Abstract
Objectives
A few studies suggest a significant prognostic value of silent myocardial ischaemia detected in asymptomatic patients. However, the current guidelines do not recommend stress testing in asymptomatic individuals. To assess the long-term prognostic value of vasodilator stress perfusion cardiovascular magnetic resonance (CMR) in asymptomatic individuals without known coronary artery disease (CAD).
Methods
Between 2009 and 2011, a retrospective cohort study with a median follow-up of 9.2 years (interquartile range: 7.8–9.6) included 1,027 consecutive asymptomatic individuals with ≥ 2 cardiovascular risk factors but without known known CAD referred for stress CMR. Major adverse cardiovascular events (MACE) included cardiovascular mortality and nonfatal myocardial infarction (MI).
Results
Among 1,027 asymptomatic subjects, 903 (87.9%) (mean age 70.6 ± 12.4 years and 46.2% males) completed the follow-up, and 91 had MACE (10.1%). Using Kaplan-Meier analysis, silent ischaemia and unrecognised MI were associated with MACE (hazard ratio [HR]: 8.70; 95% CI: 5.79–13.10 and HR: 3.40; 95% CI: 2.15–5.38, respectively; both p < 0.001). In multivariable stepwise Cox regression, silent ischaemia and unrecognised MI were independent predictors of MACE (HR: 6.66; 95% CI 4.41–9.23; and HR: 2.42; 95% CI 1.23–3.21, respectively; both p < 0.001). The addition of silent ischaemia and unrecognised MI led to improved model discrimination for MACE (change in C statistic from 0.66 to 0.82; NRI = 0.497; IDI = 0.070).
Conclusions
Silent ischaemia and unrecognised MI are good long-term predictors for the incidence of MACE in selected asymptomatic individuals with multiple risk factors and without known CAD. These stress CMR parameters have incremental long-term prognostic value to predict MACE over traditional risk factors.
Key Points
• Silent ischaemia and unrecognised myocardial infarction defined by stress CMR are good long-term predictors of cardiovascular events in asymptomatic individuals without known coronary artery disease.
• The addition of stress cardiac MR imaging led to improved model discrimination for cardiovascular events over traditional risk factors in this specific population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary artery disease (CAD) represents a leading cause of morbidity and mortality worldwide [1]. As the healthcare costs associated with CAD were projected to double between 2015 and 2030 [1], risk stratification and primary prevention of individuals without known CAD is crucial. Several studies have demonstrated the cost-effectiveness of stress cardiovascular magnetic resonance (CMR) for risk stratification in symptomatic patients with known or suspected CAD [2,3,4,5]. However, there are very few data assessing the prognostic value of silent ischaemia detected by stress CMR in asymptomatic subjects at high cardiovascular risk [6, 7]. Current American and European guidelines do not recommend systematic stress testing in asymptomatic individuals [8,9,10], except in high-risk diabetics [11]. These guidelines rely on studies that included symptomatic patients or patients with a low prevalence of silent ischaemia [12, 13]. The prevalence of silent ischaemia is highly variable ranging between 2 and 46% depending on the number of risk factors [7, 14,15,16]. Several studies have shown that asymptomatic patients with silent ischaemia have the same or even higher cardiovascular risk than symptomatic patients with typical angina [12, 13]. Therefore, risk stratifying asymptomatic subjects could be beneficial to manage therapeutic strategy and prevention.
CMR imaging has emerged as an accurate technique to assess myocardial ischaemia and scar without ionising radiation [2, 3, 17]. Although a recent study suggests that silent ischaemia by stress CMR can predict cardiovascular events in asymptomatic individuals [6], targeted prognostic data are scarce and dedicated subgroup analyses have not been performed [2, 18, 19]. This study aimed to assess the long-term prognostic value of vasodilator stress perfusion CMR in asymptomatic individuals without known CAD.
Methods
Study population
Between December 2009 and December 2011, we conducted a single-centre retrospective study of consecutive asymptomatic individuals without known CAD, referred for vasodilator stress perfusion CMR. Subjects were included if they had ≥ 2 cardiovascular risk factors including age > 50 years for men or > 60 years for women, diabetes, hypertension, smoking, dyslipidaemia, family history of CAD, and obesity defined by body mass index (BMI) ≥ 30 kg/m2. Patients with a known stenosis ≥ 50% on at least 1 epicardial coronary artery on invasive coronary angiography or computed tomography angiography; patients with a positive functional test; patients with a history of revascularisation, defined by previous percutaneous coronary intervention or coronary artery bypass graft; and patients with prior myocardial infarction (MI) and prior hospitalisation for heart failure or LV dysfunction were excluded. Other exclusion criteria are detailed in Supplementary Material 1. Clinical data were collected according to medical history and clinical examination on the day of stress CMR. The absence of symptoms was checked by a senior cardiologist on the day of stress CMR. All patients gave informed written consent for clinical CMR examination and enrolment in the clinical research study. The study was approved by the local Ethic Committee of our Institutions and conducted in accordance with the Declaration of Helsinki. This study followed the STROBE reporting guideline for cohort studies [20].
Patients follow-up and clinical outcome
The follow-up consisted of a clinical visit as part of usual care (71%) or by direct contact with the patient or the referring cardiologist (29%). Data collection was ended in January 2020. Cardiovascular events were checked by medical reports collected from the corresponding hospitals. Cardiovascular mortality was defined using the electronic French National Registry of Death (Institut National de la Statistique et des Etudes Economiques, INSEE registry). The primary endpoint was the occurrence of at least one of the combined major adverse clinical events (MACE) defined as cardiovascular mortality or nonfatal MI. The secondary endpoint was cardiovascular mortality. Nonfatal MI was defined by typical angina of ≥ 20-min duration, ECG changes, and a rise in troponin or creatine kinase level above the 99 percentile of the upper reference limit [21]. Cardiovascular mortality was defined as sudden cardiac death with documented fatal arrhythmias or any death immediately preceded by acute MI, acute or exacerbation of heart failure, or stroke. All clinical events were defined according to standardised definitions [22]. Late coronary revascularisation was defined by a revascularisation occurring > 90 days after CMR. For patients who underwent PCI < 90 days after the index examination, peri-procedural events (MI or cardiovascular mortality) were not included in the analysis.
CMR protocol
The detailed CMR protocol has been published in previous studies [23,24,25] and detailed in Supplementary Material 2. Briefly, CMR was performed in a dedicated CMR laboratory on a 1.5-T scanner (MAGNETOM Espree, Siemens). Vasodilation was induced with dipyridamole injected at 0.84 mg/kg over 3 min. After a bolus of gadolinium-based contrast agent (0.1 mmol/kg), stress perfusion imaging was performed using an ECG-triggered saturation-prepared balanced steady-state free precession sequence. A series of six slices (four short-axis views, a 2-chamber, and a 4-chamber view) were acquired every other heartbeat. Ten minutes after contrast injection, breath-hold contrast-enhanced 3D T1-weighted inversion recovery gradient echo sequence was acquired to detect late gadolinium enhancement (LGE).
CMR image analysis
LV volumes and function were quantified on the short-axis cine stack (syngo.via, Siemens). Stress perfusion and LGE images were evaluated according to the 17-segment model of the American Heart Association [26]. The analysis of perfusion images was performed visually by two experienced cardiologists (J.G. and F.S.) blinded to follow-up data. Silent ischaemia was defined as a subendocardial or transmural perfusion defect that (1) occurred in at least one myocardial segment, (2) persisted for at least three phases beyond peak contrast enhancement, (3) followed a coronary distribution, and (4) in the absence of co-location with LGE [18, 27]. An unrecognised MI was defined by LGE with ischaemic patterns defined by subendocardial or transmural LGE [28]. A myocardial segment was considered viable if the LGE thickness was < 50% of the myocardial wall [29]. The total number of ischaemic segments was assessed visually in each patient.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD), categorical variables as frequency with percentage, and follow-up as median and interquartile range (IQR). Patients with and without silent ischaemia were compared using the Student’s t test or the Wilcoxon rank-sum test for continuous variables and the chi-square or Fisher’s exact test for categorical variables. Cumulative incidence rates of the outcomes were estimated using the Kaplan-Meier method and compared with the log-rank test. Data of patients who were lost to follow-up were censored at the time of the last contact. Cox proportional hazards methods were used to identify the predictors of MACE among patients with and without silent ischaemia. The assumption of proportional hazards ratio (HR) was verified. The different multivariable models used for adjustment were as follows:
-
Model 1: used a stepwise forward Cox regression strategy to select the strongest parsimonious set of clinical covariates for MACE and cardiovascular mortality, with a p value ≤ 0.2 on univariable screening (without silent ischaemia and unrecognised MI).
-
Model 2: model 1 + presence of silent ischaemia and unrecognised MI.
-
Model 3: included the following traditional cardiovascular risk factors: age, male, BMI, hypertension, diabetes mellitus, current or previous smoking, dyslipidaemia, and LV ejection fraction (LVEF).
-
Model 4: model 3 + presence of silent ischaemia and unrecognised MI.
The discriminative capacity of each model for predicting MACE was determined according to the Harrell’s C-statistic before and after addition of silent ischaemia and MI. The additional predictive value of silent ischaemia and MI was calculated by the Harrell’s C-statistic increment, the continuous net reclassification improvement (NRI), and the integrative discrimination index (IDI).
In competitive risk analysis, cumulative incidence functions were used to display the proportion of patients with the event of interest or the competing event (non-fatal MI or cardiovascular mortality) as time progressed, and the Fine and Gray regression model was used for the subdistribution hazard. A two-tailed p value < 0.05 was considered statistically significant. Statistical analysis was performed using R software, version 3.3.1 (R Project for Statistical Computing).
Results
Patients’ characteristics
Among the 6,095 individuals referred for dipyridamole stress CMR during the inclusion period, 1,027 (16.8%) were asymptomatic and without known CAD. The flowchart of the study participants is depicted in Fig. 1. Overall, 903 asymptomatic patients without known CAD completed the clinical follow-up and constituted our study cohort. Baseline patient characteristics and baseline CMR data are presented in Table 1. Among the 903 patients (46.2% males, mean age = 70.6 ± 12.4 years), 65.8% had hypertension, 49.1% dyslipidaemia, 43.0% diabetes mellitus, 42.8% obesity, and 24.5% a family history of CAD and 23.9% were smokers.
CMR study
Of 1,027 asymptomatic patients without known CAD, 982 (95.6%) completed the stress CMR protocol. Reasons for failure to complete CMR are detailed in the study flowchart (Fig. 1). No patient died during or shortly after CMR and there was one case of unstable angina. Detailed safety results are presented in Supplementary Material 3.
CMR analysis
In the study cohort, mean LVEF was 62.0 ± 9.1%. Patients with inducible ischaemia had a lower mean LVEF than patients without inducible ischaemia (56.8 ± 8.1% vs 62.7 ± 9.2%, p < 0.001, respectively). An unrecognised MI was diagnosed in 96 (10.6%) patients, and the presence of silent ischaemia was detected in 110 (12.2%) patients (Fig. 2). Among the 96 patients with unrecognised MI, 31 (32.3%) had silent ischaemia. Patients with silent ischaemia were older (73.5 ± 11.3 vs. 70.2 ± 12.5 years, p < 0.001), more frequently males (59.1% vs. 43.1%, p < 0.001), and presented a higher cardiovascular risk using the 10-year risk for fatal CAD score (3.1 [1.4–6.2]% vs. 2.0 [0.7–5.1]%, p < 0.001) [30], and the Framingham risk score > 20% of risk of CAD at 10 years (82.7% vs. 64.6%, p < 0.001) [31]. Among the 388 diabetics, 40 (10.3%) had silent ischaemia and 29 (7.5%) unrecognised MI. Of the 110 patients with silent ischaemia, 69 (62.7%) had a coronary angiography with early revascularisation < 90 days after CMR. Among those, 2 patients were censored due to the recurrence of MI or cardiovascular mortality within 90 days after CMR.
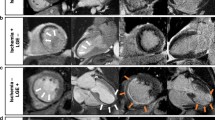
Examples of silent ischaemia and unrecognised MI on stress CMR in asymptomatic patients. Panel a: normal. Fifty-nine-year-old male without known CAD but with diabetes, hypertension, and active smoking. Stress CMR revealed no perfusion defect and LGE was negative, ruling out the diagnosis of CAD. Panel b: silent ischaemia. Sixty-nine-year-old female without known CAD but with obesity, diabetes, and hypertension. Stress CMR showed a subendocardial perfusion defect on the inferior wall on first-pass perfusion images (white arrows) without myocardial scar on LGE sequences, indicative of silent myocardial ischaemia. Coronary angiography revealed high-grade stenoses of the RCA. Panel c: unrecognised MI. Sixty-two-year-old female without known CAD but with CAD heredity, dyslipidaemia, and hypertension. Stress CMR showed a subendocardial inferior unrecognised MI on LGE (orange arrows), with a colocalisation of the perfusion defect (white arrows) and, therefore, no inducible ischaemia. Coronary angiography confirmed the chronic occlusion of the RCA and the absence of other significant stenosis. Abbreviations: CAD, coronary artery disease; CMR, cardiovascular magnetic resonance; LAD, left anterior descending; LGE, late gadolinium enhancement; MI, myocardial infarction; RCA, right coronary artery
Prognostic value
Median (IQR) follow-up was 9.2 (7.8–9.6) years. There were 91 MACE (10.1%), including 72 cardiovascular mortality (8.0%) and 19 nonfatal MI (2.1%). Furthermore, 121 all-cause mortality (13.4%), 55 late coronary revascularisations (6.1%), 12 hospitalisations for heart failure (1.3%), and 10 sustained documented ventricular tachycardia (1.1%) were recorded. Annualised event rates were 2.2% for MACE, 1.1% for cardiovascular mortality, and 2.9% for all-cause mortality.
The univariable analysis of baseline individuals and CMR characteristics for the prediction of MACE and cardiovascular mortality is presented in Table 2. Age, male gender, hypertension, diabetes, the presence of silent ischaemia, the number of ischaemic segments, the presence of unrecognised MI, LVEF, and both LV end-diastolic and end-systolic volumes indexed were all significantly associated with MACE. Using Kaplan-Meier analysis, silent ischaemia and unrecognised MI were associated with the occurrence of MACE (HR: 8.70; 95% CI: 5.79–13.10; and HR: 3.40; 95% CI: 2.15–5.38, respectively; both p < 0.001) (Fig. 3, Supplement 4). In addition, silent ischaemia was associated with cardiovascular mortality (HR: 8.92; 95% CI: 5.63–14.20), nonfatal MI (HR: 7.02; 95% CI: 3.31–14.92), and all-cause mortality (HR: 4.30; 95% CI: 3.05–6.02, all p < 0.001; Supplement 5). The presence of silent ischaemia was associated with MACE in both men (HR: 11.70; 95% CI: 6.53–20.90) and women (HR: 5.32; 95% CI: 2.74–10.30, both p < 0.001; Fig. 4).
Kaplan-Meier curves for MACE and cardiovascular mortality stratified by the presence of silent ischaemia (a and b, respectively) or by the presence of unrecognised MI (c and d, respectively). Kaplan-Meier curves of MACE (cardiovascular mortality or nonfatal MI) as a function of length of follow-up for those with and without myocardial ischaemia for the study population. Test comparing the two groups is based on the log-rank test
Kaplan-Meier curves for MACE stratified by the presence of ischaemia in women (a) and men (b). Kaplan-Meier curves of MACE (cardiovascular mortality or nonfatal MI) as a function of length of follow-up for women (a) and men (b) with and without myocardial silent ischaemia. Test comparing the two groups is based on the log-rank test
In multivariable stepwise Cox regression (model 2), the presence of silent ischaemia and unrecognised MI were independent predictors of a higher incidence of MACE (HR: 6.66; 95% CI 4.41–9.23; and HR: 2.42; 95% CI 1.23–3.21, respectively; both p < 0.001) (Table 3). Moreover, the presence of silent ischaemia and unrecognised MI were also independent predictors of cardiovascular mortality (HR: 6.21; 95% CI: 3.89–9.48; and HR: 2.19; 95% CI 1.11–3.12, respectively; both p < 0.001). In competitive risk analysis, the presence of silent ischaemia was independently associated with nonfatal MI and cardiovascular mortality (both p < 0.001) (Table 4 and Supplement 6).
The negative predictive value of the absence of silent ischaemia was homogenous regardless of the age, with an average annualised event rates of MACE of 2.2% (Supplement 7).
In patients with inducible ischaemia, early revascularisation within 90 days after CMR was not associated with significant difference in the occurrence of MACE (p = 0.77) (Fig. 5).
Incremental prognostic value of stress CMR
For the prediction of MACE, baseline C statistic values were 0.66 (95% CI, 0.62–0.69) for model 1 with stepwise variable selection and 0.72 (95% CI, 0.67–0.78) for model 3 with traditional cardiovascular risk factors. The addition of CMR-induced silent ischaemia and unrecognised MI significantly improved the C statistic to 0.82 (95% CI, 0.78–0.86; C statistic improvement for model 1: 0.16; NRI = 0.497; IDI = 0.070) and 0.79 (95% CI, 0.76–0.84; C statistic improvement for model 2: 0.07; NRI = 0.332; IDI = 0.035) (Supplement 8).
Discussion
In asymptomatic patients with cardiovascular risk factors and no known CAD, the study demonstrates that (1) the prevalence of silent ischaemia and unrecognised MI was 12.2% and 10.6%, respectively; (2) both silent ischaemia and unrecognised MI were independent long-term predictors of MACE and CV mortality; and (3) the presence of silent ischaemia and unrecognised MI improved model discrimination in predicting MACE, after adjusting for covariates or traditional cardiovascular risk factors. This is the first study showing the incremental prognostic value of stress CMR over traditional cardiovascular risk factors in this particular cohort of patients.
Prevalence of silent ischaemia and unrecognised MI
The prevalence of silent ischaemia and unrecognised MI is consistent with prior studies in patients with a similar level of cardiovascular risk [6, 14, 15, 32, 33]. The reported annualised rate of MACE (2.2%) is also in line with contemporary cohorts of patients without known CAD [5]. Similar to the Stress CMR Perfusion Imaging in the United States (SPINS) study [32], one-third of patients with unrecognised MI had silent myocardial ischaemia.
Prognostic value of silent ischaemia
The current findings confirm that silent myocardial ischaemia and unrecognised MI are good prognosticators in asymptomatic patients, as suggested by Stacey et al in a study including 347 asymptomatic patients [6]. Although some studies have emphasised the prognostic value of silent ischaemia in asymptomatic middle-aged individuals [6, 7], the current data show a good prognostic value of silent ischaemia irrespective of age.
Similarly to previous stress studies in asymptomatic patients [6, 34], the current report shows a higher prognostic value of stress CMR in men than in women, but overall demonstrates an excellent prognostic value of silent ischaemia irrespective of patient sex, which is consistent with results obtained in the general population [19].
Prognostic value of unrecognised MI
A recent multicentre cohort of patients with chest pain and suspected CAD showed that the presence of unrecognised or recognised MI portended an equally significant risk for death and/or MI [32]. The current study extends these data in asymptomatic patients with cardiovascular risk factors. In agreement, a meta-analysis assessing 2,009 participants has shown that the presence of unrecognised MI is a strong predictor of MACE and all-cause mortality in asymptomatic patients [35]. In addition, silent ischaemia and unrecognised MI improved the prediction risk model of MACE over cardiovascular risk factors after adjusting for covariates, suggesting a potential role of stress CMR in guiding the preventive management of such patients.
Risk stratification of asymptomatic patients
Although nearly a third of the cohort underwent a stress test or CCTA before the stress CMR because of a high cardiovascular risk, all subjects of the current cohort were asymptomatic. The current guidelines do not recommend stress testing in asymptomatic individuals without known CAD. But those guidelines are mostly based on studies including symptomatic patients [8,9,10, 36, 37]. In agreement with prior studies [6], the current data show that stress CMR has accurate prognostic value and excellent safety profile in asymptomatic individuals with cardiovascular risk factors [38].
This study did not show a significant effect of early revascularisation on the occurrence of MACE in patients with inducible ischaemia. These results are consistent with the ISCHEMIA trial, which strongly emphasised the roles of optimising medical therapy and the lack of benefit of an early invasive approach even in patients with chest pain [39]. However, some studies have shown a clinical interest of coronary revascularisation in patients with objective evidence of silent ischaemia [40]. In a large observational cohort study of nearly 10,000 patients with asymptomatic stable ischaemic heart disease and obstructive coronary artery disease, Czarnecki et al reported a consistent benefit of revascularisation across hospitals in patients with silent ischaemia with a reduction in mortality of 19% and in nonfatal MI of 42% at a median follow-up of 4.6 years [41]. A recent study including 1,473 patients with silent ischaemia showed a significant reduction in cardiovascular mortality at 5 years in the revascularisation group as compared with the medical therapy group (25 vs. 34%, respectively) [42].
The study suggests that the incremental prognostic value of stress CMR in asymptomatic individuals at risk could be very helpful to help optimise prevention strategies in those subjects. Whether an improved risk stratification could translate into better diagnostic and therapeutic strategies in asymptomatic individuals at risk for future cardiovascular events has yet to be demonstrated.
In patients with stable angina and risk factors for CAD, the use of stress CMR as a first-line strategy has been recently compared to an invasive approach with fractional flow reserve and was shown noninferior in terms of outcomes with a lower incidence of coronary revascularisation [43]. Along with its added prognostic value, the steadily increasing expertise and availability of stress CMR makes it a safe, reproducible, and reliable test to stratify the risk of cardiovascular events in asymptomatic patients without known CAD [44].
Study limitations
First, the study was retrospective with a risk of referral bias. There were 8.0% of patients lost to follow-up, which can be explained by a relatively long follow-up and the design of the study. The analysis of the CMR perfusion scans was visual, but it represents the most widely accepted clinical method with optimal diagnostic accuracy. Although adenosine is commonly used for stress perfusion CMR, dipyridamole was used in our centre between 2009 and 2011, as in other prognostic studies [45], mainly because of medico-economic reasons and similar or very close efficacy/safety profile compared to adenosine. Of note, there was a risk of over-estimation of both the yield and positive predictive value in a more general less highly selected cohort. Finally, this retrospective study could not capture all the confounding factors regarding the association between management decisions after the stress CMR exam and patient risks.
Conclusions
Stress perfusion CMR has a good discriminative long-term prognostic value in asymptomatic patients with cardiovascular risk factors without known CAD. Silent myocardial ischaemia and unrecognised MI are independently associated with nonfatal MI and cardiovascular mortality over a long-term follow-up and offer incremental prognostic value over traditional risk factors. Whether those findings could result in advances in decision making and ultimately turn into clinical benefits needs further evaluation.
Abbreviations
- b-SSFP:
-
Balanced steady-state free precession
- CAD:
-
Coronary artery disease
- CI:
-
Confidence interval
- CMR:
-
Cardiovascular magnetic resonance
- ECG:
-
Electrocardiogram
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricle
- MACE:
-
Major adverse cardiac events
- MI:
-
Myocardial infarction
- SD:
-
Standard deviation
References
Benjamin EJ, Muntner P, Alonso A et al (2019) Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation 139:e56–e528. https://doi.org/10.1161/CIR.0000000000000659
Heitner JF, Kim RJ, Kim HW et al (2019) Prognostic value of vasodilator stress cardiac magnetic resonance imaging: a multicenter study with 48 000 patient-years of follow-up. JAMA Cardiol 4:256–264. https://doi.org/10.1001/jamacardio.2019.0035
Kwong RY, Ge Y, Steel K et al (2019) Cardiac magnetic resonance stress perfusion imaging for evaluation of patients with chest pain. J Am Coll Cardiol 74:1741–1755. https://doi.org/10.1016/j.jacc.2019.07.074
Ge Y, Pandya A, Steel K et al (2020) Cost-effectiveness analysis of stress cardiovascular magnetic resonance imaging for stable chest pain syndromes. JACC Cardiovasc Imaging 13:1505–1517. https://doi.org/10.1016/j.jcmg.2020.02.029
Antiochos P, Ge Y, Steel K et al (2020) Evaluation of stress cardiac magnetic resonance imaging in risk reclassification of patients with suspected coronary artery disease. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.2834
Stacey RB, Vera T, Morgan TM et al (2018) Asymptomatic myocardial ischemia forecasts adverse events in cardiovascular magnetic resonance dobutamine stress testing of high-risk middle-aged and elderly individuals. J Cardiovasc Magn Reson 20. https://doi.org/10.1186/s12968-018-0492-5
Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Hansen JF (2005) Prevalence and prognostic significance of daily-life silent myocardial ischaemia in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J 26:1402–1409. https://doi.org/10.1093/eurheartj/ehi169
Knuuti J, Wijns W, Saraste A et al (2019) 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. https://doi.org/10.1093/eurheartj/ehz425
Patel MR, Bailey SR, Bonow RO et al (2012) ACCF/SCAI/AATS/AHA/ASE/ASNC/HFSA/HRS/SCCM/SCCT/SCMR/STS 2012 appropriate use criteria for diagnostic catheterization: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol 59:1995–2027. https://doi.org/10.1016/j.jacc.2012.03.003
Wolk MJ, Bailey SR, Doherty JU et al (2014) ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol 63:380–406. https://doi.org/10.1016/j.jacc.2013.11.009
Authors/Task Force Members, Rydén L, Grant PJ et al (2013) ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 34:3035–3087. https://doi.org/10.1093/eurheartj/eht108
Bonou M, Benroubis A, Kranidis A et al (2001) Functional and prognostic significance of silent ischemia during dobutamine stress echocardiography in the elderly. Coron Artery Dis 12:499–506. https://doi.org/10.1097/00019501-200109000-00009
Biagini E, Schinkel AFL, Bax JJ et al (2005) Long term outcome in patients with silent versus symptomatic ischaemia during dobutamine stress echocardiography. Heart 91:737–742. https://doi.org/10.1136/hrt.2004.041087
He ZX, Hedrick TD, Pratt CM et al (2000) Severity of coronary artery calcification by electron beam computed tomography predicts silent myocardial ischemia. Circulation 101:244–251. https://doi.org/10.1161/01.cir.101.3.244
Zellweger MJ, Haaf P, Maraun M et al (2017) Predictors and prognostic impact of silent coronary artery disease in asymptomatic high-risk patients with diabetes mellitus. Int J Cardiol 244:37–42. https://doi.org/10.1016/j.ijcard.2017.05.069
Ng M-Y, Zhou W, Vardhanabhuti V et al (2020) Cardiac magnetic resonance for asymptomatic patients with type 2 diabetes and cardiovascular high risk (CATCH): a pilot study. Cardiovasc Diabetol 19:42. https://doi.org/10.1186/s12933-020-01019-2
Knuuti J, Ballo H, Juarez-Orozco LE et al (2018) The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: a meta-analysis focused on post-test disease probability. Eur Heart J 39:3322–3330. https://doi.org/10.1093/eurheartj/ehy267
Vincenti G, Masci PG, Monney P et al (2017) Stress perfusion CMR in patients with known and suspected CAD. JACC Cardiovasc Imaging 10:526–537. https://doi.org/10.1016/j.jcmg.2017.02.006
Coelho-Filho OR, Seabra LF, Mongeon F-P et al (2011) Stress myocardial perfusion imaging by CMR provides strong prognostic value to cardiac events regardless of patient’s sex. JACC Cardiovasc Imaging 4:850–861. https://doi.org/10.1016/j.jcmg.2011.04.015
von Elm E, Altman DG, Egger M et al (2007) Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335:806–808. https://doi.org/10.1136/bmj.39335.541782.AD
Thygesen K, Alpert JS, Jaffe AS et al (2018) Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 72:2231–2264. https://doi.org/10.1016/j.jacc.2018.08.1038
Hicks KA, Tcheng JE, Bozkurt B et al (2015) 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials. J Am Coll Cardiol 66:403–469. https://doi.org/10.1016/j.jacc.2014.12.018
Pezel T, Sanguineti F, Kinnel M et al (2020) Prognostic value of dipyridamole stress perfusion cardiovascular magnetic resonance in elderly patients >75 years with suspected coronary artery disease. Eur Heart J Cardiovasc Imaging. https://doi.org/10.1093/ehjci/jeaa193
Kinnel M, Garot J, Pezel T et al (2020) Prognostic value of vasodilator stress perfusion CMR in morbidly obese patients (BMI ≥40 kg/m2) without known CAD. JACC Cardiovasc Imaging 13:1276–1277. https://doi.org/10.1016/j.jcmg.2019.12.002
Pezel T, Sanguineti F, Kinnel M et al (2020) Safety and prognostic value of vasodilator stress cardiovascular magnetic resonance in patients with heart failure and reduced ejection fraction. Circ Cardiovasc Imaging 13. https://doi.org/10.1161/CIRCIMAGING.120.01059926
Cerqueira MD, Weissman NJ, Dilsizian V et al (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105:539–542
Schwitter J, Wacker CM, van Rossum AC et al (2008) MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J 29:480–489. https://doi.org/10.1093/eurheartj/ehm617
Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ (2005) Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J 26:1461–1474. https://doi.org/10.1093/eurheartj/ehi258
Kim RJ, Wu E, Rafael A et al (2000) The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 343:1445–1453. https://doi.org/10.1056/NEJM200011163432003
Conroy RM, Pyörälä K, Fitzgerald AP et al (2003) Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 24:987–1003. https://doi.org/10.1016/s0195-668x(03)00114-3
Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB (1998) Prediction of coronary heart disease using risk factor categories. Circulation 97:1837–1847
Antiochos P, Ge Y, Steel K et al (2020) Imaging of clinically unrecognized myocardial fibrosis in patients with suspected coronary artery disease. J Am Coll Cardiol 76:945–957. https://doi.org/10.1016/j.jacc.2020.06.063
Gómez-Revelles S, Rossello X, Díaz-Villanueva J et al (2019) Prognostic value of a new semiquantitative score system for adenosine stress myocardial perfusion by CMR. Eur Radiol 29:2263–2271. https://doi.org/10.1007/s00330-018-5774-7
Greenwood JP, Motwani M, Maredia N et al (2014) Comparison of cardiovascular magnetic resonance and single-photon emission computed tomography in women with suspected coronary artery disease from the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease (CE-MARC) Trial. Circulation 129:1129–1138. https://doi.org/10.1161/CIRCULATIONAHA.112.000071
Yang Z, Fu H, Li H et al (2021) Late gadolinium enhancement is a risk factor for major adverse cardiac events in unrecognised myocardial infarction without apparent symptoms: a meta-analysis. Clin Radiol 76:79.e1–79.e11. https://doi.org/10.1016/j.crad.2020.07.038
Gibbons RJ, Abrams J, Chatterjee K et al (2003) ACC/AHA 2002 guideline update for the management of patients with chronic stable angina--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina). Circulation 107:149–158. https://doi.org/10.1161/01.cir.0000047041.66447.29
Goff DC, Lloyd-Jones DM, Bennett G et al (2014) 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129:S49–S73. https://doi.org/10.1161/01.cir.0000437741.48606.98
Monmeneu Menadas JV, Lopez-Lereu MP, Estornell Erill J, Gonzalez PG, Muñoz BI, Gonzalez AM (2016) Pharmacological stress cardiovascular magnetic resonance: feasibility and safety in a large multicentre prospective registry. Eur Heart J Cardiovasc Imaging 17:308–315. https://doi.org/10.1093/ehjci/jev153
Maron DJ, Hochman JS, Reynolds HR et al (2020) Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 382:1395–1407. https://doi.org/10.1056/NEJMoa1915922
Gada H, Kirtane AJ, Kereiakes DJ et al (2015) Meta-analysis of trials on mortality after percutaneous coronary intervention compared with medical therapy in patients with stable coronary heart disease and objective evidence of myocardial ischemia. Am J Cardiol 115:1194–1199. https://doi.org/10.1016/j.amjcard.2015.01.556
Czarnecki A, Qiu F, Elbaz-Greener G et al (2019) Variation in revascularization practice and outcomes in asymptomatic stable ischemic heart disease. JACC Cardiovasc Interv 12:232–241. https://doi.org/10.1016/j.jcin.2018.10.049
Choi KH, Lee JM, Park I et al (2019) Comparison of long-term clinical outcomes between revascularization versus medical treatment in patients with silent myocardial ischemia. Int J Cardiol 277:47–53. https://doi.org/10.1016/j.ijcard.2018.08.006
Nagel E, Greenwood JP, McCann GP et al (2019) Magnetic resonance perfusion or fractional flow reserve in coronary disease. N Engl J Med 380:2418–2428. https://doi.org/10.1056/NEJMoa1716734
Nagel E, Chandrashekhar Y (2020) Stress-Only CMR. JACC Cardiovasc Imaging 13:1296–1298. https://doi.org/10.1016/j.jcmg.2020.04.001
Pontone G, Andreini D, Bertella E et al (2016) Prognostic value of dipyridamole stress cardiac magnetic resonance in patients with known or suspected coronary artery disease: a mid-term follow-up study. Eur Radiol 26:2155–2165. https://doi.org/10.1007/s00330-015-4064-x
Acknowledgements
We thank the medical, paramedical, and research staff of Hôpital Privé J. CARTIER.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Pr Jerome Garot.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Solenn Toupin is an employee of Siemens Healthcare. Other authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 573 kb)
Rights and permissions
About this article
Cite this article
Pezel, T., Garot, P., Kinnel, M. et al. Prognostic value of stress cardiovascular magnetic resonance in asymptomatic patients without known coronary artery disease. Eur Radiol 31, 6172–6183 (2021). https://doi.org/10.1007/s00330-021-08078-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08078-3