Abstract
Objectives
To evaluate the safety and efficacy of catheter-directed hemorrhoidal embolization (CDHE) by microcoil embolization for rectal bleeding due to hemorrhoids classified as Goligher grade I–III.
Methods
Eighty patients (62.5% males) with a mean age of 48 ± 9 years were recruited prospectively. All patients had symptomatic bleeding hemorrhoids. All patients were classified according to Goligher classification: grade I (13.7%), grade II (71.1%), grade III (15%), and no grade IV were recruited in this study. In all cases, microcoils were used to embolize the superior rectal artery(SRA), and microspheres if recurrence of bleeding occurred. Follow-up evaluation (1, 3, 6, and 12 months) included clinical examination and anoscopy. A questionnaire was conducted to determine improvement regarding bleeding, quality of life before, and the degree of patient satisfaction of each participant.
Results
Technical success was achieved in 100% of the cases. Fifty-five (68.7%) participants had the absence of rectal bleeding after 12 months of embolization. VAS and QL improved 4 points and 1.5 respectively after embolization. A total of 25/80 (31.3%) had a recurrence in rectal bleeding. Seventeen (21.3%) patients underwent a second embolization, and four patients (5%) were treated with open hemorrhoidectomy. No major complications were observed. Sixteen participants had minor complications. Subjective post-treatment symptom and QL surveys showed significant differences from the baseline survey. Likewise, the degree of satisfaction in the telephone survey at 12 months revealed a high degree of patient satisfaction (8.3±1.1).
Conclusions
The present study demonstrates that CDHE is a feasible, well-tolerated, ambulatory, anal sphincter-sparing procedure for the treatment of internal hemorrhoids.
Clinical relevance statement
CDHE is a simple procedure, well tolerated and accepted by patients, that preserves the anal sphincter and presents few complications when metal devices or microspheres are used as embolic agents.
Key Points
• The technical success rate of CDHE, defined as the closure of all the SRA in their distal segment, was achieved 100% of all patients. However, a second embolization treatment was required since 21.25% of the patients experienced rectal bleeding.
• Overall, CDHE’s safety profile is acceptable. After the procedure and 1 year of follow-up, no significant complications were observed.
• Encouraging clinical outcomes have demonstrated CDHE in individuals with hemorrhoids and mild prolapse Goligher grades I–III with persistent rectal bleeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemorrhoidal diseases cause substantial negative impacts on the quality of life in patients that have these diseases. The worldwide prevalence of hemorrhoidal disease ranges from 4 to 35% [1]. Lohsiriwat [2] defined hemorrhoidal disease as symptomatic enlargement and/or distal displacement of the anal cushions, which are prominences of anal mucosa formed by loose connective tissue, smooth muscle, arterial and venous vessels.
The vascular anatomy and pathophysiology of hemorrhoids, first described by Thomson in 1975 [3], consists of terminal branches of the superior rectal artery (SRA) that contribute to the vascularization of the corpus cavernosum recti (CCR). Additionally, both submucosal and transmural branches play an essential role in the blood supply [4]. According to Goligher et al [5], there are four grades of hemorrhoids. Grade I hemorrhoids are those that appear near-normal and no prolapse is present. Grades II, III, and IV are those with bleeding and prolapse with varying levels of reducibility: grade II, spontaneously reducible; digital reduction, grade III; not reducible, grade IV.
Bleeding of varying degrees is the main sign of hemorrhoidal diseases. Other common signs and symptoms include burning, pain, pruritus, discomfort, feeling anal pressure, and the development of skin tags [6]. Treatment options vary depending on the stage of hemorrhoidal disease. Conservative procedures are usually performed for lower grade hemorrhoids (I–II), while higher grades (III–IV) require surgery [7]. The Milligan Morgan hemorrhoidectomy is considered the standard treatment for hemorrhoids [8]. At present, other surgical treatments have emerged. These include Doppler-guided hemorrhoidal ligation (DgHAL), a nonexcisional surgical procedure for the treatment of hemorrhoidal disease consisting of the ligation of the distal branches of the superior rectal artery using Doppler guidance [9], or also called transanal hemorrhoidal dearterialization (THD) Doppler technique which consists of the same procedure and can be added mucopexy (anolift) in advanced hemorrhoidal disease [10].
One hypothesis that arises from the prior success rates in ligation procedures is if embolization techniques could be used for hemorrhoid treatment. In 2014, Vidal et al [11] described a technique to embolize the hemorrhoidal arterial blood flow called the emborrhoid technique, which led to favorable results in three patients that had bleeding hemorrhoids. A prior evaluation by Galkin [12] reported on the use of embolization in 34 patients; after treatment, no patients had recurrences. To date, studies have only evaluated smaller groups of patients. To expand on the clinical understanding of embolization treatment in participants with a hemorrhoidal disease, we sought to evaluate this procedure in a non-randomized prospective, multicenter study.
Material and methods
Participant inclusion
This prospective, multicenter study (three third-level university centers) was approved by the Ethics Committee from each center. All participants that met the inclusion criteria provided informed signed consent.
The following inclusion criteria were used: (a) participants with grade I, II, and III hemorrhoidal diseases on the Goligher scale [5], (b) older than 18 years with a score of greater than 4 on the French Bleeding Score (FBS) scale [13], and (c) greater than 2 on the scale of discomfort proposed by Tradi et al and Farfallah et al [14, 15]. The exclusion criteria were failure to sign the informed consent, participants diagnosed with other causes of rectal bleeding (cancer, fissures, others), severe renal insufficiency, non-correctable coagulation abnormalities, and adverse reaction to contrast medium not correctable with medication.
All hemorrhoids from included participants were classified according to Goligher’s classification into four grades [5]: grade I, bleeding without prolapse; grade II, prolapse after straining but spontaneous reduction; grade III, prolapse on straining and need manual reduction; and grade IV, irreducible prolapse. From January 2016 to December 2020, a total of 80 participants (Table 1) were included.
The severity of the bleeding was measured with the FBS [14,15,16]. The FBS is scaled from 0 to 9, based on three separate variables: bleeding frequency (0–4), type of bleeding (0–3), and the presence of anemia (0–2) (Table 2). The included participants had been evaluated and referred by a coloproctologist. However, all participants were also evaluated at an Interventional Radiology outpatient consult [17].
Outcome measures and definitions
The primary objective of this study was to evaluate the safety and feasibility for the treatment of hemorrhoids grade I, II, and III by embolization of the SRA in a prospective manner in a large cohort. Technical success was defined as the closure of all the SRA in their distal segment. To define clinical success, three different evaluations were performed in the follow-up after the first month: rectal bleeding measured with the FBS, pain measured with the Visual Analogic score scale (VAS), and quality of life (QOL) by the scale proposed by Tradi et al and Farfallah et al [14,15,16].
For clinical outcome, five procedure outcomes were defined: 1, complete recovery (FBS = 0, VAS = 0, QOL = 0); 2, improvement (FBS 1–3, V < 3, D < 2); 3, aggravated (FBS > 3, VAS > 3, QOL > 3) and 4, no change (similar values at the beginning); and 5, bleeding recurrence. Bleeding recurrence was classified into three grades: mild, bleeding in frequency regarding FBS: 1–2; moderate bleeding frequency FBS: 3–4, and no changes compared to before treatment. Complications were reported according to the Society of Interventional Radiology classification in minor (A–B) and major (C–F) [18]. Possible complications in vascular access (hematoma and ischemia of the hand) were assessed according to the common terminological criteria for adverse events, version 5.0 [19].
Embolization technique
All procedures were performed on an outpatient basis with a stay in the preparation room for 2–4 h. Antibiotic or analgesic premedication was not routinely used.
Vascular access was primarily performed through the left radial artery (78.7%; 63 of 80). However, when it was not possible to access the left radial artery, the left brachial artery (11.2%; 9 of 80), right femoral artery (6.2%; 5 of 80), or right radial artery (3.7%; 3 of 80) were used as alternatives. With the initial intention of the left radial route, the Barbeau test was performed [20]. The radial approach was not used in cases when the wave was abnormal after 2 min of radial compression (Barbeau C or D). The radial approach was not used in participants that had a radial artery diameter of less than 1.8 mm, and the participant was taller than 185 cm.
To access the radial artery, 9 mL of lidocaine 1% with 100 μg of nitroglycerine was administered in the subcutaneous tissue near the artery. Micropuncture access set a 21 G × 7 cm echogenic needle, and a 0.018” × 40 cm guidewire (Cook Medical). A 5 F and 10 cm radial introducer (Radiofocus -Terumo Europe). To prevent brachial or radial arterial spasm or thrombosis, a total of 2.5 mg of verapamil, 200 μg of nitroglycerin, and 2000–4000 IU of unfractionated heparin mixed with 20 mL hemodilution technique.
Inferior mesenteric artery and the SRA angiography was performed. A 2.4 or 2.7Fr - 150 cm microcatheter (Progreat - Radiofocus, Terumo Europe) was advanced as far as possible, close to the CCR (Fig. 1).
a Angiogram of the inferior mesenteric artery and its branches. b Selective superior rectal artery angiogram with two right and left two main branches. c Selective right branch angiogram of the SRA and CCR. d Selective left branch of SRA. IMA, inferior mesenteric artery; rCA, right colic artery; SA, sigmoid artery; SRA, superior rectal artery; SRAr, superior rectal artery right anterior main branch; SRAl, superior rectal artery left anterior main branch; CCR, corpus cavernosum recti
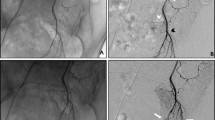
Three different types of microcoils were used according to hospital and availability: Concerto detachable (Medtronic), Ruby coil (Penumbra Inc), and Optima or Prestige (Balt) with a diameter of 2–5 mm and various lengths (10–40 cm). Three types of microcoils were also used for re-embolization procedures in participants with rebleeding, and if it was necessary, Tris-acryl Gelatin microspheres (TAGM) of 500–700 μm (Merit Medical) were used (Fig. 2). The total procedure, fluoroscopy time, air Kerma, and product area dose were also collected.
a Angiogram performed by a 5Fr MPA catheter in the SRA administering 12 mL at 4 mL/s of iodine contrast (Ioversol 320 mg/mL) to determine anatomy and possible shunting with the MRA or IRA. b Final result after microcoil embolization (black arrows) no residual flow to CCR. c Patient GIII with rebleeding previously embolized with microcoils. Distal embolization with microcatheter(white arrow) and microspheres 500–700 μm. d Final result angiogram after microspheres and microcoils (black arrows) embolization without residual flow to CCR
During and after the procedure
During the procedure, when the patient experienced anal discomfort during the embolization, 20 mg of scopolamine butylbromide was slowly administered intravenously mixed with 100 mL of saline.
After the procedure, participants remained under monitoring for at least 2 h. Participants were discharged after normal vital signs, micturition, walking, and in the absence of substantial pain. Non-steroidal anti-inflammatory drugs (NSAIDs) were prescribed for 2 to 3 days. Diet (fiber, vegetables, and plenty of fluids) was advised for a few days to avoid constipation. If a femoral approach was considered, the patients remained hospitalized and discharged after 24 h.
Participants were followed up for 1, 3, 6, and 12 months with anoscopy and clinical findings at the outpatient clinic of Interventional Radiology and Coloproctology.
Before and after embolization (between 3 and 6 months), participants voluntarily answered a questionnaire regarding symptoms and quality of life—discomfort (ANNEX I and II). The questionnaire was a modified and translated version of HD Rørvik et al. [21] to evaluate symptoms in Spanish-speaking participants regarding health-related quality of life related to hemorrhoidal disease. At the end of the study, a telephone satisfaction survey was carried out, scored from 0 to 10. Scores from 0 to 3 represented that the participant was not at all satisfied, 4–6 not very satisfied, 6–8 quite satisfied, and 8–10 very satisfied.
Statistical analysis
Qualitative variables are expressed as frequencies and percentages. Comparisons between groups were made using contingency tables with Pearson’s χ2 or Fisher’s test, depending on the magnitude of the expected frequencies. Quantitative variables are expressed as mean ± standard deviation. The relationship between the variables under study was analyzed according to their nature: quantitative variables were compared between groups using Student’s t-tests or Wilcoxon test, and categorical variables using contingency tables (Pearson’s χ2 or Fisher’s exact test, depending on the magnitude of the expected frequencies). Recurrence events were considered after a participant referred to the appearance of rectal bleeding. Statistical analyses were performed with the IBM SPSS version 21 program (SPSS Inc) and R version 3.6.3 (“The Foundation for Statistical Computing Packages”). In all cases, a p < .05 indicated statistical significance.
Results
Participant characteristics
The most prevalent clinical symptoms observed before the procedure were as follows: bleeding of varying magnitudes and the evolution ranging from months to years in 98.7% of the participants. Eight (10%) participants were diagnosed with anemia. A total of 21 participants reported tenesmus (26%), perianal pain in 15 participants (19%), and rectal pruritus in 12 participants (15%).
The mean pre-embolization hemoglobin was 12.0 ± 1.6 g/dL range (14.2–76 g/dL). A total of 42/80 (53%) of the participants had functional constipation. Eight participants (10%) had a surgical history for anal fissure, and 35 participants reported comorbidities or associated diseases: diabetes (n = 8 [10%]), fibromyalgia (n = 4 [5%]), pelvic congestion syndrome (n = 6 [7.5%]), and 17 others. Seven participants (8.7%) were being treated with oral anticoagulants.
All participants underwent a diagnostic anoscopy that demonstrated dilated and congestive internal hemorrhoids in all cases (Fig. 3). All hemorrhoids were classified using the Goligher classification: grade I, 21 (26%); grade II, 45 (56%); grade III, 13 (16%); and grade IV, 0 (0%).
a Anuscopy of a patient with GIII thrombosed and bleeding hemorrhoids. b Anuscopy of the same patient (a) embolized after 20 days without rectal bleeding. c Anuscopy showing congestive hemorrhoid in a patient with GII and rectal bleeding. d Anuscopy of the same patient (c) 15 days after SRA embolization. e Anuscopy of a female patient with rectal bleeding and GIII. f Anuscopy of the same patient (e) shows less edema and without bleeding 10 days after SRA embolization. g Anuscopy of a male patient with persistent anemia with GII bleeding hemorrhoids. h Anuscopy of the same patient 10 days after SRA embolization. GIII, Goligher - III; GII, Goligher - II; SRA, superior rectal artery
SRA embolization
All 80 participants underwent SRA embolization. A total of 17 of the 80 underwent a second embolization procedure due to recurrence of bleeding. Of these 17, 11 (64%) underwent embolization with only microcoils, four underwent embolization with coils and tris-acryl gelatin microspheres (TAGM) of 500–700 μm, and two underwent embolization procedures that targeted the inferior rectal artery with coils and TAGM of 500–700 μm. In all 17 participants, rectal bleeding either decreased or stopped after the procedures (Fig. 3).
The mean procedure time was 42.8 ± 14 min, and the mean fluoroscopy time mean was 34.2± 13.7 min. The average product radiation dose was 271,172.3±150,869 mG cm2 and the average air Kerma was 225.2± 212 mGycm2 (range, 2075–2376 mG cm2).
In all participants (80/80; 100%) the SRA was embolized. In nine participants (12%) the MRA was also embolized, and in 2 (2.5%) the IRA was embolized. An average of 8.9 ± 1.5 coils were used (range 6–12 coils per participant from three different commercial sources).
Follow-up
One participant (1 of 80; 1.3%) was lost to follow-up. When a participant did not attend the monthly outpatient consult, they were called by phone for evaluation of clinical status and reporting of any signs or symptoms. Based on the subjective assessments, at 12 months the clinical outcomes were as follows: 69% (55 of 80) healing, 21% (17 of 80) rectal bleeding with improvement, 2.5% (2 of 80) rectal bleeding with no improvement, 2.5% (2 of 80) rectal bleeding and aggravated condition, and 5% (4 of 80) reported no change in condition. FBS, VAS, and QL before and after the procedure showed statistical significance (Fig. 4).
In 7.5% (5 of 66) of participants, a radial spasm was observed, which resolved with an intravenous administration of 100 mcg nitroglycerin. One participant presented a small hematoma at the puncture site that did not require additional treatment. Six participants (7.5%) presented with hemorrhoidal rectal pain and tenesmus during embolization that resolved with the administration of NSAIDs.
Recurrence and second embolization
After the procedure, of the 25 (32.2%) who presented rebleeding at 12 months, four (5%) with mild rebleeding and marked improvement compared to baseline did not require any other treatment. Four (5%) opted for surgery, and 17 (21.2%) patients underwent a second embolization (Table 3).
In the second embolization in the 17 participants, the SRA was catheterized and if recanalization was observed in the CCR, it was embolized again with microcoils or and TAGM of 500–700 μm. If the SRA showed no vascularization to the CCR, the MRA and IRA were reviewed. A total of four participants (5%) underwent surgery with the Milligan and Morgan hemorrhoidectomy technique.
The voluntary satisfaction survey carried out before and after embolization regarding symptoms showed significant differences in the four questions asked. Four participants (5%) opted not to take the survey. The survey regarding the quality of life, also voluntary, was answered by 78 of 80 participants and showed significant differences between the responses before and after embolization. In both the satisfaction and quality of life survey, participant answers were correlated with the clinical outcomes from embolization (Fig. 5).
Discussion
The aim of this study was to evaluate the clinical outcome of SRA embolization in participants with hemorrhoidal disease. Since the first publication by Vidal et al [11] of the emborrhoid technique, additional studies have been published evaluating this procedure in patients with hemorrhoidal diseases. Although prior series have reported less than 50 patients and with a follow-up of 12 months or less, technical success has been reported between 90 and 100% and clinical success between 63 and 97% [13,14,15,16, 22,23,24,25,26,27,28,29,30,31,32,33,34]. However, it is difficult to compare the results between series since there is great variability in criteria and standards of clinical success [7]. In this cohort of 80 treated participants, the recovery and improvement at 1 year were 69% and 21%, respectively. A total of 2.5% (2 of 80) of participants reported no change in symptoms, and 2.5% of participants reported symptoms worsening after the procedure. Taken together, the embolization technique performed in this study led to hemorrhoid bleeding improvement in a majority of participants that underwent the procedure.
Milligan–Morgan and Ferguson hemorrhoidectomy is the reference standard treatment with higher clinical success rates than embolization, but it is not free of complications and requires hospitalization [35]. Other non-surgical techniques such as rubber band ligation achieve 50–70% of clinical successes in 1 year [36, 37]. Ratto et al [10] presented a single-center study with 1000 consecutive patients treated for hemorrhoidal disease with the transanal technique THD or DGHAL. This study had a follow-up period of 44 ± 29 months, and improvement of hemorrhoids was reported in 95.7% of the patients at the end of the study. In 6.8% (68 of 1000) of patients in this study, there were complications reported, of which 14 (1.4%) patients required reoperation for acute bleeding. Other complications were anal pain in 31 (3.1%) and urinary retention in 23 patients (2.3%). The mean time of hospitalization was 1 ± 0.2 days. Recurrence was 9.5% of which 70 (7.0%) required surgery.
This study shows promising results compared to DGHAL studies [36, 38] in which they show recurrence up to 30% at 1 year compared to 9.5% in the study by Ratto et al [10]. There are not very important differences when DGHAL or THD and hemorrhoidal embolization or catheter-directed hemorrhoidal embolization (CDHE) are compared [7] in relation to clinical success and recurrences. In DGHAL or THD, clinical success was 82.3–95.7% and recurrence was 9.5–30%, while in CDHE clinical success of 78.9% (66–96%) and disease recurrence of 22% (5–43%) were achieved, based mainly on rebleeding.
In general, DGHAL and CDHE did not modify or improve external hemorrhoids. In the case of DGHAL, after transanal dearterialization treatment, a mucopexy can be performed on external hemorrhoids. Embolization does not improve prolapse, but some authors [14, 16] believe that prolapse may improve over time due to decongestion by decreasing arterial flow after embolization. This phenomenon has also been observed in DGHAL without the performance of mucopexy.
CDHE has the benefit that can be performed as an outpatient procedure, the majority of patients recall it to be a not painful procedure and also has fewer complications compared with other procedures.
Radial access is one of the factors that allow outpatient treatment in addition to implying less aggression for the patient with fewer complications [39]. In our series, technical success was achieved in 100% of patients with a minor complication rate (15%) according to the Society of Interventional Radiology classification [18]. There were no major complications, which relates to the recent publication in the medical literature [11, 14, 16, 22, 40,41,42]. However, recently, Eberspacher et al [42] reported a case study on rectal sigmoid ischemia secondary to hemorrhoidal microparticle embolization in a 58-year-old patient.
Recently, Tradi et al [43] encourages us to maintain an expectant attitude regarding embolic liquids, especially since the microcoils and microspheres are so effective and safer. Additionally, this report indicated that ethylene-vinyl alcohol copolymer may be an unsafe agent for hemorrhoid embolization since in their experimental study in pigs they observed necrosis of the distal area of the rectum with mural infarction in animals that underwent embolization with ethylene vinyl alcohol copolymer.
Other authors reported pain and tenesmus between 4.6–15% and 54% with the use of polyvinyl alcohol (PVA) and TAGM as embolizing agents [15, 27,28,29]. Regarding complications in radial access (9.0 %), five spasms and one hematoma were all resolved with conservative treatment (intra-arterial spasmolytics and rest). In the pilot study of hemorrhoidal embolization with radial access by Lezzi et al [39], no major complications were recorded, only two minor complications (16%) that resolved with conservative treatment. Regarding microsphere or microparticle size embolization 500–700 μm showed significant bleeding control, but a lower complication rate was achieved with better results regarding bleeding 900–1200 μm at 12 months in a recent randomized trial comparing particle size embolization with tris-acryl gelatin microspheres [29].
This study has some limitations: it is a prospective, non-randomized study, with a low number of participants, and follow-up of only 1 year.
In conclusion, SRA embolization is feasible and a relatively simple, safe, painless procedure that does not require hospitalization. A significant percentage of participants had hemorrhoid resolution after the embolization procedures. Future evaluations comparing embolization techniques to other hemorrhoid procedures are warranted [44, 45].
Abbreviations
- CCR:
-
Corpus cavernosum recti
- CDHE:
-
Catheter-directed hemorrhoidal embolization
- DgHAL:
-
Doppler-guided hemorrhoidal ligation
- FBS:
-
French Bleeding Score
- IRA:
-
Inferior rectal artery
- MRA:
-
Middle rectal artery
- QOL :
-
Quality of life
- SRA:
-
Superior rectal artery
- TAGM:
-
Tris-acryl gelatin microspheres
- THD :
-
Transanal hemorrhoidal dearterialization Doppler
- VAS :
-
Visual Analogic score
References
De Nardi P, Maggi G (2021) Embolization of the superior rectal artery: another management option for hemorrhoids. Tech Coloproctol 25:1–2
Lohsiriwat V (2015) Treatment of hemorrhoids: a coloproctologist’s view. World J Gastroenterol 21(31):9245–9252
Thomson WHF (1975) The nature of haemorrhoids. Br J Surg 62:542–552
Aigner F, Bodner G, Gruber H et al (2006) The vascular nature of hemorrhoids. J Gastrointest Surg 10:1044–1050
Goligher JC, Leacock AG, Brossy JJ (1955) The surgical anatomy of the anal canal. Br J Surg 43:51–61
Romano FM, Sciaudone G, Canonico S, Selvaggi F, Pellino G (2021) Scoring system for haemorrhoidal disease. Rev Recent Clin Trials 16:96–100
Makris GC, Thulasidasan N, Malietzis G et al (2021) Catheter-directed hemorrhoidal dearterialization technique for the management of hemorrhoids: a meta-analysis of the clinical evidence. J Vasc Interv Radiol 32:1119–1127
Milligan ETC, Morgan CN, Jones LE, Officer R (1937) Surgical anatomy of the anal canal, and the operative treatment of hemorrhoids. Lancet 2:1119–1124
Avital S, Inbar R, Karin E, Greenberg R (2012) Five-year follow-up of Doppler-guided hemorrhoidal artery ligation. Tech Coloproctol 16(1):61–65
Ratto C, Campenni P, Papeo F, Donisi L, Litta F, Parello A (2017) Transanal hemorrhoidal dearterialization (THD) for hemorrhoidal disease: a single-center study on 1000 consecutive cases and a review of the literature. Tech Coloproctology 21:953–962
Vidal V, Louis G, Bartoli J, Sielezneff I (2014) Embolization of the hemorrhoidal arteries (the emborrhoid technique): a new concept and challenge for interventional radiology. Diagn Interv Imaging 95:307–315
Galkin EV (1994) Interventional radiology of chronic hemorrhoids. Vestnik Rentgenologii (Mosk) 4:52–56 (in Russian with English abstract)
Mousa N, Sieleznee I, Sapoval M et al (2017) Embolization of the superior rectal arteries for chronic bleeding due to hemorrhoidal disease. Color Dis 19:194–199
Tradi F, Louis G, Giorgi R et al (2018) Embolization of the superior rectal arteries for hemorrhoidal disease: prospective results in 25 patients. J Vasc Interv Radiol 29(6):884–892
Fathallah N, Beaussier H, Chatellier G et al (2020) Proposal for a new score:hemorrhoidal bleeding score (HBS). Ann Coloproctol 32:819–825
Moussa N, Bonnet B, Pereira H et al (2020) Mid-term results of superior rectal artery and coils for hemorrhoidal embolization with particles bleeding. Cardiovasc Intervent Radiol 43:1062–1069
Lee MJ, Fanelli F, Haage P, Hausegger K, Van Lienden KP (2012) Patient safety in interventional radiology: a CIRSE IR checklist. Cardiovasc Intervent Radiol 35(2):244–246
Khalilzadeh O, Baerlocher MO, Shyn PB et al (2017) Proposal of a new adverse event classification by the Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol 28:1432–1437
US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5. In: Common Terminology Criteria for Adverse Events (CTCAE) Version 5. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed 27 November 2021
Barbeau GR, Arsenault F, Dugas L, Simard S, Larivière MM (2004) Evaluation of the ulnopalmar arterial arches with pulse oximetry and plethysmography: comparison with the Allen's test in 1010 patients. Am Heart J 147(3):489–493
Rørvik HD, Styr K, Ilum L et al (2019) Hemorrhoidal Disease Symptom Score and Short Health ScaleHD: new tools to evaluate symptoms and health-related quality of life in hemorrhoidal disease. Dis Colon Rectum 62:333–342
Zakharchenko A, Kaitoukov Y, Vinnik Y et al (2016) Safety and efficacy of superior rectal artery embolization with particles and metallic coils for the treatment of hemorrhoids (emborrhoid technique). Diagn Interv Imaging 97:1079–1084
Sun X, Xu J, Zhang J, Jin Y, Chen Q (2018) Management of rectal bleeding due to internal haemorrhoids with arterial embolisation: a single-centre experience and protocol. Clin Radiol 73(11):985.e1–985.e6
Venturini M, De Nardi P, Marra P et al (2018) Embolization of superior rectal arteries for transfusion dependent haemorrhoidal bleeding in severely cardiopathic patients: a new field of application of the “emborrhoid” technique. Tech Coloproctol 22(6):453–455
Park S, Kim Y, Shin JH et al (2020) Outcome of rectal arterial embolization for rectal bleeding in 34 patients: a single-center retrospective study over 20 years. J Vasc Interv Radiol 31(4):576–583
Giurazza F, Corvino F, Cavaglià E et al (2020) Emborrhoid in patients with portal hypertension and chronic hemorrhoidal bleeding: preliminary results in five cases with a new coiling release fashion “Spaghetti technique”. Radiol Med 125(10):1008–1011
Abb El Tawab K, Salem AA, Khafagy R (2020) New technique of embolization of the hemorrhoidal arteries using embolization particles alone: retrospective results in 33 patients. Arab J Interv Radiol 4:27–31
Han X, Xia F, Chen G et al (2021) Superior rectal artery embolization for bleeding internal hemorrhoids. Tech Coloproctol 25(1):75–80
Küçükay MB, Küçükay F (2021) Superior rectal artery embolization with tris-acryl gelatin microspheres: a randomized comparison of particle size. J Vasc Interv Radiol 32(6):819–825
Stecca T, Farneti F, Balestriero G et al (2021) Superior rectal artery embolization for symptomatic grades 2 and 3 hemorrhoidal disease: 6-month follow-up among 43 patients. J Vasc Interv Radiol 32(9):1348–1357
Ferrer Puchol MD, Esteban Hernandez E, Blanco Gonzalez FJ, Ramiro Gandia R, Solaz Solaz J, Pacheco Usmayo A (2020) Selective intra-arterial embolization to treat hemorrhoids. Embolizacion intraarterial selectiva como tratamiento de la patología hemorroidal [Article in English and Spanish]. Radiol 62:313–319
Gu XP, Sun LF (2020) A retrospective study of super-selective superior rectal artery branch embolization (SRAE) and procedure of prolapse and hemorrhoids (PPH). Fudan Univ J Med Sci 47:275–279
Luo CS, Jia YP, Mao AW, Yang W (2017) Preliminary clinical study of the treatment of hemorrhoids by superselective embolization of superior rectal artery [Article in Chinese]. Zhonghua Yi Xue Za Zhi 97:1960–1963
Rebonato A, Maiettini D, Patriti A et al (2021) Hemorrhoids embolization: state of the art and future directions. J Clin Med 10(16):3537
Simillis C, Thoukididou SN, Slesser AA, Rasheed S, Tan E, Tekkis PP (2015) Systematic review and network meta-analysis comparing clinical outcomes and effectiveness of surgical treatments for haemorrhoids. Br J Surg 102:1603–1618
Brown SR, Tiernan JP, Watson AJM et al (2016) Haemorrhoidal artery ligation versus rubber band ligation for the management of symptomatic seconddegree and third-degree haemorrhoids (HubBLe): a multicentre, openlabel, randomised c ontrolled trial. Lancet 388:356–364
Gupta PJ (2003) Infrared coagulation versus rubber band ligation in early stage hemorrhoids. Braz J Med Biol Res 36:1433–1439
Pucher PH, Sodergren MH, Lord AC, Darzi A, Ziprin P (2013) Clinical outcome following Doppler-guided haemorrhoidal artery ligation: a systematic review. Color Dis 15(6):e284–e294
Iezzi R, Campenni P, Posa A et al (2021) Outpatient transradial emborrhoid technique: a pilot study. Cardiovasc Intervent Radiol 44(8):1300–1306
Vidal V, Sapoval M, Pellerin O (2015) Arterial embolization of hemorrhoids: reply. Cardiovasc Intervent Radiol 38(4):1056
Lohssirivat V (2021) Embolization of the superior rectal artery for bleeding haemorrhoids (emborrhoid technique): a summary of early results from the literature and a few cautions for the future of this innovative procedure. Color Dis 23:1584–1585
Eberspache C, Ficuccilli F, Tessieri L et al (2021) Annoyed with haemorrhoids? Risks Emborrhoid Technique Dig Dis Sci 66(11):3725–3729
Tradi F, Panneau J, Brige P et al (2022) Evaluation of multiple embolic agents for embolization of the superior rectal artery in an animal model. Cardiovasc Intervent Radiol 45(4):510–519. https://doi.org/10.1007/s00270-021-03041-7
Talaie R, Torkian P, Moghadam AD et al (2022) Hemorrhoid embolization: A review of current evidences. Diagn Interv Imaging 103(1):3–11
Moggia E, Talamo G, Gallo G et al (2021) Do we have another option to treat bleeding hemorrhoids? The emborrhoid technique: experience in 16 patients. Rev Recent Clin Trials 16(1):81–86
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Miguel A. De Gregorio.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
No study subjects or cohorts have been previously reported.
Methodology
-
prospective
-
cross sectional study /observational
-
multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 144 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
De Gregorio, M.A., Guirola, J.A., Serrano-Casorran, C. et al. Catheter-directed hemorrhoidal embolization for rectal bleeding due to hemorrhoids (Goligher grade I–III): prospective outcomes from a Spanish emborrhoid registry. Eur Radiol 33, 8754–8763 (2023). https://doi.org/10.1007/s00330-023-09923-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09923-3