Abstract
Objectives
There is growing evidence that sodium fluoride ([18F]fluoride) PET/CT can detect active arterial calcifications at the molecular stage. We investigated the relationship between arterial mineralization in the left common carotid artery (LCC) assessed by [18F]fluoride PET/CT and cardiovascular/thromboembolic risk.
Methods
In total, 128 subjects (mean age 48 ± 14 years, 51% males) were included. [18F]fluoride uptake in the LCC was quantitatively assessed by measuring the blood-pool-corrected maximum standardized uptake value (SUVmax) on each axial slice. Average SUVmax (aSUVmax) was calculated over all slices and correlated with 10-year risk of cardiovascular events estimated by the Framingham model, CHA2DS2-VASc score, and level of physical activity (LPA).
Results
The aSUVmax was significantly higher in patients with increased risk of cardiovascular (one-way ANOVA, p < 0.01) and thromboembolic (one-way ANOVA, p < 0.01) events, and it was significantly lower in patients with greater LPA (one-way ANOVA, p = 0.02). On multivariable linear regression analysis, age ( = 0.07, 95% CI 0.05 – 0.10, p < 0.01), body mass index ( = 0.02, 95% CI 0.01 – 0.03, p < 0.01), arterial hypertension ( = 0.15, 95% CI 0.08 – 0.23, p < 0.01), and LPA ( = −0.10, 95% CI −0.19 to −0.02, p=0.02) were independent associations of aSUVmax.
Conclusions
Carotid [18F]fluoride uptake is significantly increased in patients with unfavorable cardiovascular and thromboembolic risk profiles. [18F]fluoride PET/CT could become a valuable tool to estimate subjects’ risk of future cardiovascular events although still major trials are needed to further evaluate the associations found in this study and their potential clinical usefulness.
Key Points
• Sodium fluoride ([ 18 F]fluoride) PET/CT imaging identifies patients with early-stage atherosclerosis.
• Carotid [ 18 F]fluoride uptake is significantly higher in patients with increased risk of cardiovascular and thromboembolic events and inversely correlated with the level of physical activity.
• Early detection of arterial mineralization at a molecular level could help guide clinical decisions in the context of cardiovascular risk assessment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Complications of atherosclerosis are a leading cause of death and disability in the Western world. Atherosclerotic plaque formation is influenced by several risk factors such as smoking habits, arterial hypertension (HTN), diabetes, and chronic renal disease, and it is usually a progressive process, which in many cases remains asymptomatic until an acute adverse event occurs [1, 2]. Early detection of the molecular processes underlying atherosclerosis is of great clinical value in order to prevent adverse outcomes through the modification of the lifestyle and/or pharmacological treatment of associated comorbidities [3, 4]. However, in order for precautionary measures to be taken at earlier stages of cardiovascular disease onset, sensitive and specific imaging methods must be utilized in order to visualize plaque formation and assess the integrity of the vasculature, as well as to monitor changes of these factors over the length of disease progression.
In this setting, there is growing evidence that sodium fluoride positron emission tomography/computed tomography ([18F]fluoride PET/CT) has the capacity to detect arterial calcifications early at a molecular stage [5,6,7,8]. [18F]fluoride is a molecular tracer that has been utilized recently in the detection of atherosclerotic calcifications, coronary and carotid plaques, and associated localization of the tracer along calcified areas bring up the question to evaluate if [18F]fluoride could be useful in stratifying the risk of cardiovascular events [9]. In contrast to fluorodeoxyglucose ([18F]FDG), which is a marker of inflammatory processes, [18F]fluoride uptake has been identified as a marker of active microcalcification and thereby a potential predictor of cardiovascular events related to microcalcifications, such as myocardial infarction, angina, and coronary artery disease [10, 11]. Further elucidating the association between [18F]fluoride uptake and arterial calcifications will be of clinical importance to physicians, who may be able to apply this information to improve cardiovascular risk assessment of individual patients.
A positive correlation between [18F]fluoride uptake in the thoracic aorta and high-risk cardiovascular profile has previously been reported, which presents a promising development in determining the association between [18F]fluoride uptake and cardiovascular detriment [8]. However, no such data is currently available for the carotid arteries. The aim of this study was to evaluate the association of cardiovascular and thromboembolic risk factors to [18F]fluoride PET/CT uptake in the left common carotid artery (LCC). In doing so, we intended to examine the utility of this imaging modality in identifying molecular calcifications in the LCC, as well as to determine if these [18F]fluoride uptake values are consistent with cardiovascular and thromboembolic risk profile assessments.
Methods
Study population
The study population included 128 out of 139 subjects from the Cardiovascular Molecular Calcification Assessed by 18F-NaF PET/CT (CAMONA) study in whom PET/CT images had an adequate quality to allow LCC segmentation. The study has been approved by the Danish National Committee on Health Research Ethics, and is registered at ClinicalTrials.gov (NCT01724749) in accordance with the principles of the Declaration of Helsinki. All subjects gave written informed consent prior to the study.
Patient evaluation
All subjects underwent a physical examination to exclude signs of overt atherosclerotic disease. Office blood pressure measurement was also performed, and blood samples were obtained to evaluate white blood cell (WBC) count, lipid profile, fasting plasma glucose, glycated hemoglobin (HbA1c), C-reactive protein (CRP), homocysteine, fibrinogen, and creatinine levels. Renal function was estimated from the glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration equation as previously reported [12]. All patients completed a questionnaire to assess smoking status, alcohol consumption, and physical activity level (LPA), which was estimated using a list of everyday activities as previously described (Table 1) [13]. Additionally, body mass index (BMI) was assessed in all subjects. The 10-year risk of major adverse cardiovascular events was estimated in each subject using the Framingham Risk Score (FRS), and the risk of thromboembolic events was estimated by the CHA2DS2-VASc (Congestive heart failure, Hypertension, Age ( > 65 = 1 point, > 75 = 2 points), Diabetes, previous Stroke/transient ischemic attack (2 points)-vascular disease) score [14, 15].
Cardiac PET/CT acquisition protocol
[18F]fluoride PET/CT imaging was performed according to previously described methods by the CAMONA study group [3, 8]. Briefly, hybrid PET/CT scanners (GE Discovery STE, VCT, RX, and 690/710 systems) were used and the scanners were assigned randomly to every subject by the scheduling department of the hospital. [18F]fluoride PET/CT was performed 90 min after the intravenous injection of 2.2 MBq of [18F]fluoride per kilogram of body weight. PET images were corrected for attenuation, scatter, random coincidences, and scanner dead time. OSEM was used as the reconstruction method for the PET images. Low-dose CT imaging (140kV, 30–110 mA, noise index 25, 0.8 s per rotation, slice thickness 3.75 mm) was performed for attenuation correction and anatomical orientation. The effective radiation dose received from the entire imaging protocol was approximately 6.7 mSv.
Carotid PET/CT data analysis
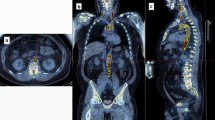
Quantitative analysis was performed on fused PET/CT images using OsiriX 7.5.1 software (Pixmeo SARL) by measuring average SUVmax (aSUVmax) as follows. A region of interest (ROI) was manually drawn on the fused axial image around the LCC, passing through the whole structure with a slice thickness of 3.75 mm. The SUVmax measurements were performed on CT attenuation corrected PET images. The uncorrected SUVmax of each slice was recorded, summed, and divided by the total number of slices to yield the aSUVmax (Fig. 1). Images were analyzed by two experienced physicians (S.C., O.A.), who were blinded to the patients’ clinical data (inter-user correlation coefficient 0.9; p < 0.001). Blood-pool correction was done by measuring the activity in the inferior vena cava only, as it was done by prior CAMONA authors because this location was least subject to spillover activity from adjacent [18F]fluoride-avid anatomical locations.
Statistical analysis
Continuous variables were expressed as mean ± SD if normally distributed, or as median (25th – 75th percentile) if not normally distributed. All continuous variables were tested for normality using the 1-sample Kolmogorov-Smirnov test. Categorical data were expressed as counts and percentages. Continuous variables were compared using an independent sample one-way ANOVA test. Categorical variables were compared using the chi-square test or Fisher’s exact test when appropriate. Multivariable linear regression analysis was used to test the association between LCC [18F]fluoride uptake and baseline covariates. All variables showing a statistically significant association at univariate analysis were initially entered into the multivariable model; then, a model reduction was performed by stepwise backward elimination of variables with a p value > 0.05. The aim of selection was to reduce the set of predictor variables to those that are necessary and account for nearly as much of the variance as is accounted for by the total set and to avoid mass significance. In order to avoid overfitting, all potential confounders were initially entered into the multivariable model on the basis of known clinical relevance, and then a model reduction was performed by excluding variables with a p value > 0.20 based on the log-likelihood test. Analysis of variance inflation factor was used to examine the presence of multicollinearity of the covariates. Independence of observations assumption was assessed using the Durbin-Watson statistic. Values of variance inflation factor exceeding 3.5 were regarded as indicators of multicollinearity. The normal distribution of residuals assumption was assessed using a normal probability plot. Two-tailed tests were considered statistically significant at the 0.05 level. All the analyses were performed using IBM SPSS version 23.0 software (SPSS Inc.).
Results
Clinical characteristics of the study population
The baseline characteristics of the study population are summarized in Table 2. One hundred twenty-eight subjects were included in the analysis (mean age 48 ± 14 years; 65 (51%) males). Overall, 13 (10%) patients were active smokers, 28 (22%) had arterial HTN, and 20 (16%) had hyperlipidemia while only 2 (2%) patients had type II diabetes mellitus. A sedentary lifestyle (LPA: 1) was reported by 28 (20%) subjects while 36 (25%) subjects reported a vigorously or extremely active lifestyle (LPA: 3–4). The mean BMI was 27 ± 4.5 with 55 (39%) being overweight (BMI 25–29.9) and 27 (19%) obese (BMI ≥30). The median 10-year FRS was 6% (2–12%), while the median CHA2DS2-VASc score was 1 (0–1).
Association between cardiovascular risk factors and arterial molecular calcification
A multiple regression analysis was carried out to investigate whether baseline covariates could be significantly associated with LCC [18F]fluoride uptake (Table 3). All variables showing a statistically significant association at univariate analysis were initially entered into the multivariable model. Then, in order to avoid overfitting, a model reduction was performed by stepwise backward elimination of variables with a p value > 0.05 (see below). A statistically significant regression equation was found (F (2, 3) = 20, p < 0.01)), which explained 51% of the variance (R2 = 0.51) (Fig. 2). Age (β= 0.07, 95% CI 0.05–0.10, p<0.01), BMI (β = 0.02, 95% CI 0.01 – 0.03, p < 0.01), and arterial HTN (β = 0.15, 95% CI 0.08 – 0.23, p < 0.01) were all significantly associated with aSUVmax, while LPA (β = −0.10, 95% CI −0.19 to −0.02, p = 0.02) was significantly and inversely associated with aSUVmax. The final predictive model was as follows:
where HTN is coded as 1 = yes or 0 = no, gender is coded 1 = male or 0 = female, age is expressed in years, BMI in kg/m2, and LPA can vary from 1 to 4 as mentioned above.
Correlation between cardiovascular/thromboembolic risk and arterial molecular calcification
Average SUVmax increased according to the 10-year risk of major adverse cardiovascular events estimated by the FRS with a mean aSUVmax of 0.95 ± 0.19 for subjects at low risk (<10%), 1.07 ± 0.22 for those at intermediate risk (10–20%), and 1.30 ± 0.23 for high-risk (≥20%) subjects (one-way ANOVA, p < 0.01) (Fig. 3). The aSUVmax also increased according to the annual risk of thromboembolic events estimated by the CHA2DS2-VASc score (Fig. 4). Subjects with very low risk (CHA2DS2-VASc: 0–1, equal to an annual risk of thromboembolic events ≤1.3%) had the lowest aSUVmax (0.95 ± 0.18), whereas those at high risk (CHA2DS2-VASc ≥4, equal to an annual risk > 4%) had the highest aSUVmax (1.50 ± 0.0.51) (one-way ANOVA, p < 0.01). On the other hand, a significant decrease in aSUVmax was observed according to LPA with a mean aSUVmax of 0.90 ± 0.33 in subjects with an extremely active lifestyle, compared to a mean aSUVmax of 1.17 ± 0.34 in sedentary subjects (one-way ANOVA, p = 0.02) (Fig. 5).
Discussion
The present study demonstrates the association between cardiovascular/thromboembolic risk and arterial molecular calcification of the LCC as assessed by [18F]fluoride PET/CT. The major findings are as follows: (1) [18F]fluoride uptake in the LCC is correlated with established atherosclerotic risk factors such as age, BMI, smoking habit, arterial HTN, diabetes, lipid profile, and chronic kidney disease. (2) Age, gender, BMI, arterial HTN, and LPA are all independently associated with LCC [18F]fluoride uptake, explaining 51% of its variance. (3) LCC [18F]fluoride uptake increases according to the estimated risk of major adverse cardiovascular events and the estimated risk of thromboembolic events.
Atherosclerosis-related diseases such as stroke, myocardial infarction, and limb ischemia are major causes of mortality and permanent disability in the Western world. Unstable plaques, characterized by a high-density lipid core with the presence of macrophages, foam cells, and active chronic inflammation, are vulnerable to ulceration and rupture [16]. In cases of plaque rupture, platelets and coagulation factors are exposed to the thrombogenic core, which allows for thrombus formation. The subsequent healing process, as well as associated chronic inflammation, leads to progressive plaque calcification [17]. In this regard, detection of vascular calcification with imaging may improve cardiovascular risk stratification by identifying or characterizing the high-risk “vulnerable” plaques. Traditionally, CT imaging has been used to detect arterial calcification with correlation to cardiovascular morbidity and mortality [18]. Unfortunately, conventional CT imaging has limited sensitivity for the detection of early-stage disease and cannot distinguish between sites of active calcium deposition, which is a potential marker of vulnerable atherosclerotic plaques, from chronic vascular calcification which is typical of stable disease [19, 20]. [18F]fluoride PET/CT has the potential to overcome these limitations. The chemisorption with the exchange of 18F-ion for OH-ion on the surface of hydroxyapatite crystals, forming fluorapatite, allows [18F]fluoride PET/CT to detect vascular calcification at a very early (molecular) stage and selectively identify sites of active calcium deposition with hydroxyapatite formation in the earliest stages of atherosclerotic plaque calcification [21, 22]. Previous studies have already demonstrated a significant positive correlation between [18F]fluoride uptake and cardiovascular risk profile at various sites such as the thoracic aorta; carotid, femoral, and coronary arteries; and aortic valve, with increasing [18F]fluoride uptake according to the number of cardiovascular risk factors [6, 8, 23,24,25]. Derlin et al have initially reported a positive correlation between [18F]fluoride uptake in the common carotid arteries and prevalence of cardiovascular risk factors in a population of neurologically asymptomatic patients, who were investigated for oncologic indications [6]. Carotid [18F]fluoride uptake has also been associated with incidence and severity of complications related to atherosclerosis: Quirce et al have compared [18F]fluoride uptake within carotid plaques in patients investigated for recent cerebrovascular accidents finding a higher [18F]fluoride uptake in symptomatic plaques compared to asymptomatic ones [26, 27]. den Harder et al demonstrated that [18F]fluoride activity is related to CT calcification and CT calcification progression in the femoral arteries, which may suggest that PET/CT can detect calcifications that are not visible using CT alone [28]. Our findings confirm and extend these results in a population of healthy adults and angina pectoris patients with a relatively low cardiovascular risk compared to previous findings which typically involved elderly patients with advanced cardiovascular disease [10].
FRS is a simple common tool for the assessment of 10-risk of cardiovascular disease events. It is a quick and easy tool used frequently in the clinical setting, which comprise of six coronary risk factors including age, gender, total cholesterol, high-density lipoprotein (HDL) cholesterol, smoking habits, and systolic blood pressure. We have identified a positive association between cardiovascular risk factors and carotid artery molecular calcification with increasing [18F]fluoride uptake according to the estimated 10-year risk of major adverse cardiovascular events. We have also found a significant decrease in [18F]fluoride uptake with increased LPA.
For the first time, we have described the relationship between arterial [18F]fluoride uptake and the risk of thromboembolic events estimated by the CHA2DS2-VASc score. This was originally developed to estimate the risk of ischemic stroke in patients with atrial fibrillation (AF) [15]. However, there is growing evidence of its capability to predict the risk of stroke even in sinus rhythm patients [25, 27]. In a large study including 12,599 patients with arterial HTN and stable sinus rhythm, a 2-fold increase in the risk of thromboembolic events was observed per each point of increase in the CHA2DS2-VASc score [29]. Lip et al have also tested the CHA2DS2-VASc score in a large community cohort of non-AF subjects (the Chin-Shan Community Cohort Study), showing a similar value in predicting stroke risk compared to AF subjects [30]. Among 20,970 patients without known AF enrolled in the Alberta Provincial Project for Outcomes Assessment in Coronary Heart disease (APPROACH) prospective registry, the CHA2DS2-VASc scores predicted ischemic stroke/transient ischemic attack (TIA) events with similar accuracy to that observed in historical populations with AF, but with lower absolute event rates (absolute annual incidence of stroke/TIA ≥1% with CHA2DS2-VASc ≥ 4) [31]. The inclusion, in the CHA2DS2-VASc score, of variables representing major risk factors for cardiovascular disease has been advocated as potential explanation of its ability to predict atherosclerosis and subsequent thromboembolic risk independent to the presence of AF [32]. In patients with high CHA2DS2-VASc score, indeed, conditions such as heart failure, older age, diabetes, and HTN all promote activation of prothrombotic factors [33, 34]. However, even if biologically plausible, these hypotheses have never been convincingly proven. Here, we have identified a clear correlation between increasing CHA2DS2-VASc scores and LCC [18F]fluoride uptake, which demonstrates the link between risk factors included in the CHA2DS2-VASc score and active arterial molecular calcification, thus offering an explanation for the increased risk of thromboembolic events regardless of the presence of AF. Our observations also find support in pathology studies correlating [18F]fluoride accumulation to the histological characterization of vascular calcification in carotid plaques. A significant correlation between tracer activity in the carotid plaques and presence of calcification in the corresponding histological sections has been observed in patients who underwent [18F]fluoride PET/CT studies before carotid endarterectomy for symptomatic carotid artery stenosis [35]. The currently available risk stratification tools to tailor primary prevention strategies in cardiovascular diseases are mainly based on clinical characteristics, and they have repeatedly overestimated the cardiovascular risk in high-risk subjects or underestimated it among those at lower risk [36]. [18F]fluoride PET/CT, which provides both anatomical and functional information on the active atheroma burden, could represent an accurate and reproducible method to identify high-risk patients, who benefit the most from aggressive risk factor modification strategies, such as high-intensity statin therapy.
The main limitation of our study is the lack of data regarding clinical outcomes; we have correlated [18F]fluoride uptake with the estimated risk of cardiovascular and thromboembolic events but it remains unknown if this result translates into a correlation with long-term incidence of major adverse cardiovascular events. Moreover, the cross-sectional nature of this study only allowed us to correlate the static cardiovascular risk profile of subjects with [18F]fluoride uptake. It lacked longitudinal data which may have assessed variations in LCC [18F]fluoride uptake within the same subjects in relation to risk factor modification that may clarify the correlation between [18F]fluoride uptake and vascular biology. Traditionally, the radiation dose administered to the patients using this imaging modality is not considered insignificant. However, recent consideration contradicts this standpoint by referring to the fact that the current guidelines on the dangers of low-dose radiation are outdated and based on a never-proven hypothesis, a view that is shared by the International Organization for Medical Physics and the American Association of Physicists in Medicine, both of which believe that the negative effects of radiation doses of this magnitude are negligible [37]. We can also predict one of the future limitations that could arise in the use of [18F]fluoride PET/CT is cost-effectiveness of using such an advanced procedure as a screening tool in CVD. Most of the current literature is based on different clinical entities [38]. We believe it is still too early in our hypothesis to provide cost-effective data analysis.
From a technical point of view, a significant limiting factor to our technique was the spatial resolution of PET. The quantitative approach we used did not consider partial volume effect which may have influenced the study results. The LCC wall is smaller than spatial resolution of PET; however, this anatomic location is relatively spared by cardiac and respiratory cycle motion, which reduces the potential partial volume effects. Moreover, [18F]fluoride uptake was determined by global assessment which partly overcomes the partial volume effect and is not affected by difficulties in localizing focal lesions, which provides a more reliable picture of the atherosclerotic burden on the arterial wall, especially in low-risk patients, who comprised the majority of our study population.
In conclusion, our findings indicate that [18F]fluoride uptake in LCC is strongly correlated with the estimated risk of cardiovascular and thromboembolic events, confirming the potential for [18F]fluoride PET/CT to provide functional information related to plaque biology by detecting sites of active arterial molecular calcification. It is hoped that NaF-PET/CT alone or as an adjunct to the above-mentioned clinical risk scores could significantly increase the ability to predict risk of development of CVD on an individual basis. The strong association with CVD risk scores points to [18F]fluoride PET/CT as a promising tool for identifying and monitoring therapeutic efficacy in subjects at high risk of cardiovascular events, who may have an opportunity to follow aggressive cardiovascular risk modification strategies and/or are offered anti-atherosclerotic medication. However, it is still too early to say and these promising results need further elucidation and confirmation in larger prospective cohort studies of patients at high risk for cardiovascular events.
Abbreviations
- [18F]FDG:
-
Fluorodeoxyglucose
- [18F]fluoride:
-
Sodium fluoride
- aSUVmax:
-
Averaged maximal standardized uptake value
- CAMONA:
-
Cardiovascular Molecular Calcification Assessed by 18F-NaF PET/CT
- CHA2DS2-VASc:
-
Congestive heart failure, Hypertension, Age ( > 65 = 1 point, > 75 = 2 points), Diabetes, previous Stroke/transient ischemic attack (2 points)-vascular disease
- CT:
-
Computed tomography
- CVD:
-
Cardiovascular disease
- FRS:
-
Framingham Risk Score
- HTN:
-
Hypertension
- LCC:
-
Left common carotid artery
- LPA:
-
Level of physical activity
- PET:
-
Positron emission tomography
- ROI:
-
Region of interest
- SUVmax:
-
Maximum standardized uptake value
References
Wardlaw JM, Stevenson MD, Chappell F et al (2009) Carotid artery imaging for secondary stroke prevention: Both imaging modality and rapid access to imaging are important. Stroke 40:3511–3517. https://doi.org/10.1161/strokeaha.109.557017
Quillard T, Libby P (2012) Molecular imaging of atherosclerosis for improving diagnostic and therapeutic development. Circ Res 111:231–244. https://doi.org/10.1161/circresaha.112.268144
Blomberg BA, Thomassen A, Takx RA et al (2014) Delayed (1)(8)F-fluorodeoxyglucose PET/CT imaging improves quantitation of atherosclerotic plaque inflammation: Results from the CAMONA study. J Nucl Cardiol 21:588–597. https://doi.org/10.1007/s12350-014-9884-6
Borja AJ, Hancin EC, Zhang V, Revheim ME, Alavi A (2020) Potential of PET/CT in assessing dementias with emphasis on cerebrovascular disorders. Eur J Nucl Med Mol Imaging 47:2493–2498. https://doi.org/10.1007/s00259-020-04697-y
Derlin T, Richter U, Bannas P et al (2010) Feasibility of 18F-sodium fluoride PET/CT for imaging of atherosclerotic plaque. J Nucl Med 51:862–865. https://doi.org/10.2967/jnumed.110.076471
Derlin T, Wisotzki C, Richter U et al (2011) In vivo imaging of mineral deposition in carotid plaque using 18F-sodium fluoride PET/CT: Correlation with atherogenic risk factors. J Nucl Med 52:362–368. https://doi.org/10.2967/jnumed.110.081208
Lee DH, Lee SJ, Lee DJ et al (2014) Carotid artery FDG uptake may serve as a biomarker for cardiovascular risk stratification in asymptomatic adults. Nucl Med Mol Imaging 48:196–202. https://doi.org/10.1007/s13139-014-0277-1
Blomberg BA, de Jong PA, Thomassen A et al (2017) Thoracic aorta calcification but not inflammation is associated with increased cardiovascular disease risk: Results of the CAMONA study. Eur J Nucl Med Mol Imaging 44:249–258. https://doi.org/10.1007/s00259-016-3552-9
Irkle A, Vesey AT, Lewis DY et al (2015) Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun 6:7495. https://doi.org/10.1038/ncomms8495
Dweck MR, Chow MW, Joshi NV et al (2012) Coronary arterial 18F-sodium fluoride uptake: A novel marker of plaque biology. J Am Coll Cardiol 59:1539–1548. https://doi.org/10.1016/j.jacc.2011.12.037
McKenney-Drake ML, Moghbel MC, Paydary K et al (2018) (18)F-NaF and (18)F-FDG as molecular probes in the evaluation of atherosclerosis. Eur J Nucl Med Mol Imaging 45:2190–2200. https://doi.org/10.1007/s00259-018-4078-0
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
Livesey G (1987) Energy and protein requirements the 1985 report of the 1981 Joint FAO/WHO/UNU Expert Consultation. Nutr Bull 12:138–149. https://doi.org/10.1111/j.1467-3010.1987.tb00040.x
Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB (1998) Prediction of coronary heart disease using risk factor categories. Circulation 97:1837–1847. https://doi.org/10.1161/01.cir.97.18.1837
Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ (2010) Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro Heart Survey on atrial fibrillation. Chest 137:263–272. https://doi.org/10.1378/chest.09-1584
Stary HC, Chandler AB, Dinsmore RE et al (1995) A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 92:1355–1374. https://doi.org/10.1161/01.cir.92.5.1355
Abdelbaky A, Corsini E, Figueroa AL et al (2013) Focal arterial inflammation precedes subsequent calcification in the same location: A longitudinal FDG-PET/CT study. Circ Cardiovasc Imaging 6:747–754. https://doi.org/10.1161/circimaging.113.000382
Budoff MJ, Shaw LJ, Liu ST et al (2007) Long-term prognosis associated with coronary calcification: Observations from a registry of 25,253 patients. J Am Coll Cardiol 49:1860–1870. https://doi.org/10.1016/j.jacc.2006.10.079
Ehara S, Kobayashi Y, Yoshiyama M et al (2004) Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: An intravascular ultrasound study. Circulation 110:3424–3429. https://doi.org/10.1161/01.Cir.0000148131.41425.E9
Joshi NV, Vesey AT, Williams MC et al (2014) 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: A prospective clinical trial. Lancet 383:705–713. https://doi.org/10.1016/s0140-6736(13)61754-7
Aikawa E, Nahrendorf M, Figueiredo JL et al (2007) Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation 116:2841–2850. https://doi.org/10.1161/circulationaha.107.732867
Czernin J, Satyamurthy N, Schiepers C (2010) Molecular mechanisms of bone 18F-NaF deposition. J Nucl Med 51:1826–1829. https://doi.org/10.2967/jnumed.110.077933
Blomberg BA, Thomassen A, de Jong PA et al (2017) Coronary fluorine-18-sodium fluoride uptake is increased in healthy adults with an unfavorable cardiovascular risk profile: Results from the CAMONA study. Nucl Med Commun 38:1007–1014. https://doi.org/10.1097/mnm.0000000000000734
Janssen T, Bannas P, Herrmann J et al (2013) Association of linear 18F-sodium fluoride accumulation in femoral arteries as a measure of diffuse calcification with cardiovascular risk factors: A PET/CT study. J Nucl Cardiol 20:569–577. https://doi.org/10.1007/s12350-013-9680-8
Rojulpote C, Borja AJ, Zhang V et al (2020) Role of (18)F-NaF-PET in assessing aortic valve calcification with age. Am J Nucl Med Mol Imaging 10:47–56
Quirce R, Martinez-Rodriguez I, De Arcocha TM et al (2013) Contribution of 18F-sodium fluoride PET/CT to the study of the carotid atheroma calcification. Rev Esp Med Nucl Imagen Mol 32:22–25. https://doi.org/10.1016/j.remn.2012.08.003
Quirce R, Martinez-Rodriguez I, Banzo I et al (2016) New insight of functional molecular imaging into the atheroma biology: 18F-NaF and 18F-FDG in symptomatic and asymptomatic carotid plaques after recent CVA. Preliminary results. Clin Physiol Funct Imaging 36:499–503. https://doi.org/10.1111/cpf.12254
den Harder AM, Wolterink JM, Bartstra JW et al (2020) Vascular uptake on (18)F-sodium fluoride positron emission tomography: Precursor of vascular calcification? J Nucl Cardiol. https://doi.org/10.1007/s12350-020-02031-5
Mazzone C, Cioffi G, Carriere C et al (2017) Predictive role of CHA2DS2-VASc score for cardiovascular events and death in patients with arterial hypertension and stable sinus rhythm. Eur J Prev Cardiol 24:1584–1593. https://doi.org/10.1177/2047487317726068
Lip GY, Lin HJ, Chien KL et al (2013) Comparative assessment of published atrial fibrillation stroke risk stratification schemes for predicting stroke, in a non-atrial fibrillation population: The Chin-Shan Community Cohort Study. Int J Cardiol 168:414–419. https://doi.org/10.1016/j.ijcard.2012.09.148
Mitchell LB, Southern DA, Galbraith D, Ghali WA, Knudtson M, Wilton SB (2014) Prediction of stroke or TIA in patients without atrial fibrillation using CHADS2 and CHA2DS2-VASc scores. Heart 100:1524–1530. https://doi.org/10.1136/heartjnl-2013-305303
Ohira T, Shahar E, Chambless LE, Rosamond WD, Mosley TH Jr, Folsom AR (2006) Risk factors for ischemic stroke subtypes: The Atherosclerosis Risk in Communities study. Stroke 37:2493–2498. https://doi.org/10.1161/01.Str.0000239694.19359.88
McClung JA, Naseer N, Saleem M et al (2005) Circulating endothelial cells are elevated in patients with type 2 diabetes mellitus independently of HbA(1)c. Diabetologia 48:345–350. https://doi.org/10.1007/s00125-004-1647-5
Chong AY, Freestone B, Lim HS et al (2007) Plasma von Willebrand factor and soluble E-selectin levels in stable outpatients with systolic heart failure: The Frederiksberg Heart Failure study. Int J Cardiol 119:80–82. https://doi.org/10.1016/j.ijcard.2006.07.085
Zhang Y, Li H, Jia Y et al (2018) Noninvasive assessment of carotid plaques calcification by (18)F-sodium fluoride accumulation: Correlation with pathology. J Stroke Cerebrovasc Dis 27:1796–1801. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.02.011
DeFilippis AP, Young R, McEvoy JW et al (2017) Risk score overestimation: The impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. Eur Heart J 38:598–608. https://doi.org/10.1093/eurheartj/ehw301
Høilund-Carlsen PF (2019) The good rays: Let them shine! Eur J Nucl Med Mol Imaging 46:271–275. https://doi.org/10.1007/s00259-018-4233-7
Gerke O, Hermansson R, Hess S, Schifter S, Vach W, Høilund-Carlsen PF (2015) Cost-effectiveness of PET and PET/computed tomography: A systematic review. PET Clin 10:105–124. https://doi.org/10.1016/j.cpet.2014.09.008
Acknowledgements
We thank the staff and participants of the CAMONA research study for their contributions.
Funding
This study has received funding from the Jørgen and Gisela Thrane’s Philanthropic Research Foundation, Broager, Denmark.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Abass Alavi (abass.alavi@pennmedicine.upenn.edu).
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Oke Gerke (Department of Nuclear Medicine, Odense University Hospital, Odense, Denmark; Research Unit of Clinical Physiology and Nuclear Medicine, Department of Clinical Research, University of Southern Denmark, Odense, Denmark) kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained. The CAMONA study was approved by the Danish National Committee on Biomedical Research Ethics, registered at ClinicalTrials.gov (NCT01274749) and conducted from 2012 to 2016 in accordance with the Declaration of Helsinki.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in Castro S, Muser D, Acosta-Montenegro O, et al Common carotid artery molecular calcification assessed by 18F-NaF PET/CT is associated with increased cardiovascular disease risk: results from the CAMONA study. J Nucl Med 2017;58:34–34. (Abstract accepted to 2017 Society of Nuclear Medicine and Molecular Imaging conference).
Methodology
• retrospective
• case-control study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Poul Flemming Høilund-Carlsen and Abass Alavi are co-last authors.
Rights and permissions
About this article
Cite this article
Castro, S.A., Muser, D., Lee, H. et al. Carotid artery molecular calcification assessed by [18F]fluoride PET/CT: correlation with cardiovascular and thromboembolic risk factors. Eur Radiol 31, 8050–8059 (2021). https://doi.org/10.1007/s00330-021-07917-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-07917-7