Abstract
Objectives
To investigate the diagnostic performance and interobserver variability in the determination of arterial invasion in pancreatic ductal adenocarcinoma (PDAC) and determine the best CT imaging criterion.
Methods
We retrospectively evaluated 128 patients with PDAC (73 men and 55 women) who underwent preoperative contrast-enhanced CT. Five board-certified radiologists (expert) and four fellows (non-expert]) independently assessed the arterial invasion (celiac, superior mesenteric, splenic, and common hepatic arteries) using a 6-point score: 1, no tumor contact; 2, hazy attenuation ≤ 180°; 3, hazy attenuation > 180°; 4, solid soft tissue contact ≤ 180°; 5, solid soft tissue contact > 180°; and 6, contour irregularity. ROC analysis was performed to evaluate the diagnostic performance and determine the best diagnostic criterion for arterial invasion, with pathological or surgical findings as references. Interobserver variability was assessed using Fleiss’s ĸ statistics.
Results
Among the 128 patients, 35.2% (n = 45/128) received neoadjuvant treatment (NTx). Solid soft tissue contact ≤ 180° was the best diagnostic criterion for arterial invasion as defined by the Youden Index both in patients who did and did not receive NTx (sensitivity, 100% vs. 100%; specificity, 90% vs. 93%; and AUC, 0.96 vs. 0.98, respectively). Interobserver variability among the non-expert was not inferior to that among the expert (ĸ = 0.61 vs 0.61; p = .39 and ĸ = 0.59 vs 0.51; p < .001 in patients treated with and without NTx, respectively).
Conclusions
Solid soft tissue contact ≤ 180° was the best diagnostic criterion for the determination of arterial invasion in PDAC. Considerable interobserver variability was seen among the radiologists.
Key Points
• Solid soft tissue contact ≤ 180° was the best diagnostic criterion for the determination of arterial invasion in pancreatic ductal adenocarcinoma.
• Interobserver agreement among non-expert radiologists was almost comparable to that among expert radiologists.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related death in both men and women [1]. The 5-year survival rate is only 10% for all stages and is the lowest among all cancers. Complete surgical resection (R0 resection) is the only curative treatment; however, only 15–20% of patients are eligible for curative surgery at the time of diagnosis [2]. Patients who have undergone surgical resection have a relatively high 5-year survival rate of 20%; in patients with R0 resection, the rate is as high as 32% [3].
Surgical resectability is determined on dynamic contrast-enhanced pancreatic protocol computed tomography (CT) images according to the National Comprehensive Cancer Network (NCCN) guidelines (Version 2. 2022). The presence and degree of solid soft tissue contact (≤ 180° or > 180°) between the tumor and vessel circumference determines the resectability status as resectable, borderline resectable (BR), or locally advanced (LA) [4]. Cross-sectional imaging reveals arterial contact with the tumor, which can be seen as increased hazy attenuation, stranding, or solid soft tissue contact. Furthermore, analysis of the vessel caliber or contour irregularity should be noted and documented in sufficient detail in standardized reports [4, 5].
Neoadjuvant treatment (NTx) is being increasingly employed for the management of BR or LA tumors, to downstage them into resectable tumors [6]. In addition, a strategy of NTx followed by surgery is currently recommended for resectable tumors with high-risk features, such as highly elevated carbohydrate antigen (CA) 19 − 9 levels, large primary tumors or regional lymph nodes, excessive weight loss, and extreme pain [4]. Following NTx, the solid soft tissue may be replaced by hazy attenuation or stranding on CT images [4, 5]. To our knowledge, no study has evaluated the relationship between individual CT findings including increased hazy attenuation or stranding, solid soft tissue, contour irregularity, and its degree, and arterial invasion in patients treated with and without NTx. Therefore, the purpose of this study was to investigate the diagnostic performance of preoperative CT imaging findings for diagnosing arterial invasion in patients with PDAC and to determine the best imaging criterion. Additionally, we aimed to evaluate the interobserver variability of the assessment of the CT imaging findings and to determine if a radiologist’s experience level impacts evaluation.
Materials and Methods
Patients
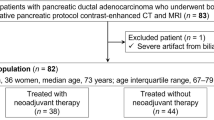
Our institutional review board approved this retrospective study and written informed consent was waived. Data related to the study are available at Gifu University Hospital. Between January 2008 and July 2020, 238 consecutive patients with pathologically proven PDAC who had undergone pancreatic protocol dynamic contrast-enhanced CT, regardless of the resectability status and NTx administration, were identified. Patients who had metastatic disease and/or LA tumors and had not yet undergone surgery were excluded (Fig. 1).
CT Protocols and contrast material injection
We used a 256-, 64-, or 16-detector CT scanner (Revolution CT [n = 35], Discovery CT750 HD [n = 58], or Light Speed Ultra 16 [n = 24] from GE Healthcare or Brilliance CT 64 [n = 11] from Philips Healthcare) with an automatic tube current modulation program. CT imaging parameters are summarized in Table 1.
The contrast material, 350-mg iomeprol per milliliter, was intravenously injected over 30 s. In all patients, 600 mg of iodine per kilogram of body weight was administered. A 15–20 mm-diameter circle was placed on the abdominal aorta at the level of the first lumbar vertebral body, as a region-of-interest. Real-time fluoroscopic monitoring scans (120 kVp, 10 mA) were initiated 10 s after the start of the injection of contrast material. The diagnostic CT scanning was started with an additional delay of 20 and 60 s for the pancreatic and portal venous phases after the abdominal aorta enhancement had reached 100 HU threshold on a bolus-tracking program (Smart-Prep from GE Healthcare or Bolus tracking from Philips Healthcare). The raw CT data at 1.25 mm section thickness with 50% overlap was reconstructed using a filtered back projection or hybrid iterative reconstruction algorithm (adaptive statistical iterative reconstruction [ASiR]-Veo 40%, ASiR 40% from GE Healthcare, or iDose3 from Philips Healthcare). Reconstructed images at 2.5 mm thickness in the coronal plane were also available.
Image analysis
The CT images of each patient were independently analyzed by nine radiologists, of whom five were board-certified radiologists (N.M., Y.N., N.K., T.A., and M.K., with 7–13 years of experience [expert]) and four were fellows (S.N., K.F., F.N., and T.K., 3–5 years of experience [non-expert]). CT images analyzed in this study were those obtained before surgery, even in patients treated with NTx. The radiologists were aware that all patients had PDAC; however, they were blinded to the treatment received and the intraoperative or pathological findings. the preset window setting for the axial and coronal CT images was fixed at 350-HU width and 40-HU level. The radiologists were allowed to adjust the window settings at their own discretion during analysis, which consisted of two separate reading sessions with a two-week interval in between to minimize recall bias.
During the first reading session, all radiologists independently evaluated the presence of arterial invasion using a 2-point scale: 0, absence; and 1, presence. The arterial invasion was assessed for the celiac, superior mesenteric, splenic, and common hepatic arteries, respectively. In the second reading session, all radiologists independently evaluated the CT findings for the aforementioned four arteries using a 6-point scale (Fig. 2): 1, no tumor contact; 2, increased hazy attenuation/stranding ≤ 180°; 3, increased hazy attenuation/stranding > 180°; 4, solid soft tissue contact ≤ 180°; 5, solid soft tissue contact > 180°; and 6, contour irregularity based on the NCCN guidelines [4, 6].
Reference standard
The interval between the preoperative contrast-enhanced CT and pancreatectomy ranged from 1 to 84 days, with a median of 21 days. Surgical resection was performed by an experienced team of pancreaticobiliary surgeons at our institution. Histopathological findings (n = 107) or surgical records (n = 21) were the reference standard for confirmation of arterial invasion. Unresectability (n = 21) was confirmed based on intraoperative findings; R2 resection was performed due to marked infiltration of the PDAC. In these patients, the final diagnoses for the arterial invasion were obtained from their surgical records. In the remaining 107 patients, the arterial invasion was histopathologically evaluated and confirmed using hematoxylin–eosin-stained specimens obtained during resection. Experienced pathologists examined the specimens for arterial invasion, and made a diagnosis based on tumor infiltration into the layers beyond the adventitia.
Statistical analysis
The sample size required for this study was calculated based on a sufficient number of arterial invasion cases required for Clopper-Pearson’s exact lower confidence limit for sensitivity to be > 60%. Thus, assuming a sensitivity of 90%, the number of pathologically proven arterial invasion cases required would be ≥ 12. For the without NTx group, assuming an arterial invasion prevalence of 4–5% and an independent assessment of four arteries from a patient, the sample size was calculated as 75. For the with NTx group, assuming an arterial invasion prevalence of 7–8% and an independent assessment of four arteries from a patient, the sample size was calculated as 43. The actual number of patients collected was increased until 12 arterial invasions were observed in each group.
Patients’ demographics and tumor characteristics were summarized based on NTx status using mean ± standard deviation (SD) for continuous variables and frequencies and percentages for categorical variables. To evaluate the diagnostic performance for arterial invasion of the 6-point scale in the second reading session, receiver operating characteristic (ROC) curves and area under the ROC curves (AUCs) were calculated for patients who did and did not receive NTx, using the assessment data of all four arteries. The 6-point scale for the construction of the ROC curve was based on a consensus by the five board-certified radiologists. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated using the cut-off points, Youden index, and the 6-point scale. The above parameters were also calculated during the first reading session.
The best statistical and clinical value of the 6-point scale was defined as the best cut-off point for predicting arterial invasion for each patient group treated with and without NTx. Sensitivity, specificity, PPV, and NPV were also calculated for all the radiologists during the first reading session; binary evaluation using a 6-point scale was conducted at the second reading session. The diagnostic performance of the experts and non-experts, using the 6-point scale, was compared using the AUC of the MRMC ROC [7, 8]. To evaluate the interobserver variability during the first and second reading sessions, Fleiss’s kappa coefficient was calculated for all radiologists, a group comprising between expert and non-expert radiologists. The coefficient for all the radiologists was 0.95. All statistical analyses were performed using R version 4.0.3 (R Foundation for Statistical Computing).
Results
Patients’ demographics and tumor characteristics
Of the 238 patients, 101 were excluded as were not ideal candidates for surgery, nine were excluded as surgical resection was not attempted because of patients not wishing to receive (n = 8) or lost to follow-up (n = 1) (Fig. 1). The final sample consisted of 128 patients (73 men and 55 women; mean age ± SD, 71 ± 9 years). Among them, 45 patients received NTx and the remaining 83 did not. The NTx options included 39.6 Gy administered in 22 fractions of external-beam radiation and gemcitabine plus S-1 (n = 15), gemcitabine plus albumin-bound paclitaxel (n = 11), gemcitabine single agent (n = 9), modified FOLFIRINOX (fluorouracil, leucovorin, irinotecan, and oxaliplatin) [9] (n = 7), and S-1 single agent (n = 3). Pancreaticoduodenectomy with pancreatojejunostomy reconstruction was performed in 79 patients and distal pancreatectomy was performed in 49. The patients’ demographics and tumor characteristics are summarized in Table 2.
Determination of the best imaging criterion for arterial invasion
The 6-point scale assigned to each artery, and its sensitivity, specificity, PPV, and NPV for diagnosing arterial invasion in patients treated with and without NTx are shown in Table 3. In the first reading session, the sensitivity, specificity, PPV, and NPV were 87% vs. 92%, 92% vs. 93%, 50% vs. 34%, and 99% vs. 99% in patients treated with and without NTx, respectively. In the second reading session, solid soft tissue contact ≤ 180° was the best diagnostic criterion for arterial invasion in patients treated with and without NTx, as defined by the Youden Index (sensitivity, 100% vs. 100%; specificity, 90% vs. 93%; PPV, 48% vs. 36%; NPV, 100% vs. 100%; and AUC, 0.96 vs. 0.98) (Figs. 3 and 4).
The AUCs for diagnosing arterial invasion were 0.99 (95% confidence interval [CI]: 0.99, 1.00), 0.99 (95% CI: 0.98, 0.99), 0.88 (95% CI: 0.85, 0.91), and 0.92 (95% CI: 0.88, 0.96) for the celiac, superior mesenteric, splenic, and common hepatic arteries, respectively.
Diagnostic performance of all radiologists
The sensitivity, specificity, PPV, and NPV for diagnosing arterial invasion during the first reading session and for solid soft tissue contact ≤ 180° and > 180° in the second reading session are shown in Table 4. In the first reading session, the sensitivity ranged from 42 to 100% in all radiologists, 42 to 100% in expert radiologists, and 58 to 83% in non-expert radiologists; the specificity ranged from 89 to 99% in all radiologists, 89 to 98% in expert radiologists, and 92 to 99% in non-expert radiologists. For the criterion of solid soft tissue contact ≤ 180°, the sensitivity ranged from 58 to 100% in all radiologists, 58 to 100% in expert radiologists, and 67 to 93% in non-expert radiologists; the specificity ranged from 87 to 95% in all reviewers, 87 to 95% in expert radiologists, and 90 to 95% in non-expert radiologists. For the criterion of solid soft tissue contact > 180°, the sensitivity ranged from 27 to 92% in all radiologists, 33 to 92% in expert radiologists, and 27 to 83% in non-expert radiologists; the specificity ranged from 93 to 99% in all radiologists, 93 to 99% in expert radiologists, and 93 to 98% in non-expert radiologists.
Interobserver variability for the assessment of arterial nvasion
In the second reading session, the AUCs for diagnosing arterial invasion in patients treated with NTx were 0.93 (95% CI: 0.86, 1.00) and 0.89 (95% CI: 0.87, 0.92) among expert and non-expert radiologists, respectively; there was no significant difference (p = 0.31). The AUC for diagnosing arterial invasion in patients treated without NTx were 0.95 (95% CI: 0.88, 1.00) and 0.96 (95% CI: 0.90, 1.00) among expert and non-expert radiologists, respectively; there was no significant difference (p = 0.84).
The interobserver variability for arterial invasion assessment for all radiologists, expert, and non-expert radiologists is summarized in Table 5. In the first reading session, all radiologists showed moderate agreement when using the 2-point scale for patients treated with (ĸ = 0.71; 95% CI: 0.68, 0.73) and without NTx (ĸ = 0.66; 95% CI: 0.65, 0.68). The expert radiologists showed a higher agreement than the non-expert radiologists in patients treated without NTx (ĸ = 0.70 vs 0.63, p = 0.005); however, there was no such difference in patients treated with NTx (ĸ = 0.71 vs 0.75, p = 0.15). In the second reading session, all radiologists showed a fair to moderate agreement when using the 6-point scale for patients treated with (ĸ = 0.61; 95% CI: 0.60, 0.63) and without NTx (ĸ = 0.54; 95% CI: 0.53, 0.56). The non-expert radiologists showed a higher agreement than the expert radiologists in patients treated without NTx (ĸ = 0.59 vs 0.51, p < 0.001); however, there was no such difference in patients treated with NTx (ĸ = 0.61 vs 0.61, p = 0.39).
Discussion
The presence and degree of tumor contact with peripancreatic arteries are crucial in determining surgical resectability in patients with pancreatic ductal adenocarcinoma (PDAC). According to the National Comprehensive Cancer Network (NCCN) guidelines, solid soft tissue contact of > 180° with the celiac or superior mesenteric arteries denotes unresectability. Our study revealed that the presence of solid soft tissue contact ≤ 180° showed the highest diagnostic performance for arterial invasion in patients treated with and without neoadjuvant treatment (NTx). Contour deformity demonstrated the highest specificity; contour deformity is not a criterion for resectability in the NCCN guideline. Furthermore, CT assessment of arterial invasion when using the 6-point scale provided fair to moderate interobserver agreement among nine radiologists; interobserver variability among non-expert radiologists was not inferior to that among expert radiologists.
Vascular involvement of > 180° is defined as LA tumors according to NCCN criteria [10]. The positive predictive value (PPV) of preoperative CT for predicting unresectability was high (89–100%); however, the PPV for predicting resectability was low (45–79%) [11]. The imaging criterion for diagnosing vascular invasion gives importance to specificity over sensitivity to avoid denying curative surgery to patients with resectable tumors. The diagnostic performance of CT for detecting vascular invasion reportedly has a specificity of 82 to 100% and a sensitivity of 70 to 96% [12,13,14,15,16]. Two relatively recent meta-analyses showed a pooled sensitivity of 65% and 85% [17, 18]. Although these studies were published about 10 years ago, the sensitivity and specificity were almost the same as our results in patients who did not receive NTx.
The treatment strategy for BR and LA PDACs has dramatically changed in recent years. The surgical resection rates have exceeded 60% after NTx with FOLFIRINOX and 30% with gemcitabine plus albumin-bound paclitaxel [19]. Following NTx, solid soft tissue contact may be replaced by increased hazy attenuation/stranding; as a result, accurate assessment of arterial invasion and/or resectability on CT images becomes a challenge due to the mixing of viable tumor and posttreatment fibrosis [20, 21]. By comparing the diagnostic performance for arterial invasion between solid soft tissue contact ≤ 180° and > 180°, a relatively huge difference in sensitivity was seen, especially in patients treated with NTx (100% vs 60%) in this study. Our results indicate that a great number of patients with potentially resectable tumors based on post-NTx CT images may undergo incomplete resections (R1 or R2 resections). In fact, 22% of patients (n = 10/45) treated with NTx underwent R2 resections, which was higher than in those without NTx (13%, n = 11/83) in our study. To predict the presence of arterial invasion, solid soft tissue contact ≤ 180° might be more useful than solid soft tissue contact > 180°.
The criterion of solid soft tissue contact ≤ 180° has a marginally lower specificity (90% vs. 93% in patients treated with vs. without NTx) than the criterion for solid soft tissue contact > 180° (97% vs. 97% in patients treated with vs. without NTx). However, we believe that a significant difference in sensitivity has a higher clinical impact than a lesser difference in specificity. At present, there is no reliable criterion for evaluating the treatment response. Therefore, the following strategies are suggested in the event of downstaging or stable disease [22]: (a) reliance on clinical evaluation and CA 19 − 9 levels over time for making further treatment decisions; and (b) surgical assessment of resectability in patients with clinical improvement and decline in CA 19 − 9 levels to ≤ 200 U/mL. Only 3 factors are independently associated with good prognosis: post-NTx CA 19 − 9 response, extended duration (≥ 6 cycles) of NTx, and pathologic response [23]. Only 28% of patients showed radiological downstaging; however, 64% of patients showed a decline of post-NTx CA 19 − 9 levels to normal limits. Although the optimal NTx duration remains controversial, continued NTx might be considered to lower the CA 19 − 9 levels when solid soft tissue contact ≤ 180° to a major branch is observed on preoperative CT images.
Our study had several limitations. First, the study population was relatively small, and the investigation was conducted at a single institution. Additionally, we only included patients who underwent surgery to confirm the pathological findings. Therefore, the selection bias excludes patients with > 180° circumferential vessel involvement. Second, CT acquisition and image reconstruction protocols were heterogeneous because of the retrospective nature of the study with a long study period. Given the retrospective nature of the study, sagittal images were not available for all the cases during image analyses. The lack of sagittal images could potentially impact the accurate assessment of vascular involvement. Finally, we evaluated the CT diagnostic performance for arterial invasion in all four arteries combined. Further studies with a homogeneous CT protocol are required to validate our results for each individual artery.
In conclusion, the presence of solid soft tissue contact ≤ 180° was the best diagnostic criterion for arterial invasion in patients with pancreatic ductal adenocarcinoma. Additionally, fair to moderate interobserver variability exists in the CT assessment of arterial invasion, even among non-expert radiologists.
Abbreviations
- NPV:
-
Negative predictive value
- NTx:
-
Neoadjuvant treatment
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PPV:
-
Positive predictive value
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72:7–33
Kamarajah SK, Burns WR, Frankel TL, Cho CS, Nathan H (2017) Validation of the American Joint Commission on Cancer (AJCC) 8th Edition Staging System for Patients with Pancreatic Adenocarcinoma: A Surveillance, Epidemiology and End Results (SEER) Analysis. Ann Surg Oncol 24:2023–2030
Giovinazzo F, Soggiu F, Jang JY et al (2020) Gemcitabine-based neoadjuvant treatment in borderline resectable pancreatic ductal adenocarcinoma: a meta-analysis of individual patient data. Front Oncol 10:1112
NCCN clinical practice guidelines in oncology: pancreatic adenocarcinoma, version 2. 2022. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
Al-Hawary MM, Francis IR, Chari ST et al (2014) Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology 270:248–260
Weniger M, Moir J, Damm M et al (2020) Neoadjuvant therapy in elderly patients receiving FOLFIRINOX or gemcitabine/nab-paclitaxel for borderline resectable or locally advanced pancreatic cancer is feasible and lead to a similar oncological outcome compared to non-aged patients - Results of the RESPECT-Study. Surg Oncol 35:285–297
Dendumrongsup T, Plumb AA, Halligan S, Fanshawe TR, Altman DG, Mallett S (2014) Multi-reader multi-case studies using the area under the receiver operator characteristic curve as a measure of diagnostic accuracy: systematic review with a focus on quality of data reporting. PLoS One 9:e116018
Hadjiiski L, Chan HP, Sahiner B, Helvie MA, Roubidoux MA (2007) Quasi-continuous and discrete confidence rating scales for observer performance studies: Effects on ROC analysis. Acad Radiol 14:38–48
Mavros MN, Moris D, Karanicolas PJ, Katz MHG, O’Reilly EM, Pawlik TM (2021) Clinical trials of systemic chemotherapy for resectable pancreatic cancer: a review. JAMA Surg. https://doi.org/10.1001/jamasurg.2021.0149
Lu DS, Reber HA, Krasny RM, Kadell BM, Sayre J (1997) Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am J Roentgenol 168:1439–1443
Wong JC, Lu DS (2008) Staging of pancreatic adenocarcinoma by imaging studies. Clin Gastroenterol Hepatol 6:1301–1308
Zamboni GA, Kruskal JB, Vollmer CM, Baptista J, Callery MP, Raptopoulos VD (2007) Pancreatic adenocarcinoma: value of multidetector CT angiography in preoperative evaluation. Radiology 245:770–778
Fletcher JG, Wiersema MJ, Farrell MA et al (2003) Pancreatic malignancy: value of arterial, pancreatic, and hepatic phase imaging with multi-detector row CT. Radiology 229:81–90
Catalano C, Laghi A, Fraioli F et al (2003) Pancreatic carcinoma: the role of high-resolution multislice spiral CT in the diagnosis and assessment of resectability. Eur Radiol 13:149–156
Karmazanovsky G, Fedorov V, Kubyshkin V, Kotchatkov A (2005) Pancreatic head cancer: accuracy of CT in determination of resectability. Abdom Imaging 30:488–500
Koelblinger C, Ba-Ssalamah A, Goetzinger P et al (2011) Gadobenate dimeglumine-enhanced 3.0-T MR imaging versus multiphasic 64-detector row CT: prospective evaluation in patients suspected of having pancreatic cancer. Radiology 259:757–766
Zhao WY, Luo M, Sun YW et al (2009) Computed tomography in diagnosing vascular invasion in pancreatic and periampullary cancers: a systematic review and meta-analysis. Hepatobiliary Pancreat Dis Int 8:457–464
Yang R, Lu M, Qian X et al (2014) Diagnostic accuracy of EUS and CT of vascular invasion in pancreatic cancer: a systematic review. J Cancer Res Clin Oncol 140:2077–2086
Chapman BC, Gleisner A, Rigg D et al (2018) Perioperative and survival outcomes following neoadjuvant FOLFIRINOX versus gemcitabine abraxane in patients with pancreatic adenocarcinoma. JOP 19:75–85
Morgan DE, Waggoner CN, Canon CL et al (2010) Resectability of pancreatic adenocarcinoma in patients with locally advanced disease downstaged by preoperative therapy: a challenge for MDCT. AJR Am J Roentgenol 194:615–622
Kim YE, Park MS, Hong HS et al (2009) Effects of neoadjuvant combined chemotherapy and radiation therapy on the CT evaluation of resectability and staging in patients with pancreatic head cancer. Radiology 250:758–765
Zins M, Matos C, Cassinotto C (2018) Pancreatic adenocarcinoma staging in the era of preoperative chemotherapy and radiation therapy. Radiology 287:374–390
Truty MJ, Kendrick ML, Nagorney DM et al (2021) Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for borderline/locally advanced pancreatic cancer. Ann Surg 273:341–349
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Yoshifumi Noda.
Conflict of Interest
AK: Grant support for research activities from Philips, GE Healthcare, and PanCAN. Other authors report no relevant disclosures or conflicts of interest.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived because this is a retrospective study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Noda, Y., Mizuno, N., Kawai, N. et al. Determination of arterial invasion in pancreatic ductal adenocarcinoma: what is the best diagnostic criterion on CT?. Eur Radiol 33, 3617–3626 (2023). https://doi.org/10.1007/s00330-023-09521-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09521-3