Abstract
Objectives
Target therapy with BRAF/MEK inhibitors in metastatic melanoma is characterised by a high response rate; however, acquired resistance to treatment develops in many cases. We aimed to investigate if baseline total metabolic tumour volume (TMTV) and therapy-response assessment by [18F]FDG PET/CT have a prognostic role on progression-free survival (PFS) and overall survival (OS) in patients with metastatic melanoma receiving BRAF ± MEK inhibitors.
Methods
Fifty-seven patients who performed an [18F]FDG PET/CT at baseline and on treatment were retrospectively evaluated. A Cox proportional-hazard model was used to examine associations between OS and PFS with baseline clinical/PET parameters as well as for PET response.
Results
According to EORTC criteria, 34 patients were classified as responders (partial/complete metabolic response [PMR/CMR]) and 23 as non-responders (progressive/stable metabolic disease [PMD/SMD]). Baseline characteristics associated with a shorter PFS were more than two metastatic organ sites and TMTV > 56 cm3; the latter was the only independent feature at multivariate analysis. Patients achieving a CMR were associated with a prolonged PFS compared with those with PMR (median PFS 42.9 vs 8.8 months; p = 0.009). Disease progression occurred in new-onset disease sites in 87.5% of CMR, 7.1% of PMR and 34.8% of PMD/SMD (p < 0.001). High baseline TMTV and lack of treatment response were independent prognostic factors for OS, stratifying patients in three different prognostic classes (median OS 6.7, 18.3 and 102.2 months, respectively).
Conclusions
Baseline TMTV and metabolic response may be useful prognostic indicators for PFS and OS in patients with advanced melanoma treated with BRAF/MEK inhibitors.
Key Points
• In a retrospective cohort of 57 metastatic melanoma patients treated with BRAF/MEK inhibitors, a TMTV > 56 cm 3 at baseline [ 18 F]FDG PET/CT was significantly correlated with a shorter PFS and OS.
• The combined use of baseline TMTV along with PET response during treatment allowed for the identification of three groups of patients with very different median OS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melanoma is a very aggressive and highly metastatic malignancy; the presence of ulceration, high Breslow thickness and loco-regional lymph node metastases at diagnosis are known adverse prognostic factors [1]. In advanced disease, the role of chemotherapy alone or in combination with immunomodulating agents such as interleukin-2 and interferon-alpha has been invariably palliative and without impact on the prognosis with a median overall survival of about 6–7 months and less than 10% of patients alive at 5 years [2]. The clinical outcome of melanoma is dramatically changed upon the introduction of new highly active approaches such as immune checkpoint inhibitors (ICIs) like anti-PD1 and anti-CTLA4 antibodies and targeted therapy with BRAF inhibitors (BRAFi) ± MEK inhibitors (MEKi) in BRAF-mutated patients with 5-year OS of about 40–50% [3]. Target therapy with BRAFi/MEKi is characterised by a high rate of response of around 50–55% for BRAFi treatment alone and 65–70% for the combination of both inhibitors [3]. However, resistance (either primary or acquired) develops in many patients resulting in treatment failure, with a median progression-free survival of about 11–14 months [1]. Several factors including age, sex, performance status, number of organ sites of metastases and lactate dehydrogenase (LDH) levels showed an association with progression-free survival (PFS) and overall survival (OS) [4]. [18F]FDG PET/CT is extensively used for staging, restaging and monitoring of therapy response in melanoma [5]. The rationale for its use to assess treatment response to BRAFi derives from the early reduction of FDG uptake, which occurs in metastatic melanoma cells as documented in preclinical models [6]. Indeed, it has been demonstrated that patients treated with BRAFi (vemurafenib or dabrafenib) show a significant decrease in FDG uptake within 2 weeks from starting treatment [7, 8]. On the other hand, the onset of resistance to treatment with BRAFi/MEKi in the course of treatment is often observed in daily practice by PET/CT, with very different scintigraphic patterns ranging from metabolic progression of few metastatic foci to the appearance of new disease sites associated with partial or even complete metabolic response of the existing lesions. As previously reported, several baseline factors (e.g., performance status, age, sex, number of organ metastatic sites, and lactate dehydrogenase level) can inform a priori the clinician about the likelihood of achieving and maintaining a response to BRAFi ± MEki [4], but to date, there are no studies evaluating a possible prognostic role of [18F]FDG PET/CT performed before and during treatment. The purpose of this retrospective study was to investigate if baseline total metabolic tumour volume (TMTV), a marker of tumour burden, as well as therapy-response assessment by [18F]FDG PET/CT have a prognostic role in PFS and OS in patients with advanced melanoma receiving BRAF/MEK inhibition therapy.
Patients and methods
Patients
The study was approved by the local institutional ethics committee (prot. n° 1460/21), and it was performed in accordance with ethical standards. Given the retrospective design of the study, the ethics committee allowed the use and processing of the patients’ clinical data even in the absence of written informed consent. A database search was performed for patients with metastatic cutaneous melanoma treated with targeted therapy with anti-BRAF ± anti-MEK and who underwent [18F]FDG PET/CT at our institution between June 2012 and June 2020. The choice for a BRAFi monotherapy schedule rather than the combined BRAFi + MEKi treatment depended on the approved indications at the time of initiation of the target treatment or on the clinical choice for frail patients or those with comorbidities. The treatment was administered as the first or subsequent line until disease progression or unacceptable toxicity. Eligible patients (Fig. 1) were moreover required to have undergone an [18F]FDG PET/CT scan less than 1 month before treatment onset (PET0), a second scan (PET1) 2–6 months from the start of treatment and a minimum follow-up of 6 months. Due to the known limits of the technique for the evaluation of brain metastases, patients showing exclusive metastatic brain involvement were excluded. A final cohort of 57 consecutive patients was then analysed. Serial contrast-enhanced CT or MRI scans of the head were regularly performed for brain metastasis surveillance as per the clinical practice of our institution. In the case of disease progression with BRAFi ± MEKi, patients could have been treated with subsequent anticancer therapies or best supportive care according to the treating oncologist’s clinical judgement. The study period for data collection corresponded to patients’ treatment period that lasted from the first dose of target therapy until patient discontinuation for any reason or the patient’s death, whichever comes first. Patient clinical outcome was measured by PFS and OS defined as the period starting from the date of the first treatment cycle to the date of disease progression or death, respectively. ‘Disease progression’ for PFS was considered by metabolic disease progression on PET/CT and by contrast-enhanced head MRI/CT scan for brain metastases.
[18F]FDG PET/CT imaging and response evaluation
A combined PET/CT imaging was performed using a Siemens Biograph 16 (Siemens Healthineers). Patients fasted for a minimum of 6 h before the scan, and glucose levels below 150 mg/dl were required at the time of tracer injection. PET/CT acquisition was performed 60 ±10 min after intravenous (i.v.) injection of an average dose of 5 MBq/kg (0.14 mCi/kg) of [18F]FDG. A non-contrast-enhanced whole-body CT scan was acquired for anatomic localisation and attenuation correction of PET images. The following parameters were used: 120–140 Kev, 4-mm slice thickness using CAREDose software to reduce radiation dose and optimise image quality. PET data were acquired on a 3D mode immediately after the CT scan 2–3 min for each bed position. PET images were reconstructed by an ordered subset expectation maximisation (OSEM) algorithm (TrueX, Siemens Healthineers) with point spread function modelling (3 iterations, 21 subsets). After reconstruction, the images were filtered by a Gaussian filter with a full width at half maximum of 4 mm. Analysis of PET/CT images was performed by two experienced nuclear medicine physicians, who had knowledge of patient clinical data and of imaging time, but who were blinded on patient clinical outcome. Areas of pathologic FDG uptake were assessed qualitatively and semi-quantitatively using the maximum standardised uptake value (SUVmax) of the hottest pixel within a volume of interest (VOI) placed on the lesion.
Up to five target lesions (max two per organ) were selected for analysis at PET0, choosing those with maximum FDG avidity and a functional tumour diameter larger than 8 mm. The SUVmax of the same target lesions were then calculated at PET1. The results of PET1 were compared with the baseline scan (PET0) and classified according to the EORTC criteria [9], as follows: progressive metabolic disease (PMD): increase of > 25% in the sum of SUVmax in target lesions or visible increase in the extent of FDG tumour uptake (20% in the longest dimension) or appearance of new metastatic sites; partial metabolic response (PMR): reduction of the sum of SUVmax of at least 25%; stable metabolic disease (SMD): response between PMD and PMR; and complete metabolic response (CMR): complete resolution of FDG uptake within all lesions.
The patients were dichotomised as responders (PMR and CMR) or non-responders (SMD and PMD). To assess the heterogeneity of the PET response, all lesions were individually examined and the PET study was classified as follows: homogeneous response: absence of non-responding lesions; homogeneous non-response: no lesions achieved CMR/PMR; and heterogeneous response: the simultaneous presence of responding and non-responding lesions or responding lesions alongside the appearance of new metastatic foci.
Baseline TMTV was computed with a semiautomatic software package (LIFEX v. 6.3), [10] as the sum of the metabolic volumes of all pathologic lesions. To this aim, pathologic findings were automatically identified by the software by selecting regions with a SUVmax > 2 and with a volume > 0.5 cm3; then, a contrast-based threshold was applied for volume delineation. A slightly lower SUVmax threshold than the standard provided by the software (2.2) was applied considering the low FDG uptake of metastatic skin lesions. Finally, areas of physiologic FDG uptake were then excluded by an experienced nuclear medicine physician.
Statistical analysis
Statistical analyses were performed by R software (ver. 4.0.3). Differences among PET and clinical parameters versus response to therapy were assessed by independent T test for normally distributed data (e.g. age), by Mann–Whitney U test for skewed data (e.g. SUVmax, MTV, TLG) and with chi-squared test or Fisher’s exact test for qualitative variables. Parameters were dichotomised for survival analysis using the maximally selected rank statistics (Package ‘maxstat’ in R). PFS and OS curves were analysed by the Kaplan–Meier method and tested for statistical significance using the log-rank test. A Cox proportional-hazard univariate regression analysis was performed to determine hazard ratios (HRs) of prognostic factors for PFS and OS. A multivariate Cox proportional-hazard model was then applied to assess the potential independent effects of prognostic factors, including all variables statistically significant (p < 0.05) in the univariate model.
RESULTS
Baseline patient characteristics and treatment
In total, 20 out of 57 patients (35.1%) showed more than two metastatic organ sites, 80% of whom also had elevated LDH values (p < 0.001). The most frequent metastatic sites were lymph nodes (73.7%) followed by skin/subcutis (49.1%), lung (36.5%), bone (26.3%) and liver (22.8%). A total of 20 patients received monotherapy with a BRAFi (vemurafenib or dabrafenib); 37 were treated with a combination of a BRAFi + MEKi (vemurafenib + cobimetinib or dabrafenib + trametinib). In total, 47 patients received the BRAFi as a first-line treatment, while the remaining patients were previously treated with ICIs (ipilimumab n = 6, nivolumab n = 2, pembrolizumab n =1) or chemotherapy (n = 1). The characteristics of the patients enclosed in the analysis are summarised in Table 1.
PET/CT response assessment
A total of 157 target lesions were analysed. The percentage of response to treatment was quite variable among metastatic sites, although not statistically significant (p = 0.38), ranging from 73% for lymph nodes to 93% for bone lesions. On the contrary, other less frequent sites of disease, including the spleen and other abdominal localisations, were less likely to respond (55.5%). According to EORTC criteria, 34 patients were classified as responders (CMR in 17 patients and PMR in 17 patients) and 23 as non-responders (SMD in one patient and PMD in 22 patients) at PET1. A higher percentage of responders was found among females vs males (70.8 vs 51.5%) although not statistically significant (p = 0.178). No differences were observed for the number of disease sites, LDH values nor for the type of treatment (anti-BRAF alone vs combined anti-BRAF/MEK therapy). The median TMTV at PET0 was significantly lower in responders (28.2 cm3, interquartile range [IQR]: 9.5–79.9 cm3) versus non-responders (80.3 cm3, IQR 32–248.7 cm3; p = 0.011), while the median SUVmax at PET0 was similar in the two groups (13.4 vs 14.5). A homogeneous response on metastases at PET1 was in 32 patients, a homogeneous progression in 10 and a heterogeneous response in 15 (26.3%). In total, 19 of the 22 patients with PMD (86.4%) showed new metastatic foci at PET1, 11 of them (57.9%) had concurrent progression on existing metastases and eight (42.1%) showed a partial or complete metabolic response on existing disease sites (Fig. 2).
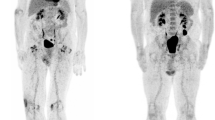
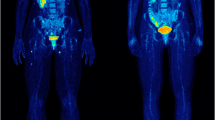
Maximum intensity projections (MIP) of [18F]FDG PET views in a 64-year-old male patient with metastatic melanoma. Baseline PET scan (a) shows multiple lung, liver and mediastinal lymph node metastases. The first evaluation PET/CT scan performed 12 weeks after starting treatment with vemurafenib + cobimetinib (b) shows almost complete disappearance of existing metastatic foci, with the appearance of pathologic lymph nodes in the right cervical and inguinal levels
Progression-free survival (PFS)
During the observation period 45 out of 57 patients showed a disease progression; 22 of them already at PET1. At the time of the present analysis, 38% and 22.9% of patients were progression-free at 12 and 36 months, respectively, with a median PFS of 7.7 months (95% CI: 4.9–15.1 months). A total of 12 patients are still on treatment with BRAFi alone (n = 4) and BRAFi ± MEKi (n = 8).
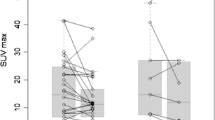
Baseline characteristics associated with a shorter PFS were more ≥ 3 metastatic organ sites and TMTV > 56 cm3. A trend was observed for the male sex, to the limits of statistical significance (p = 0.1). At the multivariate analysis (Table 2), only a high TMTV was associated with a shorter PFS (median 4.7 vs 18 months, Fig. 3). In the subgroup of responders, patients showing a CMR were significantly associated with a prolonged PFS compared with those with PMR (p = 0.009) with a median PFS of 42.9 versus 8.8 months (Fig. 3). Overall, disease progression occurred in new sites in 16 of 45 patients (35.6%), although with a significantly higher rate in patients achieving CMR (7/8, 87.5%), compared with those with PMD/SMD (8/23, 34.8%) and with PMR (1/14, 7.1%, p < 0.001). Brain and lymph nodes were the most frequent sites of disease progression in this subgroup (Table 3). After disease progression, 18 patients received a new systemic therapy line, four patients were submitted to local treatment of disease progressive sites maintaining the ongoing treatment with BRAFi/MEKi, and three patients received a combined local and systemic therapy. Finally, 20 patients who experienced a rapid disease progression with impairment of performance status were admitted to palliative care. Treatment of patients after disease progression is described in detail in Table 4.
Overall survival (OS)
In total, 34 out of 57 patients died during the observation period. The median OS was 21.5 months (95% CI: 12.2–57.5), with a survival rate of 65.7% at 1 year and 41.3% at 3 years.
At the univariate analysis, features significantly associated with a shorter OS were ≥ 3 metastatic organ sites, elevated LDH, TMTV > 56 cm3 and the lack of response at PET1. Patients showing a CMR were associated with a slightly prolonged OS compared to those with PMR, although the curves overlap after 3 years (Fig. 4a). In a multivariate analysis, TMTV, LDH and PET responses were associated with the OS (Table 2). By combining the parameters, TMTV and PET response, it was possible to identify three different prognostic classes (Fig. 4d): a very favourable prognosis (low baseline TMTV and PET response to treatment, median OS 102.2 months); a very poor outcome (high baseline TMTV and no response to treatment, median OS 6.7 months); and an intermediate prognosis (low baseline TMTV/no response to treatment, median OS 20.1 months or high baseline TMTV/response to treatment, median OS 18.3 months).
Discussion
Our retrospective study demonstrates that baseline TMTV and metabolic response may be useful prognostic indicators for PFS and OS in patients with advanced melanoma treated with BRAF/MEK inhibitors.
Although tumour response to BRAFi can be extremely rapid, most patients develop an acquired resistance to treatment due to several mechanisms occurring in neoplastic cells [11, 12]. The reactivation of the mitogen-activated protein kinase (MAPK) pathway is the most frequent mechanism of acquired resistance, and this can occur for mutation upstream and downstream of BRAF. The concomitant use of MEKi proved to be a strategy to prevent or delay resistance to BRAFi, as demonstrated in recent trials [4, 13, 14]. MEKi act through the MAPK pathway, which is immediately downstream of BRAF, and it has been identified as the gene target of several resistance mechanisms. The rapid tumour response to BRAFi has been already demonstrated in vivo by [18F]FDG PET/CT. Indeed, almost all patients treated with vemurafenib or dabrafenib show a significant decrease in FDG uptake within 2 weeks from starting treatment [7, 8], possibly reflecting the inhibition of signalling downstream of BRAF and the consequent suppression of glycolysis [6, 15]. The finding of a significant metabolic response in almost all patients makes the use of PET/CT at such an early stage of limited value as a predictive tool for patient outcome, although in the publication by McArthur et al [7], a median reduction in SUVmax of 82% correlated with PFS. In the same study, it was observed that all 27 patients showed a metabolic response to treatment (24 PMR and 3 CMR), with minimal variability in response among lesions, suggesting no evidence of drug resistance at this timing. In contrast, in a study by Carlino on 23 patients treated with dabrafenib, a heterogeneous response of lesions was observed in 26% of cases [8]. The intra-patient heterogeneity of PET response was confirmed in our cohort with a similar rate (15/57, 26.3%). Interestingly, in half of the patients showing a PMD on PET1, disease progression was only due to the onset of new metastatic sites, while existing lesions showed at least a PMR.
To date, it is not known whether there is a relationship between the type of metabolic response and the duration of treatment response to BRAFi and MEKi. Results of this study indicated that patients achieving a CMR are significantly less prone to relapse compared with those with PMR, with a median PFS of 42.9 vs 8.8 months and a PFS rate of 87.8% vs 41.2% at 1 year and of 62.9 vs 17.6% at 3 years. This behaviour differs from that observed in a recent article on the use of [18F]FDG PET/CT to monitor ICI treatment in metastatic melanoma [16], in which all patients showing a PET response after 3–4 months from the start of treatment were progression-free at 1 year regardless of whether they achieved a PMR or a CMR, with a slight difference at 2 years (83.9 vs 100%, respectively). The type of metabolic response at the first PET/CT assessment may have a practical clinical implication, namely that patients who do not achieve a CMR could benefit from a closer clinical/radiological surveillance while on treatment for rapid therapeutic crossover in the event of progression. Interestingly, in our retrospective analysis, the high majority of patients with CMR (87.5%) relapsed in sites different from those already present before treatment; on the contrary, the high majority of the patients with PMR (92.9%) relapsed on existing lesions. It can be postulated that the persistence of FDG avidity on metastatic lesions after few months of treatment could suggest the onset of cell clonality resistance to BRAF inhibition. Lymph nodes and brain metastases were the most frequent sites of disease progression in patients showing a metabolic response in existing lesions, as previously observed in a larger cohort [17]. In particular, brain metastases were found in five of the eight patients who achieved a CMR. It is indeed known that melanoma brain metastases might have distinct molecular alterations, such as increased activation of the phosphatidylinositol 3-kinase-protein kinase B (PI3K-AKT) signalling pathway, which has also been associated with resistance to BRAFi and MEKi [18, 19]. The degree of metabolic response (CMR vs PMR) correlated with PFS but not OS. This discrepancy may be related to the small sample size and/or to the higher incidence of progression at the brain level in patients with CMR, which represents a poor prognostic factor for survival [20].
Baseline TMTV represents a surrogate of tumour burden, and it has been demonstrated to be a strong prognostic imaging marker in several oncologic conditions [21,22,23,24]. The prognostic value of baseline TMTV was described in a cohort of 142 melanoma patients treated with ipilimumab, where the median survival of patients showing a TMTV above the median (26.85 cm3) was 10.8 months compared with 26.0 months for patients with lower TMTV values [25]. At present, there is no clear definition of tumour burden in patients with metastatic melanoma; therefore, the number of metastatic organ sites and LDH are the only parameters currently used to determine which treatment should be used as first line [26, 27]. In the present study, the TMTV had a low accuracy to predict response to treatment (positive predictive value 60.8%; negative predictive value 67.6%), but it was found to be a strong prognostic factor for both PFS and OS. Interestingly, only TMTV and LDH remain independent prognostic factors on multivariate analysis, as opposed to the number of metastatic organ sites, which is strongly associated with PFS and OS overall survival in previous reports [14, 17, 22]. TMTV seems to have a greater prognostic weight than LDH for the OS (HR 4.4 vs 2.5), as shown by a trend of the curves in Fig. 4b, c. If the prognostic value of TMTV will be confirmed in prospective studies with larger populations, it could be proposed to select high-risk patients who can benefit from combination therapies with ICIs plus BRAFi [28, 29], although these treatment protocols are not currently recommended outside of clinical studies [27].
Analysis of survival curves showed that the prognostic strength of baseline TMTV is comparable to that of PET response to treatment, so that by combining them together, it was possible to stratify patients into three prognostic categories: a group with a very favourable prognosis for low baseline TMTV and PET response to treatment (median OS 102.2 months), a cohort with a very poor outcome in case of high baseline TMTV and no response to treatment (median OS 6.7 months) and a group with an intermediate behaviour, when only one of the two prognostic factors was present, indifferently, as highlighted by the overlapping trend of the curves in Fig. 4c.
The present study has some drawbacks. First, the patients analysed received different schedules of treatment enclosing a BRAFi as a single agent (vemurafenib or dabrafenib) or associated with a MEKi (cobimetinib or trametinib, respectively). Secondly, the retrospective design of the study may have led to a selection bias. Another possible limitation of the study is that 10 out of 57 patients received a systemic treatment (ICIs n = 9; CHT n = 1) before target therapy with BRAFi/MEKi. Nonetheless, to the best of the authors’ knowledge, the present study is the first to demonstrate a prognostic role of baseline TMTV on PFS and OS in patients with metastatic melanoma on treatment with BRAFi/MEKi and to identify a different behaviour of disease progression in patients achieving CMR as compared to PMR.
Change history
09 March 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00330-022-08672-z
Abbreviations
- [18F]FDG PET/CT:
-
2-[18F]fluoro-2-deoxy-d-glucose positron emission/computed tomography
- BRAFi:
-
BRAF inhibitors
- CMR:
-
Complete metabolic response
- MEKi:
-
MEK inhibitors
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PMD:
-
Progressive metabolic disease
- PMR:
-
Partial metabolic response
- SMD:
-
Stable metabolic disease
- TMTV:
-
Total metabolic tumour volume
References
Michielin O, van Akkooi ACJ, Ascierto PA, Dummer R, Keilholz U, ESMO Guidelines Committee (2019) Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 30:1884–1901. https://doi.org/10.1093/annonc/mdz411
Barth A, Wanek LA, Morton DL (1995) Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg 181:193–201
Ugurel S, Röhmel J, Ascierto PA et al (2020) Survival of patients with advanced metastatic melanoma: the impact of MAP kinase pathway inhibition and immune checkpoint inhibition - update 2019. Eur J Cancer 130:126–138. https://doi.org/10.1016/j.ejca.2020.02.021
Robert C, Grob JJ, Stroyakovskiy D et al (2019) Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 381:626–636. https://doi.org/10.1056/NEJMoa1904059
Bisschop C, de Heer EC, Brouwers AH, Hospers GAP, Jalving M (2020) Rational use of 18F-FDG PET/CT in patients with advanced cutaneous melanoma: a systematic review. Crit Rev Oncol Hematol 153:103044. https://doi.org/10.1016/j.critrevonc.2020.103044
Theodosakis N, Held MA, Marzuka-Alcala A et al (2015) BRAF Inhibition decreases cellular glucose uptake in melanoma in association with reduction in cell volume. Mol Cancer Ther 14:1680–92. https://doi.org/10.1158/1535-7163.MCT-15-0080
McArthur GA, Puzanov I, Amaravadi R et al (2012) Marked, homogeneous, and early [18F]fluorodeoxyglucose-positron emission tomography responses to vemurafenib in BRAF-mutant advanced melanoma. J Clin Oncol 30:1628–34. https://doi.org/10.1200/JCO.2011.39.1938
Carlino MS, Saunders CA, Haydu LE et al (2013) (18)F-labelled fluorodeoxyglucose-positron emission tomography (FDG-PET) heterogeneity of response is prognostic in dabrafenib treated BRAF mutant metastatic melanoma. Eur J Cancer 49:395–402. https://doi.org/10.1016/j.ejca.2012.08.018
Young H, Baum R, Cremerius U et al (1999) Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 35:1773–82. https://doi.org/10.1016/s0959-8049(99)00229-4
Nioche C, Orlhac F, Boughdad S, Reuzé S, Goya-Outi J, Robert C, Pellot-Barakat C, Soussan M, Frouin F, Buvat I (2018) LIFEx: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Research 78(16):4786–4789
Trunzer K, Pavlick AC, Schuchter L et al (2013) Pharmacodynamic effects and mechanisms of resistance to vemurafenib in patients with metastatic melanoma. J Clin Oncol 31:1767–74. https://doi.org/10.1200/JCO.2012.44.7888
Rizos H, Menzies AM, Pupo GM et al (2014) BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res 20:1965–77. https://doi.org/10.1158/1078-0432.CCR-13-3122
Long GV, Flaherty KT, Stroyakovskiy D et al (2017) Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol 28:1631–1639. https://doi.org/10.1093/annonc/mdx176
Schadendorf D, Long GV, Stroiakovski D et al (2017) Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur J Cancer 82:45–55. https://doi.org/10.1016/j.ejca.2017.05.033
Parmenter TJ, Kleinschmidt M, Kinross KM et al (2014) Response of BRAF-mutant melanoma to BRAF inhibition is mediated by a network of transcriptional regulators of glycolysis. Cancer Discov 4:423–33. https://doi.org/10.1158/2159-8290.CD-13-0440
Annovazzi A, Vari S, Giannarelli D et al (2020) Comparison of 18F-FDG PET/CT criteria for the prediction of therapy response and clinical outcome in patients with metastatic melanoma treated with ipilimumab and PD-1 inhibitors. Clin Nucl Med 45:187–194. https://doi.org/10.1097/RLU.0000000000002921
Long GV, Weber JS, Infante JR et al (2016) Overall survival and durable responses in patients with BRAF V600-mutant metastatic melanoma receiving dabrafenib combined with trametinib. J Clin Oncol 34:871–878. https://doi.org/10.1200/JCO.2015.62.9345
Chen G, Chakravarti N, Aardalen K et al (2014) Molecular profiling of patient-matched brain and extracranial melanoma metastases implicates the PI3K pathway as a therapeutic target. Clin Cancer Res 20:5537–5546. https://doi.org/10.1158/1078-0432.CCR-13-3003
Amaral T, Sinnberg T, Meier F et al (2017) The mitogen-activated protein kinase pathway in melanoma part I - activation and primary resistance mechanisms to BRAF inhibition. Eur J Cancer 73:85–92. https://doi.org/10.1016/j.ejca.2016.12.010
Gutzmer R, Vordermark D, Hassel JC et al (2020) Melanoma brain metastases - interdisciplinary management recommendations 2020. Cancer Treat Rev 89:102083. https://doi.org/10.1016/j.ctrv.2020.102083
Woff E, Hendlisz A, Ameye L et al (2019) Validation of metabolically active tumor volume and total lesion glycolysis as 18F-FDG PET/CT–derived prognostic biomarkers in chemorefractory metastatic colorectal cancer. J Nucl Med 60:178–184. https://doi.org/10.2967/jnumed.118.210161
Lim R, Eaton A, Lee NY et al (2012) 18F-FDG PET/CT metabolic tumor volume and total lesion glycolysis predict outcome in oropharyngeal squamous cell carcinoma. J Nucl Med 53:1506–13. https://doi.org/10.2967/jnumed.111.101402
Van de Wiele C, Kruse V, Smeets P, Sathekge M, Maes A (2013) Predictive and prognostic value of metabolic tumour volume and total lesion glycolysis in solid tumours. Eur J Nucl Med Mol Imaging 40:290–301. https://doi.org/10.1007/s00259-012-2280-z
Kwee SA, Lim J, Watanabe A, Kromer-Baker K, Coel MN (2014) Prognosis related to metastatic burden measured by 18F-fluorocholine PET/CT in castration-resistant prostate cancer. J Nucl Med 55:905–10. https://doi.org/10.2967/jnumed.113.135194
Ito K, Schöder H, Teng R et al (2019) Prognostic value of baseline metabolic tumor volume measured on 18F-fluorodeoxyglucose positron emission tomography/computed tomography in melanoma patients treated with ipilimumab therapy. Eur J Nucl Med Mol Imaging 46:930–939. https://doi.org/10.1007/s00259-018-4211-0
Gershenwald JE, Scolyer RA, Hess KR, et al (2017) Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 67:472–492. https://doi.org/10.3322/caac.21409
Keilholz U, Ascierto PA, Dummer R et al (2020) ESMO consensus conference recommendations on the management of metastatic melanoma: under the auspices of the ESMO Guidelines Committee. Ann Oncol 31:1435–1448. https://doi.org/10.1016/j.annonc.2020.07.004
Ascierto PA, Ferrucci PF, Fisher R et al (2019) Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat Med 25:941–946. https://doi.org/10.1038/s41591-019-0448-9
Ribas A, Lawrence D, Atkinson V et al (2019) Combined BRAF and MEK inhibition with PD-1 blockade immunotherapy in BRAF-mutant melanoma. Nat Med 25:936–940. https://doi.org/10.1038/s41591-019-0476-5
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Alessio Annovazzi.
Conflict of Interest
Dr. Virginia Ferraresi: consulting or advisory role—Bristol Myers Squibb, Novartis, MSD; speaker—Bristol Myers Squibb, Novartis, Pierre Fabre; travel/accommodations: Bristol Myers Squibb, Pierre Fabre, MSD. Dott. Michelangelo Russilo: speaker—Novartis, Pierre Fabre. All remaining authors have declared no conflicts of interest.
Statistics and Biometry
Two of the authors have significant statistical expertise.
Informed Consent
Given the retrospective design of the study, the ethics committee allowed the use and processing of the patients’ clinical data even in the absence of written informed consent.
Ethical Approval
The study was approved by the local institutional ethics committee (prot. n° 1460/21) and it was performed in accordance with ethical standards.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The reference 'C Nioche, F Orlhac, S Boughdad, S Reuzé, J Goya-Outi, C Robert, C Pellot-Barakat, M Soussan, F Frouin, and I Buvat. LIFEx: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Research 2018; 78(16):4786-4789' was added.
Rights and permissions
About this article
Cite this article
Annovazzi, A., Ferraresi, V., Rea, S. et al. Prognostic value of total metabolic tumour volume and therapy-response assessment by [18F]FDG PET/CT in patients with metastatic melanoma treated with BRAF/MEK inhibitors. Eur Radiol 32, 3398–3407 (2022). https://doi.org/10.1007/s00330-021-08355-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08355-1