progression-free survival.
Our study has several limitations. Since we investigated data from a single center, our study population was limited. However, this approach ensured consistent high-quality MRI and PET data and minimized the risk that the observed differences could be due to technical reasons. Further studies with larger prospective cohorts are required to validate our results. According to the power analysis in our study, we were able to compare the results for patients with HL and NHL. However, we did not have a sufficiently large number of patients to further evaluate NHL subtypes. DWI has been proven to be comparable to [18F]FDG PET in adult DLBCL [17, 26]. Further studies are required to investigate the role of DWI in NHL subtypes. However, from a practical point of view, our data provide initial evidence to suggest an abbreviated [18F]FDG PET/MR protocol for patients with HL, and a more comprehensive [18F]FDG PET/MR protocol for patients with NHL, including DWI. In addition, a complete protocol of WB MRI including DWI might be considered an alternative for patients with HL in hospitals where [18F]FDG PET/CT or PET/MRI is not available.
In conclusion, WB-DWI is comparable to [18F]FDG PET/MRI for response assessment in patients with Hodgkin lymphoma, whereas patients with non-Hodgkin lymphoma will benefit from an integrated imaging approach.
Abbreviations
- AC:
-
Agreement coefficient
- ADC:
-
Apparent diffusion coefficient
- CR:
-
Complete response
- [18F]FDG:
-
Fluorine-18 fluorodeoxyglucose
- HL:
-
Hodgkin lymphoma
- NHL:
-
Non-Hodgkin lymphoma
- PD:
-
Progressive disease
- PR:
-
Partial response
- SD:
-
Stable disease
- SUV:
-
Standardized uptake value
- WB-DWI:
-
Whole-body diffusion-weighted imaging
References
Sandlund JT, Guillerman RP, Perkins SL et al (2015) International pediatric non-Hodgkin lymphoma response criteria. J Clin Oncol 33:2106–2111
Riad R, Omar W, Kotb M et al (2010) Role of PET/CT in malignant pediatric lymphoma. Eur J Nucl Med Mol Imaging 37:319–329
Adams MC, Turkington TG, Wilson JM, Wong TZ (2010) A systematic review of the factors affecting accuracy of SUV measurements. AJR Am J Roentgenol 195:310–320
Römer W, Hanauske AR, Ziegler S et al (1998) Positron emission tomography in non-Hodgkin’s lymphoma: assessment of chemotherapy with fluorodeoxyglucose. Blood 91:4464–4471
Sher AC, Seghers V, Paldino MJ et al (2016) Assessment of sequential PET/MRI in comparison with PET/CT of pediatric lymphoma: a prospective study. AJR Am J Roentgenol 206:623–631
Muehe AM, Theruvath AJ, Lai L et al (2018) How to provide gadolinium-free PET/MR cancer staging of children and young adults in less than 1 h: the Stanford approach. Mol Imaging Biol 20:324–335
Schäfer JF, Gatidis S, Schmidt H et al (2014) Simultaneous whole-body PET/MR imaging in comparison to PET/CT in pediatric oncology: initial results. Radiology 273:220–231
Hirsch FW, Sattler B, Sorge I et al (2013) PET/MR in children. Initial clinical experience in paediatric oncology using an integrated PET/MR scanner. Pediatr Radiol 43:860–875
Abdulqadhr G, Molin D, Aström G et al (2011) Whole-body diffusion-weighted imaging compared with FDG-PET/CT in staging of lymphoma patients. Acta Radiol 52:173–180
Padhani AR, Liu G, Koh DM et al (2009) Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11:102–125
Sumi M, Ichikawa Y, Nakamura T (2007) Diagnostic ability of apparent diffusion coefficients for lymphomas and carcinomas in the pharynx. Eur Radiol 17:2631–2637
Herneth AM, Guccione S, Bednarski M (2003) Apparent diffusion coefficient: a quantitative parameter for in vivo tumor characterization. Eur J Radiol 45:208–213
Afaq A, Fraioli F, Sidhu H et al (2017) Comparison of PET/MRI with PET/CT in the evaluation of disease status in lymphoma. Clin Nucl Med 42:e1–e7
Giraudo C, Karanikas G, Weber M et al (2018) Correlation between glycolytic activity on [18F]-FDG-PET and cell density on diffusion-weighted MRI in lymphoma at staging. J Magn Reson Imaging 47:1217–1226
Atkinson W, Catana C, Abramson JS et al (2016) Hybrid FDG-PET/MR compared to FDG-PET/CT in adult lymphoma patients. Abdom Radiol (NY) 41:1338–1348
Bernstine H, Domachevsky L, Nidam M et al (2018) 18F-FDG PET/MR imaging of lymphoma nodal target lesions: comparison of PET standardized uptake value (SUV) with MR apparent diffusion coefficient (ADC). Medicine (Baltimore) 97:e0490
Siegel MJ, Jokerst CE, Rajderkar D et al (2014) Diffusion-weighted MRI for staging and evaluation of response in diffuse large B-cell lymphoma: a pilot study. NMR Biomed 27:681–691
Mayerhoefer ME, Karanikas G, Kletter K et al (2015) Evaluation of diffusion-weighted magnetic resonance imaging for follow-up and treatment response assessment of lymphoma: results of an 18F-FDG-PET/CT-controlled prospective study in 64 patients. Clin Cancer Res 21:2506–2513
Theruvath AJ, Siedek F, Muehe AM et al (2020) Therapy response assessment of pediatric tumors with whole-body diffusion-weighted MRI and FDG PET/MRI. Radiology 296:143–151
Hagtvedt T, Seierstad T, Lund KV et al (2015) Diffusion-weighted MRI compared to FDG PET/CT for assessment of early treatment response in lymphoma. Acta Radiol 56:152–158
Latifoltojar A, Punwani S, Lopes A et al (2019) Whole-body MRI for staging and interim response monitoring in paediatric and adolescent Hodgkin’s lymphoma: a comparison with multi-modality reference standard including (18)F-FDG-PET-CT. Eur Radiol 29:202–212
Shapira-Zaltsberg G, Wilson N, Trejo Perez E et al (2020) Whole-body diffusion-weighted MRI compared to (18 F)FDG PET/CT in initial staging and therapy response assessment of Hodgkin lymphoma in pediatric patients. Can Assoc Radiol J 71:217–225
Mayerhoefer ME, Raderer M, Jaeger U et al (2018) Ultra-early response assessment in lymphoma treatment: [(18)F]FDG PET/MR captures changes in glucose metabolism and cell density within the first 72 hours of treatment. Eur J Nucl Med Mol Imaging 45:931–940
Lymphoma: regional lymph nodes, National Cancer Institute, SEER training module. Available via https://training.seer.cancer.gov/lymphoma/anatomy/lymph-nodes.html. Accessed 8 Jun 2023
Cheson BD, Fisher RI, Barrington SF et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32:3059–3068
Lin C, Itti E, Luciani A et al (2011) Whole-body diffusion-weighted imaging with apparent diffusion coefficient mapping for treatment response assessment in patients with diffuse large B-cell lymphoma: pilot study. Invest Radiol 46:341–349
Klenk C, Gawande R, Uslu L et al (2014) Ionising radiation-free whole-body MRI versus (18)F-fluorodeoxyglucose PET/CT scans for children and young adults with cancer: a prospective, non-randomised, single-centre study. Lancet Oncol 15:275–285
De Paepe KN, Van Keerberghen CA, Agazzi GM et al (2021) Quantitative whole-body diffusion-weighted MRI after one treatment cycle for aggressive non-Hodgkin lymphoma is an independent prognostic factor of outcome. Radiol Imaging Cancer 3:e200061
Maggialetti N, Ferrari C, Minoia C et al (2016) Role of WB-MR/DWIBS compared to 18F-FDG PET/CT in the therapy response assessment of lymphoma. Radiol Med (Torino) 121:132–143
Spijkers S, Littooij AS, Kwee TC et al (2021) Whole-body MRI versus an [(18)F]FDG-PET/CT-based reference standard for early response assessment and restaging of paediatric Hodgkin’s lymphoma: a prospective multicentre study. Eur Radiol 31:8925–8936
Xue HD, Li S, Sun HY, Jin ZY, Sun F (2008) Experimental study of inflammatory and metastatic lymph nodes with diffusion weighted imaging on animal model: comparison with conventional methods. Chin Med Sci J 23:166–171
Riad R, Omar W, Sidhom I et al (2010) False-positive F-18 FDG uptake in PET/CT studies in pediatric patients with abdominal Burkitt’s lymphoma. Nucl Med Commun 31:232–238
Moskowitz CH, Schöder H, Teruya-Feldstein J et al (2010) Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in advanced-stage diffuse large B-cell lymphoma. J Clin Oncol 28:1896–1903
Bailly C, Eugène T, Couec ML et al (2014) Prognostic value and clinical impact of (18)FDG-PET in the management of children with Burkitt lymphoma after induction chemotherapy. Front Med (Lausanne) 1:54
De Paepe K, Bevernage C, De Keyzer F et al (2013) Whole-body diffusion-weighted magnetic resonance imaging at 3 Tesla for early assessment of treatment response in non-Hodgkin lymphoma: a pilot study. Cancer Imaging 13:53–62
Tsuji K, Kishi S, Tsuchida T et al (2015) Evaluation of staging and early response to chemotherapy with whole-body diffusion-weighted MRI in malignant lymphoma patients: a comparison with FDG-PET/CT. J Magn Reson Imaging 41:1601–1607
Acknowledgements
We acknowledge stipend support of the first author by the Faculty of Medicine, Chiang Mai University, Thailand.
Funding
This study has been supported by a grant from the National Cancer Institute, grant number R01CA269231.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Heike E. Daldrup-Link, MD, PhD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
All patients or their legally authorized representative signed a written informed consent, and all children between 7 and 18 years of age signed an assent form.
Ethical approval
Institutional Review Board approval was obtained (IRB-44706).
Study subjects or cohorts overlap
There is no overlap with previously published work.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morakote, W., Baratto, L., Ramasamy, S.K. et al. Comparison of diffusion-weighted MRI and [18F]FDG PET/MRI for treatment monitoring in pediatric Hodgkin and non-Hodgkin lymphoma. Eur Radiol 34, 643–653 (2024). https://doi.org/10.1007/s00330-023-10015-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10015-5