Abstract
Background and aims
Transarterial 90Y radioembolization (TARE) is increasingly being used for hepatocellular carcinoma (HCC) treatment. However, tumor response assessment after TARE may be challenging. We aimed to assess the diagnostic performance of gadoxetate disodium MRI for predicting complete pathologic necrosis (CPN) of HCC treated with TARE, using histopathology as the reference standard.
Methods
This retrospective study included 48 patients (M/F: 36/12, mean age: 62 years) with HCC treated by TARE followed by surgery with gadoxetate disodium MRI within 90 days of surgery. Two radiologists evaluated tumor response using RECIST1.1, mRECIST, EASL, and LI-RADS-TR criteria and evaluated the percentage of necrosis on subtraction during late arterial, portal venous, and hepatobiliary phases (AP/PVP/HBP). Statistical analysis included inter-reader agreement, correlation between radiologic and pathologic percentage of necrosis, and prediction of CPN using logistic regression and ROC analyses.
Results
Histopathology demonstrated 71 HCCs (2.8 ± 1.7 cm, range: 0.5–7.5 cm) including 42 with CPN, 22 with partial necrosis, and 7 without necrosis. EASL and percentage of tumor necrosis on subtraction at the AP/PVP were independent predictors of CPN (p = 0.02–0.03). Percentage of necrosis, mRECIST, EASL, and LI-RADS-TR had fair to good performance for diagnosing CPN (AUCs: 0.78 – 0.83), with a significant difference between subtraction and LI-RADS-TR for reader 2, and in specificity between subtraction and other criteria for both readers (p-range: 0.01–0.04). Radiologic percentage of necrosis was significantly correlated to histopathologic degree of tumor necrosis (r = 0.66 – 0.8, p < 0.001).
Conclusions
Percentage of tumor necrosis on subtraction and EASL criteria were significant independent predictors of CPN in HCC treated with TARE. Image subtraction should be considered for assessing HCC response to TARE when using MRI.
Key Points
• Percentage of tumor necrosis on image subtraction and EASL criteria are significant independent predictors of complete pathologic necrosis in hepatocellular carcinoma treated with 90 Y radioembolization.
• Subtraction, mRECIST, EASL, and LI-RADS-TR have fair to good performance for diagnosing complete pathologic necrosis in hepatocellular carcinoma treated with 90 Y radioembolization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary hepatic malignancy, accounting for up to 90% of cases, and is the second leading cause of cancer-related death worldwide [1]. According to the Barcelona Clinic Liver Cancer (BCLC) current guidelines, patients diagnosed with early-stage HCC can be curatively treated by surgical resection, radiofrequency ablation, or liver transplantation [2]. However, 80% of patients are not eligible for curative treatment at the time of HCC diagnosis because of advanced tumor stage and/or background liver disease [3]. In the past two decades, multiple locoregional therapies (LRTs) have been developed for advanced HCC or with a curative prospect in patients who are not candidate for liver transplantation. LRT can also be used for tumor downstaging to make patients eligible for transplantation [4]. Yttrium-90 (90Y) transarterial radioembolization (TARE) is a growing LRT technique with data supporting its use in curative intent but also as neoadjuvant therapy before surgical resection or transplantation [5,6,7,8,9,10,11,12] and in cases of advanced-stage HCC with macrovascular invasion [13, 14].
Radiologic assessment of the response after LRT can be challenging as the lesion may demonstrate heterogeneous necrosis and typically no reduction in size at least initially. Among LRT techniques, TARE is particular as the radiation emitted may increase blood flow that may translate into increased enhancement in the treated area (the so-called radiation effect) [15], which may lead to uncertainty over the presence/absence of residual disease [16]. Traditional criteria for response to therapy assessment such as the Response Evaluation Criteria in Solid Tumor (RECIST) have been adapted in order to achieve a more accurate evaluation of lesions after LRT. As such, the European Association for the Study of Liver (EASL) criteria and the modified RECIST criteria (mRECIST) that only assess the viable tumor portion instead of the whole lesion have been adapted to LRT assessment [17,18,19,20]. In addition, the use of post-contrast image subtraction with MRI has been shown to be useful for tumor necrosis assessment [21,22,23]. Radiopathologic correlation is crucial in order to understand and interpret correctly the imaging spectrum present in treated tumors after TARE. The assessment of the degree of necrosis is of particular interest as it determines tumor viability. Published data on pathologic correlation in patients with HCC treated with TARE is limited (9–35 patients) [8, 19, 24,25,26]. Previous studies have emphasized the challenge of assessing HCC response post TARE [8, 24], with poor to moderate inter-reader agreement using mRECIST or LI-RADS-TR in one study [19]. King et al showed a correlation between mRECIST, qEASL (3D measurement of the lesion), and LI-RADS-TR criteria and percentage of necrosis at pathology [19]. Additional imaging features including rim enhancement, lack of hepatocyte-specific contrast uptake, T2-hyperintensity, and plateau or persistent enhancement were described as having potential added value to classic criteria of response assessment [8, 24]. With the increased use of TARE for HCC, there is a need for a clear definition of tumor response/necrosis.
Our objective was to assess the diagnostic performance of gadoxetate disodium–enhanced MRI for predicting complete pathologic necrosis (CPN) of HCC treated with TARE, using histopathology as the reference standard.
Material and methods
Patients
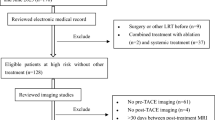
This single-center HIPAA compliant retrospective study was approved by the local Institutional Review Board. A waiver for informed consent was obtained. All consecutive patients with HCC treated with TARE who underwent liver transplantation or resection and contrast-enhanced MRI using gadoxetate disodium, ≤ 90 days prior to surgery between November 2012 and March 2019, have been identified through the institutional liver transplant database. Inclusion criteria were as follows: (1) adult cirrhotic patients that underwent liver transplant or resection, (2) MRI with gadoxetate disodium performed with adequate technique and quality ≤ 90 days prior to liver transplantation or resection, (3) segmental/lobar TARE prior to imaging with a minimal delay of 12 weeks between TARE and imaging. Among 57 potentially eligible patients, 10 patients were excluded (Fig. 1). Clinical variables including demographics, body mass index, etiology of liver disease, Child-Pugh score, Model for End Stage Liver Disease score, BCLC classification, serum alfa-feto protein, Y90 dose, and type of treatment (lobar/segmental) and surgery type (resection/transplantation) were recorded.
MRI acquisition
We analyzed the MRI closest to surgery (< 90 days) and we only included patients with a minimal delay of 12 weeks between TARE and imaging, so to assess the response adequately. However, most patients got a longer delay between TARE and surgery with multiple MRIs performed. Of those, only the last MRI before surgery (performed < 90 days before surgery) was considered for the present study.
MRI of the abdomen was performed using various 1.5-T/3.0-T clinical systems with abdominal phase-array coils, using parallel imaging with a field of view of 300–400mm (Magnetom Avanto [n = 6], Skyra [n = 8], Aera [n = 16], Siemens Healthineers; and Signa 1.5T [n = 17], GE Healthcare). The liver MRI was a standard-of-care contrast-enhanced exam including dynamic phase images (pre-contrast, early and late arterial phases [AP], portal venous phase [PVP], transitional phase) as well as hepatobiliary phase (HBP) at 10 and 20 min post-injection of a fixed dose of 10 mL of gadoxetate disodium (Eovist/Primovist, Bayer Healthcare). MRI protocol is presented in Table 1. Enhanced phases–precontrast image subtraction datasets were generated automatically for all MRI exams.
Index lesion selection
The study coordinator (N.V.V., a radiologist with 2 years of experience in abdominal imaging) reviewed the MRI exams using a picture archiving and communication system (PACS) and the pathology reports to identify the index lesions. If multiple lesions were present in the same patient, up to three treated HCCs (≥ 1 cm) were included. Size, segment location, and series/image numbers of the lesion were recorded and labeled as lesion1/lesion2/lesion3 to ensure that radiologists and pathologist assessed the same lesion. Anonymized evaluation sheets were filled by the observers.
Image analysis
Two radiologists (J.G.,A.L.), both with 3 years of experience in abdominal imaging, independently reviewed the images on PACS. The readers were aware of the study aims but blinded to all clinical radiological and pathological reports. Exams were reviewed in a random order. If multiple lesions were present, all lesions were scored in the same reading session. The readers were asked to report the following parameters on late AP, PVP, and HBP (20 min post-contrast): LI-RADS v2018 treatment response algorithm (nonviable/equivocal/viable [27]), RECIST (cm), mRECIST (cm), EASL (cm2) on non-subtracted images as well as degree of tumor necrosis (defined visually as non-enhancing tissue) on subtracted post-contrast images, in 10% increments. The readers used late AP, PVP, and HBP (20 min post-contrast) for assessment of response. As the characteristics of tumor enhancement during the transitional phase are not well understood, we decided to not assess transitional phase images in our study [28].
Histopathological analysis
An experienced liver pathologist (M.I.F., 15 years of experience) blinded to the MRI results and clinical pathologic report retrospectively reviewed gross and microscopic finding in all index lesions, and reported degree of pathologic necrosis in 10% increments. Percentage of necrosis and size at pathology were considered the reference standard. For each lesion, tumor grade, presence/absence of microvascular invasion, satellite lesions, and T-stage were reported.
Statistical analysis
Imaging data was compared to histopathological data on a per-lesion basis. Reader assessments were pooled across the two readers by averaging their values when comparing them to the reference standard.
Reader agreement was assessed using the kappa coefficient (K) for the binary assessment of LI-RADS-TR, and the intraclass correlation coefficient (ICC) for the numeric measures (RECIST, EASL, m-RECIST, percentage of necrosis). Κ was interpreted as an indication of poor agreement when < 0, as slight agreement when 0 ≤ K ≤ 0.2, as fair agreement when 0.2 < K ≤ 0.4, as moderate agreement when 0.4 < K ≤ 0.6, and as substantial agreement when K > 0.6. The ICC was interpreted as follows: poor reliability (< 0.5), moderate reliability (0.5–0.75), good reliability (0.75–0.9), and excellent reliability (0.90) [29].
Spearman rank correlations were used to characterize the association of measures with degree of necrosis on pathology, and ICC was calculated between degree of necrosis on image subtraction and on pathology.
Demographic and clinical variables, as well as TARE (including TARE type, 90Y doses), imaging, and pathological (including number of HCC, HCC size, percentage of necrosis and histological grade) variables, were considered predictors of CPN using generalized estimating equations to account for the correlation among lesions from the same patient. Predictors were entered one at a time to obtain unadjusted odds ratios and 95% confidence intervals. For multivariable analysis, a backward selection procedure was employed, taking as candidate predictors any variables whose unadjusted association had a significance level of p < 0.10. The final model thus selected was then assessed for collinearity. When predictors were sufficiently correlated to indicate a collinearity problem (variance inflation factor > 10), they were removed one at a time, and the model with the best fit to the data as determined by the smallest QIC (Quasi-likelihood under the Independence model Criterion) statistic was chosen [30]. Receiver operating characteristic (ROC) analyses were used to assess the significant measure’s (based on univariate analysis) ability to predict CPN, with area under the ROC curve, and corresponding confidence limits, as a performance metric. Optimal cutoffs from the ROC analyses were established for each measure based on the value that minimized the Euclidian distance from the point on the ROC curve representing sensitivity and specificity of one (100%). All statistical tests were conducted at the two-sided 5% significance level using SAS 9.4 (SAS Institute).
Results
The final study population included 47 patients (M/F: 35/12, mean age: 62 years), with a total of 71 HCCs (mean size at pathology: 2.8 ±1.7 cm, 0.5–7.5 cm). TARE was delivered at the lobar level in 31 patients and segmental level in 12 patients, while 4 patients underwent other treatment distributions: whole liver (n = 1), right lobe + caudate lobe (n = 2), and right lobe + segment IV (n = 1). The mean delay between TARE and MRI was 6 months (range: 4–27 months), and the mean delay between TARE and surgery was 8 months (range: 5–29 months). Patient characteristics are presented in Table 2.
Histopathologic findings
There were 28 patients with 1 HCC, 17 with 2 HCCs, and 3 with 3 HCCs. Fifty-seven tumors were in the right hepatic lobe, 12 in the left hepatic lobe, and 2 in both hepatic lobes. Mean percentage of necrosis was 82% ± 32% (range 0–100%). CPN was observed in 42 (59.2%) HCCs, 90–99% necrosis in 10 (14.1%), 80–89% necrosis in 3 (4.2%), 70–79% necrosis in 2 (2.8%), 60–69% necrosis in 4 (5.6%), 30–39% necrosis in 2 (2.8%), and 10–19% necrosis in 1 (1.4%), while 7 HCCs were completely viable (0% necrosis, 9.9%).
Of the 29 HCCs with a viable component, 7 were well differentiated, 19 were moderately differentiated, and 3 were poorly differentiated. Cirrhosis was present in 44/47 patients, the 3 remaining patients had hepatitis B virus infection without cirrhosis.
MRI findings
Inter-reader agreement
There was good inter-reader agreement for assessing radiologic degree of necrosis using subtraction on AP/PVP (ICC: 0.85/0.86) and for RECIST (ICC: 0.82), while it was moderate for HBP subtraction (ICC: 0.7), mRECIST (ICC: 0.65), and EASL (ICC: 0.62). Agreement was fair for LI-RADS-TR (K: 0.4).
Prediction of CPN
Univariate logistic regression showed that mRECIST, EASL, LI-RADS-TR, and subtraction (at all assessed phases) were all significant predictive factors of CPN (Table 3). In multivariable analysis, only EASL [OR: 0.74 (confidence intervals, CIs: 0.57–0.96), p = 0.02], subtraction on AP [OR: 0.73 (0.55–0.97), p = 0.03], and PVP [OR: 0.71 (0.51–0.89), p = 0.02] were independent predictive factors of CPN. No other variable was predictive of CPN, including DWI signal.
mRECIST, EASL, LI-RADS-TR, and subtraction were all significant predictors of CPN (pooled AUC: 0.83–0.84 for radiologic percentage of necrosis with pooled AUCs of 0.79–0.8 for mRECIST, EASL, and LI-RADS-TR, p < 0.0001), with a significant difference observed between subtraction and LI-RADS-TR (p = 0.04) for reader 2 (Figs. 2 and 3). While we observed no difference in sensitivity, the specificity of subtraction on AP/PVP was significantly higher compared to mRECIST (p = 0.04), EASL (p = 0.04), and LI-RADS-TR (p = 0.01) for prediction of CPN. The specificity of subtraction on HBP was significantly higher compared to LI-RADS-TR (p = 0.01) for the prediction of CPN. RECIST was not a significant predictor of CPN (AUC: 0.51, p = 0.17) (Table 4, Fig. 4).
A 62-year-old man with HCV cirrhosis and right hepatic lobe HCC (arrows) treated with lobar TARE. Non-subtracted images are shown (A: AP, B: PVP, and C: HBP). On image subtraction, the degree of tumor necrosis was rated as 100% on AP (D), PVP (E), and HBP (F) by readers 1 and 2, respectively. Measurements of both observers were for mRECIST: 0/0 cm, EASL: 0/0 cm2, LI-RADS-TR: LR-TR nonviable (both observers), RECIST: 3.8/4 cm. Note decreased hepatobiliary uptake in surrounding peritumoral liver (asterisks on C and F). Corresponding gross pathologic image shows completely necrotic lesion (arrow) measuring 4 cm in cirrhotic liver (G). Corresponding microscopic H&E image (×10) shows completely necrotic HCC with embolization beads noted (H, arrow). Abbreviations: HCV: hepatitis C virus; HCC: hepatocellular carcinoma; TARE: transarterial radioembolization; AP: arterial phase; PVP: portal venous phase; HBP: hepatobiliary phase; RECIST: Response Evaluation Criteria in Solid Tumors; mRECIST: modified RECIST; EASL: European Association for the Study of the Liver; LI-RADS-TR: liver imaging reporting and data system for treated tumor; LR-TR: LI-RADS treatment response
A 58-year-old man with HCV cirrhosis and right hepatic lobe HCC (arrows) treated with segmental TARE. Non-subtracted images are shown (A: AP, B: PVP and C: HBP). On image subtraction, tumor necrosis was rated as 30% on AP (D), PVP (E), and HBP (F) by observers 1 and 2, respectively. Note some misregistration artifact on PVP (E). Measurements of both observers were for mRECIST: 2.2/2.4 cm, EASL: 3.8/4.3 cm2, LI-RADS: LR-TR viable (both observers), RECIST: 2.9/3 cm. Corresponding gross pathologic picture shows 2.7-cm HCC (arrows) with 30% necrosis in cirrhotic liver (G). Corresponding microscopic H&E image (×10) shows viable HCC (arrows) and a portion of necrotic tumor around (H). Abbreviations: HCV: hepatitis C virus; HCC: hepatocellular carcinoma; TARE: transarterial radioembolization; AP: arterial phase; PVP: portal venous phase; HBP: hepatobiliary phase; RECIST: Response Evaluation Criteria in Solid Tumors; mRECIST: modified RECIST; EASL: European Association for the Study of the Liver; LI-RADS-TR: liver imaging reporting and data system for treated tumor; LR-TR: LI-RADS treatment response
Receiver operating characteristics curves for significant MRI criteria (selected based on univariate analysis). ROC curves for image subtraction at the AP (sub AP), PVP (sub PVP) and HBP (sub HBP), mRECIST, EASL and LI-RADS-TR as individual predictors of complete pathologic necrosis. Abbreviations: MRI: magnetic resonance imaging; ROC: receiver operating characteristic; AP: arterial phase; PVP: portal venous phase; HBP: hepatobiliary phase; mRECIST: modified RECIST; EASL: European Association for the Study of the Liver; LI-RADS-TR: liver imaging reporting and data system treatment response
Degree of tumor necrosis on subtraction was significantly correlated to histopathologic degree of tumor necrosis at all phases (AP r = 0.63, PVP and HBP r = 0.64, all p < 0.0001), without significant difference between phases (p-range: 0.33–0.92).
Discussion
In our study, we observed that TARE is an efficient therapy for HCC with 59% (42/71) of tumors demonstrating CPN, in line with previous reports [8, 24]. When considering radiologic assessment of tumor response, we found that image subtraction, mRECIST, EASL, and LI-RADS-TR have excellent performance for predicting CPN in HCC post TARE, confirming the results from previous studies assessing different LRTs [8, 21]. In addition, the degree of tumor necrosis on image subtraction demonstrated a strong correlation with histopathologic degree of tumor necrosis. In addition, image subtraction on late AP and PVP had good inter-reader agreement, superior to that of mRECIST, EASL, and LI-RADS-TR. Inter-reader agreement for assessment of tumor necrosis on subtraction on HBP was in the lower range than based on AP and PVP, likely due to the fact that HBP is not typically used for the assessment of necrosis, with consecutively more reader variation. Finally, demographic, clinical, and qualitative radiological (other than those mentioned above) and tumor size characteristics were poor predictors of CPN, while EASL and subtraction at AP and PVP were independent predictors of CPN in multivariable analysis.
Evaluation of tumor response after TARE can be challenging due to the following: (1) necrosis rather than shrinkage of the tumor and (2) the presence of radiation effect in the surrounding liver parenchyma [31]. The radiation effect relates to diffuse increase in blood flow secondary to TARE that may translate as increased contrast enhancement in the surrounding liver, which may lead to confusion over the presence/absence of residual tumor [16]. Typically, the enhancement of the surrounding peritumoral liver decreases approximately 3 months post-treatment, and residual tumor and/or local tumor progression become easier to assess [32]. Thus, a delay of at least 3 months after TARE is recommended for imaging follow-up [32, 33]. In the present study, the mean delay between TARE and MRI was 6 months, with decreased effect of radiation. Chronic appearance of the treated zone is retraction and fibrosis (with enhancement on late post-contrast phases when using extracellular contrast media) [31]. There are a few studies assessing HCC response to TARE with MRI compared to histopathology (between 9 and 35 patients) [8, 19, 24,25,26]. These studies evaluated the diagnostic performance of EASL and mRECIST criteria for the diagnosis of CPN. Riaz et al showed that EASL and mRECIST criteria were predictive of the degree of pathologic necrosis and the presence of rim enhancement was correlated with histopathologic necrosis [8]. Ahmed et al also demonstrated a correlation between each of EASL and mRECIST criteria with histopathologic degree of necrosis. Additional features that correlated with histopathologic necrosis included the following: absence of HBP uptake, increased T2 signal, and plateaued or persistent enhancement after contrast injection [24]. King et al found a correlation between LI-RADS-TR criteria with histopathologic necrosis in 9 patients [19]. None of these studies assessed image subtraction. In another study including various LRT techniques with only 5 patients treated by TARE, Gordic et al showed the predictive value of image subtraction which strongly correlated with histopathologic necrosis [21]. The present study confirms these results, highlighting the potential benefit of image subtraction for the assessment of necrosis after TARE and showing the predictive value of EASL criteria for diagnosis of CPN. Our results demonstrate good specificity (82.8%) for the prediction of CPN using subtraction while sensitivity was lower (61.9–69.1%). This could be due to the interval between imaging and surgery with a possible ongoing necrosis phenomenon (even if we selected patients with < 90 days between imaging and surgery), and the possibility of residual enhancement due to granulation tissue. Also, as in by Gordic et al [21], we did not find any predictive role of DWI for diagnosis of CPN.
There is limited data on the role of image subtraction in HCC post LRT with histopathologic correlation [21,22,23]. These previous studies included mostly patients treated with ablation or transarterial chemoembolization and demonstrated that image subtraction had excellent diagnostic performance for diagnosis of tumor necrosis, with AUC ranging from 0.82 to 0.90 for the AP and 0.85 to 0.88 for the PVP, equivalent to our results. These studies also showed good inter-reader agreement, as in our current study, in which subtraction was better than mRECIST, EASL, and LI-RADS-TR in terms of inter-reader agreement (fair or moderate agreement). Correlation between necrosis at image subtraction and necrosis at histopathology reported by Mannelli et al and Gordic et al (AP, r = 0.71–0.89, PVP, r = 0.72–0.91, p < 0.001 for both studies) was slightly better than ours, possibly due to different LRT assessed. These previous studies did not use gadoxetate disodium, thus did not assess the percentage of necrosis using HBP. In the present study, we showed no difference in the diagnostic performance of subtraction obtained during different post-contrast phases (excluding transitional phase). Our results suggest that gadoxetate disodium could be used for the follow-up of patients treated with TARE as it allows index tumor response assessment and detection of new lesions with high sensitivity on the HBP [34]. However, subtraction based on HBP was not an independent predictive factor of CPN on multivariable logistic regression, unlike subtraction based on AP/PVP and EASL. Consequently, we suggest that subtraction should be assessed on AP and PVP and could be part of tumor response assessment to TARE, providing a linear assessment of response that mirrors histopathology. One limitation of subtraction is that is not systematically used with CT imaging, which limits its application to MRI [35]. Moreover, subtraction is limited by the need of good dynamic image quality with reproducible breath-holds in order to avoid misregistration between pre- and post-contrast phases [36]. In our study, we did not assess the effect of subtraction misregistration on diagnostic performance. Future directions will need to assess the effect of misregistration quality on response assessment, particularly with regard to the risk of AP respiratory artifacts that are known to be associated with the use of gadoxetate disodium [37, 38].
Our study has several limitations. First, it is a retrospective single-center study with a relatively small sample size. However, all tumors were verified at pathology. Second, there was an inherent selection bias for patients eligible for surgery and transplantation, who are more likely to have smaller tumors. This selection bias was counterbalanced by a uniform standard of reference. Third, contrast-enhanced sequences were assessed during the same reading session, which limits the comparison between sequences. Fourth, there is a possibility of interval tumor progression/response between MRI and surgery in the 90-day window. Fifth, we assessed various TARE treatment options, including segmental and lobar, reflecting our evolving experience, with the trend towards more segmental treatments. Future studies should focus on segmental TARE in small tumors.
In conclusion, subtraction (on the AP and PVP) and EASL criteria are independent predictors of CPN with excellent performance for diagnosing CPN in HCC treated with TARE. Image subtraction demonstrated good interreader agreement and showed strong correlation with histopathologic degree of necrosis. Thus, we recommend the use of subtraction when assessing HCC response to TARE with MRI.
Abbreviations
- AFP:
-
Alfa-feto protein
- AP:
-
Arterial phase
- AUC:
-
Area under the curve
- BCLC:
-
Barcelona Clinic Liver Cancer
- CPN:
-
Complete pathologic necrosis
- DWI:
-
Diffusion-weighted imaging
- EASL:
-
European Association for the Study of Liver
- HBP:
-
Hepatobiliary phase
- HCC:
-
Hepatocellular carcinoma
- ICC:
-
Interclass correlation coefficient
- LI-RADS-TR:
-
Liver Imaging Reporting and Data System treatment response
- LRT:
-
Locoregional therapy
- MELD:
-
Model for end-stage liver disease
- MRI:
-
Magnetic resonance imaging
- OR:
-
Odds ratio
- PVP:
-
Portal venous phase
- RECIST:
-
Response Evaluation Criteria in Solid Tumor
- SD:
-
Standard deviation
- TARE:
-
Transarterial radioembolization with Y90
- WI:
-
Weighted imaging
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Forner A, Reig ME, de Lope CR, Bruix J (2010) Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 30:61–74
Galun D, Basaric D, Zuvela M et al (2015) Hepatocellular carcinoma: from clinical practice to evidence-based treatment protocols. World J Hepatol 7:2274–2291
Pompili M, Francica G, Ponziani FR, Iezzi R, Avolio AW (2013) Bridging and downstaging treatments for hepatocellular carcinoma in patients on the waiting list for liver transplantation. World J Gastroenterol 19:7515–7530
Gabr A, Abouchaleh N, Ali R et al (2018) Outcomes of surgical resection after radioembolization for hepatocellular carcinoma. J Vasc Interv Radiol 29:1502–1510 e1501
Lewandowski RJ, Kulik LM, Riaz A et al (2009) A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant 9:1920–1928
Riaz A, Gates VL, Atassi B et al (2011) Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys 79:163–171
Riaz A, Kulik L, Lewandowski RJ et al (2009) Radiologic-pathologic correlation of hepatocellular carcinoma treated with internal radiation using yttrium-90 microspheres. Hepatology 49:1185–1193
Salem R, Lewandowski RJ, Kulik L et al (2011) Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 140:497–507 e492
Salem R, Lewandowski RJ, Mulcahy MF et al (2010) Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 138:52–64
Vouche M, Lewandowski RJ, Atassi R et al (2013) Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol 59:1029–1036
Bhangoo MS, Karnani DR, Hein PN et al (2015) Radioembolization with Yttrium-90 microspheres for patients with unresectable hepatocellular carcinoma. J Gastrointest Oncol 6:469–478
Titano J, Noor A, Kim E (2017) Transarterial chemoembolization and radioembolization across Barcelona clinic liver cancer stages. Semin Intervent Radiol 34:109–115
Salem R, Johnson GE, Kim E et al (2021) Yttrium-90 radioembolization for the treatment of solitary, unresectable hepatocellular carcinoma: the LEGACY study. Hepatology. https://doi.org/10.1002/hep.31819
Yaghmai V, Besa C, Kim E, Gatlin JL, Siddiqui NA, Taouli B (2013) Imaging assessment of hepatocellular carcinoma response to locoregional and systemic therapy. AJR Am J Roentgenol 201:80–96
Singh P, Anil G (2014) Yttrium-90 radioembolization of liver tumors: what do the images tell us? Cancer Imaging 13:645–657
European Association For The Study Of The L, European Organisation For R, Treatment Of C (2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56:908–943
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30:52–60
King MJ, Tong A, Dane B, Huang C, Zhan C, Shanbhogue K (2020) Response assessment of hepatocellular carcinoma treated with yttrium-90 radioembolization: inter-reader variability, comparison with 3D quantitative approach, and role in the prediction of clinical outcomes. Eur J Radiol 133:109351
Lee JS, Choi HJ, Kim BK et al (2020) The Modified Response Evaluation Criteria in Solid Tumors (RECIST) yield a more accurate prognoses than the RECIST 1.1 in hepatocellular carcinoma treated with transarterial radioembolization. Gut Liver. https://doi.org/10.5009/gnl19197
Gordic S, Corcuera-Solano I, Stueck A et al (2017) Evaluation of HCC response to locoregional therapy: validation of MRI-based response criteria versus explant pathology. J Hepatol 67:1213–1221
Kim S, Mannelli L, Hajdu CH et al (2010) Hepatocellular carcinoma: assessment of response to transarterial chemoembolization with image subtraction. J Magn Reson Imaging 31:348–355
Mannelli L, Kim S, Hajdu CH, Babb JS, Clark TW, Taouli B (2009) Assessment of tumor necrosis of hepatocellular carcinoma after chemoembolization: diffusion-weighted and contrast-enhanced MRI with histopathologic correlation of the explanted liver. AJR Am J Roentgenol 193:1044–1052
Ahmed AF, Samreen N, Grajo JR et al (2018) Angiosomal radiopathologic analysis of transarterial radioembolization for the treatment of hepatocellular carcinoma. Abdom Radiol (NY) 43:1825–1836
Vouche M, Habib A, Ward TJ et al (2014) Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology 60:192–201
Vouche M, Kulik L, Atassi R et al (2013) Radiological-pathological analysis of WHO, RECIST, EASL, mRECIST and DWI: imaging analysis from a prospective randomized trial of Y90 +/- sorafenib. Hepatology 58:1655–1666
CT/MRI LI-RADS v2018. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018
Choi J-Y, Lee J-M, Sirlin CB (2014) CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology 273:30–50
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163
Pan W (2001) Akaike's information criterion in generalized estimating equations. Biometrics 57:120–125
Young S, Taylor AJ, Sanghvi T (2018) Post locoregional therapy treatment imaging in hepatocellular carcinoma patients: a literature-based review. J Clin Transl Hepatol 6:189–197
Semaan S, Makkar J, Lewis S, Chatterji M, Kim E, Taouli B (2017) Imaging of hepatocellular carcinoma response after (90)Y Radioembolization. AJR Am J Roentgenol 209:W263–W276
Vogel A, Cervantes A, Chau I et al (2018) Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:iv238–iv255
Besa C, Kakite S, Cooper N, Facciuto M, Taouli B (2015) Comparison of gadoxetic acid and gadopentetate dimeglumine-enhanced MRI for HCC detection: prospective crossover study at 3 T. Acta Radiol Open 4:2047981614561285
Willatt J, Ruma JA, Azar SF, Dasika NL, Syed F (2017) Imaging of hepatocellular carcinoma and image guided therapies - how we do it. Cancer Imaging 17:9
An C, Park MS, Kim D et al (2013) Added value of subtraction imaging in detecting arterial enhancement in small ( < 3 cm) hepatic nodules on dynamic contrast-enhanced MRI in patients at high risk of hepatocellular carcinoma. Eur Radiol 23:924–930
McClellan TR, Motosugi U, Middleton MS et al (2017) Intravenous gadoxetate disodium administration reduces breath-holding capacity in the hepatic arterial phase: a multi-center randomized placebo-controlled trial. Radiology 282:361–368
Vietti Violi N, Argiriadi P, Rosen A et al (2020) Gadoxetate disodium-enhanced MRI: assessment of arterial phase artifacts and hepatobiliary uptake in a large series. Eur J Radiol 132:109313
Funding
Naik Vietti Violi: Swiss National Science Foundation, fellowship P2LAP3_178053. The authors state no other funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Bachir Taouli.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Bachir Taouli: Research grants: Bayer Healthcare, Takeda; Consultant: Bayer Healthcare, Alexion. The other authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• case-control study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vietti Violi, N., Gnerre, J., Law, A. et al. Assessment of HCC response to Yttrium-90 radioembolization with gadoxetate disodium MRI: correlation with histopathology. Eur Radiol 32, 6493–6503 (2022). https://doi.org/10.1007/s00330-022-08732-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08732-4