Abstract
Objectives
To evaluate clinical outcomes of thoracic duct embolization (TDE) for the management of postoperative chylothorax with the aid of the bail-out retrograde approach for thoracic duct cannulation (TDC).
Materials and methods
Forty-five patients with postoperative chylothorax underwent Lipiodol lymphangiography (LLG) between February 2016 and November 2019. If targetable central lymphatic vessels were identified in LLG, TDC, a prerequisite for TDE, was attempted. While the conventional antegrade transabdominal approach was the standard TDC method, the retrograde approach was applied as a bail-out method. Embolization, the last step of TDE, was performed after confirming leakages in the trans-TDC catheter lymphangiography. Technical and clinical success rates were determined retrospectively.
Results
TDC was attempted in 40 among 45 patients based on LLG findings. The technical success rate of TDC with the conventional antegrade approach was 78% (31/40). In addition, six more patients were cannulated using the bail-out retrograde approach, which raised the technical success rate to 93% (37/40). While 35 patients underwent embolization (TDE group), ten patients did not (non-TDE group) for the following reasons: (1) lack of targetable lymphatics for TDC in LLG (n = 5), (2) technical failure of TDC (n = 3), and (3) lack of discernible leakages in the transcatheter lymphangiography (n = 2). The clinical success of the TDE group was 89% (31/35), compared with 50% (5/10) of the non-TDE group. One major procedure-related complication was bile peritonitis caused by the needle passage of the distended gallbladder.
Conclusions
Bail-out retrograde approach for TDC could improve the overall technical success of TDC significantly.
Key Points
• Bail-out retrograde thoracic duct access may improve the overall technical success of thoracic duct access, thus improving the clinical success of thoracic duct embolization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Postoperative chylothorax may occur as a complication after various thoracic, cardiac, and neck surgeries [1, 2]. Low-output chylothorax (< 1000 mL/day) is typically treated by conservative means, whereas high-output states call for thoracic duct ligation, carrying substantial morbidity and mortality risk [1, 3, 4]. Once introduced by Cope et al, thoracic duct embolization (TDE) was accepted as the treatment of choice or at least as a viable alternative to surgical ligation in treating intractable postoperative chylothorax [3, 5,6,7].
Among three steps of TDE—Lipiodol lymphangiography (LLG), thoracic duct cannulation (TDC), and embolization—TDC is the major determining factor for the technical success of TDE due to its technical difficulty [3]. The previous studies reported the conventional antegrade TDC’s technical success rate to be about 70% [5, 8, 9]. Hence, significant numbers of patients for whom TDC had failed were forced to settle for the unproven therapeutic effect of Lipiodol during the LLG or thoracic duct disruption, although neither procedure is as clinically efficient as TDE is [10, 11].
Recently, novel retrograde transvenous or percutaneous cervical TDC techniques have been instituted [12,13,14,15,16]. The present study was thus conducted to evaluate clinical outcomes of TDE for the management of postoperative chylothorax with the aid of a bail-out retrograde approach for TDC.
Materials and methods
Our Institutional Review Board approved this retrospective study. The requirement to obtain informed consent was waived per board protocol. All interventional procedures were conducted by one interventionalist (S.H.) with 10-year experience of interventional radiology. Radiological and clinical data were retrospectively reviewed by three radiologists (S.H., H.J., and H.L.) in consensus.
Study population
Patients with postoperative chylothorax undergoing LLG between February 2016 and November 2019 for intended TDC and TDE were included in this study. In each instance, typical “milky” chylous effusions > 110 mg/dL were recorded. Conservative measures, including a fat-free or low-fat diet or total parenteral nutrition, failed to stop the chylous leakage.
Electronic medical records and picture archiving and communication systems (PACS) were accessed for the retrospective review of demographic data, etiologies of lymphatic leaks, initial triglyceride levels in leaked fluid, volumes of daily chest tube drainage, technical details, complications, follow-up periods, and clinical outcomes.
Interventional procedures
Intranodal Lipiodol lymphangiography
Lymph nodes of both inguinal regions were directly accessed under ultrasound guidance using a 26-gauge needle fitted with a short accessory tube and a 3-mL polycarbonate syringe. After positioning the needle tip at the transition between the nodal cortex and hilum, ethiodized oil (Lipiodol Ultra-Fluid; Guerbet) was injected manually under intermittent fluoroscopy until lymphatics along the upper lumbar region and cisterna chyli were opacified [17, 18]. If lymphatic opacification was inadequate or sluggish, iliac nodes at higher levels were accessed under fluoroscopy, as detailed in earlier reports [19].
Thoracic duct cannulation
Antegrade TDC
Once upper lumbar lymphatics and cisterna chyli were opacified, antegrade percutaneous transabdominal TDC was attempted under fluoroscopic guidance, using a 22-gauge, 15-cm or 21-gauge, 20-cm needle (Cook Medical) to puncture cisterna chyli or a prominent retroperitoneal duct. Anteroposterior projections served for needle advancement upon skin entry (at ~45° angle), using orthogonal projections for location adjustments.
Next, a relatively stiff guidewire, 0.014 in. (Command ES; Abbott Vascular) or 0.018 in. (V18 Control; Boston Scientific), was passed through the needle to cisterna chyli and into the thoracic duct. A microcatheter (Progreat Alpha; Terumo Corp) was then inserted over the guidewire to the same position. If greater traceability was needed after a hostile course of the wire passage, a dedicated supporting catheter (CXI Support Catheter [Cook Medical] or Rubicon Support Catheter [Boston Scientific]) was first engaged and later replaced by an ordinary microcatheter.
Retrograde TDC
If antegrade TDC failed, retrograde TDC was undertaken in either of two ways: direct puncture of the thoracic duct at neck level (n = 5) or transvenous access at the junction of the thoracic duct and subclavian vein (n = 6). Having the distal part of the thoracic duct immediately before entering the subclavian vein opacified by Lipiodol, a 21-gauge micropuncture needle (Micropuncture Access Set; Cook Medical) was introduced under fluoroscopic guidance for direct retrograde TDC, usually in the left supraclavicular fossa. If successful, a 0.016-in. guidewire (Asahi Meister; ASAHI INTECC Co.) and a microcatheter (Progreat Alpha; Terumo Corp) were advanced for further intervention. For transvenous retrograde access, a 5- or 6-Fr sheath was inserted into the left basilic or right common femoral vein, using a 5-F multipurpose, cobra-shaped or hook catheter; a microcatheter (Progreat Alpha, Terumo Corp); and a 0.016-in guidewire (Asahi Meister; ASAHI INTECC Co.) to reach and access the junction of the thoracic duct.
Embolization
After the successful antegrade or retrograde TDC, trans-TDC catheter lymphangiography using digital subtraction angiography function of the angiographic machine was performed while injecting 5–10 mL of iodinated water-soluble contrast (0.3–1 mL/s) via a microcatheter to identify the point(s) of leakage from the thoracic duct or its tributaries. If the hallmarks of leakage, such as extravasated contrast or lymphopseudoaneurysm, were lacking in the transcatheter lymphangiography, the final embolization step was abandoned, even after strenuous TDC because there is a possibility of symptom aggravation by occluding the most important drainage route of lymphatic fluid back to the venous system.
On the other hand, if the leakage was confirmed in the lymphangiography, Microcoils (Interlock [Boston Scientific] or Concerto [Covidien]) were deployed at the cervical portion of the thoracic duct via the established access catheter (Progreat Alpha; Terumo Corp) to prevent overflow of the glue material into the systemic vein. Then, minimal amount (less than 1 mL) of 5% dextrose water was flushed through the microcatheter system to prevent premature glue polymerization during the embolization. The whole segment of the thoracic duct was embolized by casting it with n-butyl-2-cyanoacrylate (NBCA, Histoacryl; B. Braun Medical Inc.) mixed with Lipiodol (Lipiodol Ultra-Fluid; Guerbet) at a ratio of 1:1.5 to 1:3 (mostly 1:2). The latter was added to opacify the glue and prevent premature microcatheter blockage. Injection of the NBCA/Lipiodol mixture took place while withdrawing the catheter. In the cases of retrograde TDC, the basic principle of the embolization was the same as antegrade TDC: placing Microcoils at the cervical portion of the thoracic duct, and then glue embolization of the whole segment of the thoracic duct from the lower part of the injured segment.
In selected cases, superselective embolization (rather than occlusion of the entire thoracic duct) was carried out using NBCA/Lipiodol mixture alone, when the culprit lymphatic vessel was visualized, and further superselection of the vessel with microcatheter was feasible.
Assessment of the clinical outcomes and complications
The clinical outcome was analyzed, and any medical records indicating the possible complications after the procedures were reviewed. The technical success rates of TDC procedures (overall, antegrade, and retrograde) were determined. Patients were classified as TDE group if they underwent all three steps of TDE, which are LLG, TDC, and embolization. Otherwise, they were classified as non-TDE group. The clinical success was defined as resolving the lymphatic leakages within 2 weeks after the final TDE attempts, regardless of its technical success. The clinical success rates of TDE and non-TDE groups were compared, respectively.
Results
Overall, 45 patients (men, 28; women, 17; mean age, 62 years) were referred for treatment during the study period (Fig. 1). In Table 1, underlying pathologic conditions and original operations performed are listed. Four patients demonstrated secondary chylous leakage into surgical neck wounds, and three showed coexisting chylous ascites.
Flowchart of the therapeutic process. Lipiodol lymphangiography was performed in all 45 referred patients who suffered from high-output postoperative chylothorax. Thoracic duct cannulation (TDC), which is the prerequisite step before the embolization, was pursued only when the targetable central lymphatic structure such as cisterna chyli or thoracic duct is seen in the Lipiodol lymphangiography (n = 40). The antegrade approach through the transabdominal route was the standard method. The retrograde approach was applied if the antegrade approach was unsuccessful. This bail-out procedure increased the overall technical success rate of TDC from 78 to 93% by salvaging six failed patients
Technical success of TDC
Antegrade TDC
Intranodal lymphangiography was successful in all patients (45/45, 100%), but TDC was attempted in 40 among 45 patients based on LLG findings; TDC was attempted in five patients due to a lack of targetable lymphatics (i.e., cisterna chyli or thoracic duct). One patient had a history of prior pelvic node dissection and was devoid of opacified inguinal lymph nodes; three patients showed interruptions of the thoracic duct; and another harbored a lymphopseudoaneurysm below cisterna chyli, without any points of thoracic duct leakage.
The technical success rate of antegrade TDC was 78%, succeeding in 31 of 40 patients. TDE was then performed in 30 of these 31 patients (96%).
Retrograde TDC
Retrograde TDC was attempted in 11 patients as bail-out procedures (n = 8) after difficult or failed standard antegrade TDC efforts or as adjunctive measures (n = 3) (Fig. 2). The bail-out procedures were successful in 6 of 8 cases. The technical success rate of retrograde TDC was 82% (9/11). In one patient for whom retrograde TDC failed, repeat antegrade TDC led to successful TDE during a subsequent separate session.
Overall TDC
As a result of bail-out retrograde TDC, the overall technical success rate of TDC improved to 93% (37/40) in the subgroup of patients subjected to TDC by achieving success in additional six patients. On an intention-to-treat basis, regarding non-attempts as failures, the success rate was 82% (37/45) (Table 2).
Clinical success of TDE
In 37 patients with successful TDC, 35 proceeded to embolization (TDE group) either in the form of extensive embolization of thoracic duct (n = 27) or superselective (n = 8) embolization of culprit lymphatic channels (Fig. 3). TDE was abandoned even after the successful TDC in two patients, who showed no evidence of leakage in LLG or trans-TDC catheter lymphangiography.
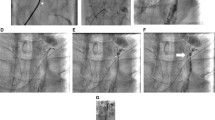
Obtained images during superselective embolization: (a) digital subtraction angiography showing contrast leakage (arrowhead) with lymphopseudoaneurysm by a branch of the thoracic duct; and (b) fluoroscopic spot image after selective embolization of the culprit branch with NBCA/Lipiodol mixture alone (arrow) while preserving main thoracic duct (white arrow)
Clinical success was 89% (31/35) in the TDE group. Among the four patients regarded as clinical failures, three patients were able to remove chest drains more than 2 weeks after the final TDE attempts. However, the other patient died from the medical complication of persistent lymphatic leakage.
Ten patients did not undergo TDE (non-TDE group) due to (1) lack of targetable central lymphatics in LLG (n = 5), (2) technical failures of TDC (n = 3), or (3) lack of discernible leakage in trans-TDC catheter lymphangiography (n = 2). The clinical success rate in this group was 50% (5/10).
Based on intention-to-treat, the overall clinical success was 80% (36/45).
Procedural complications
Only one major procedural complication was encountered during the follow-up period (average 434 days). The patient complained of extreme right upper quadrant pain immediately after successful TDE, likely due to bile peritonitis. Emergency computed tomography revealed a dilated gallbladder with scant surrounding fluid at the midsection. Following cholecystostomy performed on the next day, the abdominal pain resolved. There were also five minor procedure-related complications. Four patients developed fever that responded to conservative treatment, and one experienced asymptomatic non-target glue embolization of the pulmonary artery, which was asymptomatic.
Discussion
Wire insertion and catheterization of central lymphatic channels during TDC are the most technically challenging aspects of the three-step (LLG → TDC → embolization) process of TDE. Failure at this juncture accounts for the majority of technical failures [9]. Even after the failure of TDC, LLG alone is known to have some degree of potential therapeutic effects on lymphatic leakage in one meta-analysis data [9]. Furthermore, thoracic duct disruption was performed as a bail-out procedure to overcome the technical failure of TDC in some previous studies [3, 10, 11, 20]. Its pooled clinical success rate was 60.8% in the meta-analysis, but this is considerably lower than that of TDE (79.4%) and only slightly higher than that of LLG (56.6%) in the same analysis [5, 9, 10]. Thus, the clinical outcomes of TDE can be significantly enhanced only by bolstering the technical success of TDC.
According to previous reports, antegrade TDC’s technical success rates range from 60 to 80% [5, 8, 9]. Herein, we achieved a comparable rate (78%) that may have edged higher through repeated antegrade TDC attempts. The main issue with antegrade TDC is poor opacification of lymphatic targets (i.e., cisterna chyli or thoracic duct) due to insufficient Lipiodol injection or washout of cisternal Lipiodol by a rapid inflow of non-opacified intestinal lymphatic fluid. Large body habitus is yet another obstacle that reduces fluoroscopic image quality. Once initial wire insertion fails, the punctured Lipiodol-filled lymphatics also begin to leak, further impeding the visualization and lymphatic targeting.
Retrograde TDC may be a welcome alternative in these problematic situations because it is unaffected by washout of Lipiodol or large body habitus. Despite the dearth of information on this relatively new technique, our data indicate a viable approach with a fair (82%) technical success rate. Nevertheless, antegrade TDC remains the first choice, reserving retrograde TDC for alternate or bail-out use. This convention is based on flow considerations and valvular hurdles when passing a wire through cervical thoracic duct segments via retrograde direction. In our study, 93% of overall TDC technical success could be achieved by combining antegrade transabdominal access and bail-out retrograde approach.
Superselective embolization of damaged lymphatics, rather than complete occlusion of the thoracic duct, was performed in eight of our patients. All of them recovered without any complications or recurrence of symptoms. Unlike arterial bleeding, in which such practice is routinely pursued, the standard approach to TDE entails extensive segmental blockade of the thoracic duct. Although proven safe in most traumatic chylothorax cases, the potential complications of TDE, such as chylous ascites, lower extremity lymphedema, and protein-losing enteropathy, are well-known. These complications largely result from related pressure increases, because the thoracic duct is the primary lymphatic conduit to the venous system [8, 21,22,23,24]. In theory, selective embolization of the thoracic duct may be preferable to conventional TDE in that thoracic duct flow is sustained, causing no temporary pressure elevations. Further investigation is warranted to identify the actual benefits and the future role of this particular strategy.
Because the conventional antegrade approach for TDC involves the penetration of a long needle through the intervening abdominal organs before it reaches the deeply seated thoracic duct, cisterna chyli, or their tributaries, some practitioners still do not feel comfortable with this conventional route of access [25,26,27], regardless of the existing published reports on its safety. However, one major complication in our series warranted special attention. Bile peritonitis triggered extreme abdominal pain immediately after successful TDE, requiring emergency percutaneous cholecystostomy. Even though needle penetration of various abdominal organs during TDC is generally innocuous, the gallbladder is undoubtedly the one organ to avoid [28]. Therefore, pre-procedural computed tomography or ultrasound screening for anomalous central location or dilation of the gallbladder is advised.
A limitation of this study is its retrospective design. Some patients were transferred to our institute expressly only for TDE procedure and have returned to outside hospitals for clinical follow-up. We lost two patients to follow-up and could not retrieve sufficient post-procedural information. Also, the availability of comprehensive follow-up data was highly variable, so long-term complications, such as lower extremity edema, could not be assessed in terms of prevalence.
Conclusion
Bail-out retrograde approach for TDC could improve the overall technical success of TDC significantly. Successful TDE resulted in a quick resolution of postoperative chylothorax in the majority of patients.
Abbreviations
- LLG:
-
Lipiodol lymphangiography
- PACS:
-
Picture archiving and communication systems
- TDC:
-
Thoracic duct cannulation
- TDE:
-
Thoracic duct embolization
References
Cerfolio RJ, Allen MS, Deschamps C, Trastek VF, Pairolero PC (1996) Postoperative chylothorax. J Thorac Cardiovasc Surg 112:1361–1366
Dougenis D, Walker WS, Cameron EW, Walbaum PR (1992) Management of chylothorax complicating extensive esophageal resection. Surg Gynecol Obstet 174:501–506
Cope C, Kaiser LR (2002) Management of unremitting chylothorax by percutaneous embolization and blockage of retroperitoneal lymphatic vessels in 42 patients. J Vasc Interv Radiol 13:1139–1148
Merigliano S, Molena D, Ruol A et al (2000) Chylothorax complicating esophagectomy for cancer: a plea for early thoracic duct ligation. J Thorac Cardiovasc Surg 119:453–457
Itkin M, Kucharczuk JC, Kwak A, Trerotola SO, Kaiser LR (2010) Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients. J Thorac Cardiovasc Surg 139:584–589
Nadolski GJ, Itkin M (2013) Thoracic duct embolization for nontraumatic chylous effusion: experience in 34 patients. Chest 143:158–163
Boffa DJ, Sands MJ, Rice TW et al (2008) A critical evaluation of a percutaneous diagnostic and treatment strategy for chylothorax after thoracic surgery. Eur J Cardiothorac Surg 33:435–439
Laslett D, Trerotola SO, Itkin M (2012) Delayed complications following technically successful thoracic duct embolization. J Vasc Interv Radiol 23:76–79
Kim PH, Tsauo J, Shin JH (2018) Lymphatic interventions for chylothorax: a systematic review and meta-analysis. J Vasc Interv Radiol 29:194–202
Pamarthi V, Stecker MS, Schenker MP et al (2014) Thoracic duct embolization and disruption for treatment of chylous effusions: experience with 105 patients. J Vasc Interv Radiol 25:1398–1404
Binkert CA, Yucel EK, Davison BD, Sugarbaker DJ, Baum RA (2005) Percutaneous treatment of high-output chylothorax with embolization or needle disruption technique. J Vasc Interv Radiol 16:1257–1262
Mittleider D, Dykes TA, Cicuto KP, Amberson SM, Leusner CR (2008) Retrograde cannulation of the thoracic duct and embolization of the cisterna chyli in the treatment of chylous ascites. J Vasc Interv Radiol 19:285–290
Koike Y, Hirai C, Nishimura J, Moriya N, Katsumata Y (2013) Percutaneous transvenous embolization of the thoracic duct in the treatment of chylothorax in two patients. J Vasc Interv Radiol 24:135–137
Chung A, Gill AE, Rahman FN, Hawkins CM (2015) Retrograde thoracic duct embolization in a pediatric patient with total cavopulmonary connection and plastic bronchitis. J Vasc Interv Radiol 26:1743–1746
Kariya S, Nakatani M, Ueno Y et al (2018) Transvenous retrograde thoracic ductography: initial experience with 13 consecutive cases. Cardiovasc Intervent Radiol 41:406–414
Guevara CJ, Rialon KL, Ramaswamy RS, Kim SK, Darcy MD (2016) US–guided, direct puncture retrograde thoracic duct access, lymphangiography, and embolization: feasibility and efficacy. J Vasc Interv Radiol 27:1890–1896
Kariya S, Komemushi A, Nakatani M, Yoshida R, Kono Y, Tanigawa N (2014) Intranodal lymphangiogram: technical aspects and findings. Cardiovasc Intervent Radiol 37:1606–1610
Nadolski GJ, Itkin M (2012) Feasibility of ultrasound-guided intranodal lymphangiogram for thoracic duct embolization. J Vasc Interv Radiol 23:613–616
Pieper CC, Hur S, Sommer C-M et al (2019) Back to the future: lipiodol in lymphography—from diagnostics to theranostics. Invest Radiol 54:600–615
Le Pimpec-Barthes F, D’Attellis N, Dujon A, Legman P, Riquet M (2002) Chylothorax complicating pulmonary resection. Ann Thorac Surg 73:1714–1719
Christodoulou M, Ris H-B, Pezzetta E (2006) Video-assisted right supradiaphragmatic thoracic duct ligation for non-traumatic recurrent chylothorax. Eur J Cardiothorac Surg 29:810–814
Raguse JD, Pfitzmann R, Bier J, Klein M (2007) Lower-extremity lymphedema following neck dissection–an uncommon complication after cervical ligation of the thoracic duct. Oral Oncol 43:835–837
Marshall WH Jr, Neyazaki T, Abrams HL (1965) Abnormal protein loss after thoracic-duct ligation in dogs. N Engl J Med 273:1092–1094
Le Pimpec-Barthes F, Pham M, Jouan J, Bel A, Fabiani J-N, Riquet M (2009) Peritoneoatrial shunting for intrac chylous ascites complicating thoracic duct ligation. Ann Thorac Surg 87:1601–1603
Jayasinghe SA, Srinivasa RN, Hage AN, Gemmete JJ, Majdalany BS, Chick JFB (2018) Thoracic duct embolization: analysis of practice patterns. Ann Vasc Surg 52:168–175
Hur S, Shin JH, Lee IJ et al (2016) Early experience in the management of postoperative lymphatic leakage using Lipiodol lymphangiography and adjunctive glue embolization. J Vasc Interv Radiol 27:1177–1186
Hur S (2020) Facing the truth: penetration of vital organs during thoracic duct embolization. J Vasc Interv Radiol 31:80–81
Schild HH, Pieper CC (2020) Where have all the punctures gone? An analysis of thoracic duct embolizations. J Vasc Interv Radiol 31:74–79
Acknowledgements
This research was supported by the Basic Science Research Program of the National Research Foundation of Korea (Grant No. NRF-2018R1C1B6007875), funded by the Ministry of Science, ICT and Future Planning.
Funding
This study has received funding by the Ministry of Science, ICT and Future Planning of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Saebeom Hur.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board (IRB number: 1711-080-900).
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jun, H., Hur, S., Jeong, Y.S. et al. Thoracic duct embolization in treating postoperative chylothorax: does bail-out retrograde access improve outcomes?. Eur Radiol 32, 377–383 (2022). https://doi.org/10.1007/s00330-021-08145-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08145-9