Abstract
Objective
To determine the sensitivity and specificity of high-resolution (HR) MRI for detecting signal abnormalities of cranial nerves (CN) in giant cell arteritis (GCA) patients presenting with diplopia.
Methods
This IRB-approved retrospective single-center study included GCA patients who underwent 3-T HR MRI from December 2014 to January 2020. Two radiologists, blinded to all data, individually assessed for the presence of enhancement of the 3rd, 4th, and/or 6th CN on post-contrast HR imaging and high signal intensity on HR T2-WI, for signal abnormalities of extraocular muscles and the brainstem, and for inflammatory changes of the ophthalmic and extracranial arteries. A Fisher’s exact test was used to compare patients with or without diplopia.
Results
In total, 64 patients (42/64 (66%) women and 22/64 (34%) men, mean age 76.3 ± 8 years) were included. Of the 64 patients, 14 (21.9%) presented with diplopia. Third CN enhancement was detected in 7/8 (87.5%) patients with 3rd CN impairment, as compared to no patients with 4th or 6th CN impairment or to patients without diplopia (p < 0.001). Third CN abnormal high signal intensity on HR T2-WI was detected in 4/5 patients (80%) with 3rd CN impairment versus none of other patients (p < 0.001). Sensitivity, specificity, positive predictive value, and negative predictive value for detecting 3rd CN signal abnormalities were of 0.88, 1, 1, and 0.99 and 0.8, 1, 1, and 0.98 for post-contrast HR imaging and HR T2-WI, respectively.
Conclusions
HR MRI had excellent diagnostic sensitivity and specificity when detecting signal abnormalities of the 3rd CN in GCA patients presenting with 3rd CN impairment.
Key Points
• Third cranial nerve enhancement was detected in all patients with 3rd cranial nerve impairment except for one with transient diplopia.
• The “check mark sign” might be useful to identify 3rd cranial nerve signal abnormalities in the orbital apex.
• No signal abnormalities of the 4th or 6th cranial nerves could be detected on high-resolution MRI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Giant cell arteritis (GCA) is the most common form of vasculitis of large- and medium-sized arteries in people older than 50 [1, 2]. Histological features of GCA include polymorphic granulomatous infiltrate affecting the three tunics of arterial walls, more often at the intima-media junction, in a segmented and multifocal distribution [1, 2]. While temporal artery biopsy remains the reference standard for diagnosing GCA, recent studies on extracranial and intracranial arteries with high-resolution (HR) MRI revealed high sensitivity and specificity for a non-invasive diagnosis of GCA [3,4,5,6,7,8,9,10]. Recently, the European League Against Rheumatism (EULAR) stated that HR MRI could be used as a first-line imaging modality for diagnosing GCA [11, 12].
GCA can be associated with several ophthalmological diseases, such as loss of vision due to anterior ischemic optic neuropathy (AION) and less often central retinal artery occlusion, or diplopia, the latter occurring in 1 to 19% of GCA patients [13]. Binocular diplopia in GCA can be due to lesions involving different parts of the oculomotor system, but its main cause is an impairment of cranial nerves, mostly the 3rd and/or 6th cranial nerves and rarely the 4th [14, 15].
Recent studies showed that HR MRI was accurate for diagnosing AION in GCA patients or for distinguishing arteritic from non-arteritic AION [16, 17]. It might also reveal abnormal findings in patients with central retinal artery occlusion [18]. However, to our knowledge, no study has yet evaluated the performance of MRI in GCA patients with diplopia. Cranial nerves are challenging to image on conventional MRI due to their small size. HR MRI might overcome this issue and allows direct visualization and analysis of signal abnormalities of the 3rd, 4th, and/or 6th cranial nerves.
We hypothesized that HR MRI, which is performed for the analysis of vessel walls, could be useful to highlight signal abnormalities of the 3rd, 4th, and/or 6th cranial nerves in GCA patients presenting with diplopia. Highlighting such abnormalities could make it possible to diagnose GCA-related diplopia early so as to start anti-inflammatory treatment quickly.
Therefore, the aim of our study was to determine the diagnostic performance of HR MRI for detecting signal abnormalities of cranial nerves in GCA patients presenting with binocular diplopia.
Materials and methods
Study design
We conducted a retrospective analysis of a prospectively acquired cohort (IRB CE_20200204_3_ALR, NCT 02473029) of GCA patients in a single center specializing in ophthalmic diseases. This retrospective analysis was approved by an Institutional Review Board and adhered to the tenants of the Declaration of Helsinki (IRB CE_20200204_3_ALR). Signed informed consent was obtained from all subjects. This study follows the Standards for Reporting of Diagnostic Accuracy Studies (STARD) guidelines [19].
Study population
From December 2014 to January 2020, 64 consecutive GCA patients were included in the study.
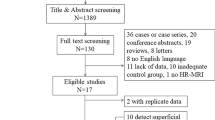
Inclusion criteria for this study were as follows: (a) age over 18 years, (b) definite diagnosis of GCA based on a positive temporal artery biopsy or a review of the clinical chart performed by an interdisciplinary panel of rheumatologists, internists, and ophthalmologists not involved in the management of the patient, based on ACR criteria [20], (c) presence of a HR MRI at 3 T performed within 7 days after onset of symptoms and before or within 5 days after the beginning of a treatment by corticosteroids. Selection of patients is shown in Supplementary Figure 1. The presence of binocular diplopia was not an inclusion criterion; thus, GCA patients with or without binocular diplopia were included.
Clinical data
Patient charts were systematically reviewed by a senior neuro-ophthalmologist, noting ophthalmological findings such as the presence of binocular diplopia as well as the number, side, and type of cranial nerves involved.
MR imaging
All MRIs were performed on a 3-T Philips INGENIA device or on a 3-T Philips ELITION (Philips Medical Systems) device with a 32-channel head coil. The MRI protocol including pre- and post-contrast 3D HR MR sequences is displayed in Supplementary Table 1. HR MRI sequences covered the whole brain, the orbit, and the entire course of 3rd, 4th, and 6th cranial nerves from the brainstem to the orbit. Post-contrast imaging was performed after intravenous injection of a single bolus (0.1 mmol/kg) of gadobutrol (Gadovist; Bayer HealthCare).
Imaging analysis
Two radiologists, blinded to all data, individually read anonymized MR images: one junior radiologist with 8 months of experience in neuroradiology (S.M.) and one senior neuroradiologist with 12 years of experience (F.C.). Six weeks after the first reading session, a consensus reading session was performed with a third reader, a second senior neuroradiologist with 9 years of experience (A.L.), also blinded to all data. This consensus session was used as reference for analysis. All reading sessions were completed on a dedicated workstation with the OsiriX software (Pixmeo).
The readers assessed the following characteristics of MRIs for all patients included in the study, irrespective of the presence of diplopia:
The primary judgment criterion was the presence of enhancement of the 3rd, 4th, and/or 6th cranial nerves on post-contrast HR imaging, as compared to the 5th cranial nerve which was used as a reference.
Secondary judgment criteria were as follows:
-
The location of the enhancement of cranial nerves along their course, according to the following locations: cistern, cavernous sinus, superior orbital fissure, orbit. The orbit included the orbital apex.
-
High signal intensity of the 3rd, 4th, and/or 6th cranial nerves on HR T2-WI, as compared to the 5th cranial nerve which was used as a reference. The four portions of the cranial nerves were adopted for this evaluation.
-
Enhancement or signal abnormalities of the brainstem, focusing on the nuclei of the 3rd, 4th, and 6th cranial nerves.
-
Enhancement or signal abnormalities of extraocular muscles, defined as an enhancement superior to that of the nasal mucosa or a signal superior to that of the temporal muscle.
-
Inflammatory changes of the ophthalmic artery, based on the evaluation of its wall thickening and mural enhancement, using the following 4-point scale: a score of 0 corresponds to no wall thickening and no mural enhancement, a score of 1 corresponds to no wall thickening and slight mural enhancement, a score of 2 corresponds to a wall thickening and substantial mural enhancement, and a score of 3 corresponds to a marked wall thickening and strong mural enhancement with perivascular inflammatory infiltration. Scores 0 and 1 were considered negative for arteritis whereas scores 2 and 3 were considered positive [3].
-
Inflammatory changes of the posterior ciliary arteries.
-
Enhancement of the optic disc.
-
Inflammatory changes of extracranial arteries, based on the evaluation of wall thickening and mural enhancement of the following six extra-cranial arterial segments: left and right frontal and parietal branches of the superficial temporal artery and occipital artery, using the previously published 4-point scale. Scores 0 and 1 were considered negative for arteritis whereas scores 2 and 3 were considered positive.
-
Artifacts scored as follows: 0 corresponding to an absence of artifacts, 1 corresponding to the presence of minor artifacts not preventing the MR analysis, 2 corresponding to the presence of major artifacts not preventing MR analysis, and 3 corresponding to the presence of major artifacts preventing MR analysis.
-
Self-confidence in reading was evaluated as follows: a score of 1 for low confidence, a score of 2 for moderate confidence, and a score of 3 for excellent confidence.
Statistical analysis
Quantitative variables were presented as mean (standard deviation or SD) or median (interquartile range or IQR) as appropriate, and categorical variables as percentages. Normality was assessed for continuous variables using the Shapiro-Wilk test. Categorical variables were compared using Fisher’s exact test, while continuous variables were compared using a t test or Mann-Whitney U test as appropriate. Interobserver and intraobserver agreement for MRI reading was assessed using non-weighted Cohen kappa statistics and interpreted as follows: 0.0 to 0.2, poor correlation; 0.21 to 0.4, fair correlation; 0.41 to 0.6, moderate correlation; 0.61 to 0.8, good correlation; and 0.81 to 1, almost perfect correlation. A Benjamini-Hochberg correction was used to take into account multiple testing. A p value below 0.05 was considered statistically significant. Data were analyzed using the R software package version 3.6.1.
Results
Demographic, clinical characteristics
In total, 64 patients (42/64 [66%] women and 22/64 [34%] men, mean age 76.3 ± 8 years) with GCA were included from December 2014 to January 2020. Among them, 14/64 patients (21.9%) presented with diplopia. Ophthalmological examination confirmed 3rd, 4th, or 6th cranial nerve impairment for 8/14 (57.1%), 1/14 (7.1%), and 5/14 (35.7%) patients, respectively. Ocular motor palsy was unilateral for all patients, involving the right eye for 8/14 (57%) patients and the left eye for 6/14 (43%) patients. Of 8 patients, 1 (12.5%) had transient 3rd cranial nerve impairment only, lasting 48 h as compared to 7/8 (87.5%) with fixed impairment. Of 14 patients with diplopia, 7 (50%) had a definite diagnosis of GCA proven by biopsy.
Delay between onset of ophthalmological symptoms and MRI was 4.5 days (IQR 2–6.8). Median delay between corticosteroids and MRI was 2 days (IQR 0.3–2.2).
Cranial nerve signal abnormalities on HR MRI (Figs. 1 and 2)
Among the 64 MRI exams of patients with GCA, 3rd cranial nerve enhancement was detected in 7/8 (87.5%) patients with 3rd cranial nerve impairment, all with a fixed impairment, as compared to no patients with 4th or 6th cranial nerve impairment or in patients without diplopia (p < 0.001). No patient with 3rd, 4th, or 6th cranial nerve impairment had 4th or 6th cranial nerve enhancement.
A 62-year-old woman with giant cell arteritis presenting with diplopia due to right 3rd cranial nerve impairment. Post-contrast fat-suppressed high-resolution 3D T1-WI MRI in axial (a) and sagittal (b, right side on the top and left side below) plane and fat-suppressed high-resolution 3D T2-WI in axial plane (c) showing right 3rd cranial nerve enhancement (black arrows) and high signal intensity (white arrows), respectively. Note the typical and conspicuous “check mark sign” at the orbital apex corresponding to the two branches of the 3rd cranial nerve after its division in the superior orbital fissure. The superior branch, which appears short and lateral in the axial plane, provides motor innervation to the superior rectus and levator palpebrae superioris, whereas the inferior branch, which appears long and medial in the axial plane, provides motor innervation to the inferior rectus, medial rectus, and inferior oblique. Coronal T2-WI MRI (d) showing high signal intensity of the right medial rectus and inferior rectus extraocular muscles (black arrowheads). 3D T1-WI MRI in coronal plane showing enhancement of the right 3rd cranial nerve (white arrowheads) (e), its superior and inferior branches after its division (f), and its distal branches after the division of the inferior branch into two branches providing motor innervation to the medial rectus and inferior rectus muscles (g).
A 76-year-old woman with giant cell arteritis presenting with diplopia due to right 3rd cranial nerve impairment. Post-contrast fat-suppressed high-resolution 3D T1-WI MRI in axial (a) and sagittal (b), right side on the top and left side below) plane and fat-suppressed high-resolution 3D T2-WI in axial plane (c) showing right 3rd cranial nerve enhancement (black arrows) and high signal intensity (white arrows), respectively. Right 3rd cranial nerve MRI signal abnormalities are extending from the lateral side of the cavernous sinus through the superior orbital fissure and the orbit. The typical “check mark sign” at the orbital apex is more conspicuous on post-contrast high-resolution T1-WI than on high-resolution T2-WI MRI. Note the subtle enhancement (black arrowhead) and high signal intensity (white arrowhead) of the right rectus extraocular muscle
Third cranial nerve abnormal high signal intensity on HR T2-WI was detected in 4/5 (80%) patients with 3rd cranial nerve impairment versus no patients with 4th or 6th cranial nerve impairment or in patients without diplopia (p < 0.001). HR T2-WI was unusable due to artifacts preventing interpretation for 11 patients with no diplopia and in 5 patients presenting with diplopia: 3 patients with 3rd cranial nerve impairment and 2 patients with 4th or 6th cranial nerve impairment. The patient without 3rd cranial nerve abnormal high signal intensity on HR T2-WI was the same with no 3rd cranial nerve enhancement. No patient with 3rd, 4th, or 6th cranial nerve impairment had 4th or 6th cranial nerve signal abnormality. Detailed imaging characteristics are displayed in Table 1.
Sensitivity, specificity, positive predictive value, and negative predictive value for detecting 3rd cranial nerve signal abnormalities in patients with a clinical 3rd nerve palsy were of 0.88, 1, 1, and 0.99 and 0.8, 1, 1, and 0.98 for post-contrast HR imaging and HR T2-WI, respectively.
Check mark sign
Signal abnormalities were located in the orbit and the superior orbital fissure for all patients with 3rd cranial nerve enhancement. Division into two branches of distinct size was observed at the orbital apex for all of them, one superior branch and one inferior branch appearing short and lateral and long and medial in the axial plane, respectively. We referred to this striking conspicuous sign as the “check mark sign.”
Inflammatory changes of orbital arteries on HR MRI
There were no significant differences between patients presenting with or without diplopia regarding the presence of inflammatory changes of the ophthalmic artery or the posterior ciliary arteries: 9/14 (64.3%) versus 44/50 (88%) (p = 0.051) and 2/14 (14.3%) versus 21/50 (42%) (p = 0.09), respectively. Detailed imaging characteristics are displayed in Table 1.
Presence of artifacts and self-reported confidence
HR MRI displayed only minor artifacts for 51/64 (79.7%) patients or none at all with a median artifact score of 1 (IQR 1).
Self-reported confidence was evaluated as moderate to excellent for 51/64 (79.7%) patients, with a median score of 2 (IQR 0).
Inter- and intrareader agreement
Inter- and intrareader agreement was moderate for assessing enhancement of any of the 3rd, 4th, and/or 6th cranial nerves on post-contrast HR MRI: κ = 0.54 [0.19–0.82] and 0.55 [0.05–0.88], respectively. It was good for assessing enhancement of the 3rd cranial nerve alone: κ = 0.74 [0.29–0.89] and 0.79 [0.35–0.95], respectively. Detailed inter- and intrareader agreements are provided in Table 1.
Discussion
Our study showed that high-resolution MRI had excellent sensitivity and specificity when detecting signal abnormalities of the 3rd cranial nerve in GCA patients presenting with 3rd cranial nerve impairment. To the best of our knowledge, our study is the first one showing MRI signal abnormalities of cranial nerves in GCA patients presenting with diplopia.
The prevalence of diplopia in our cohort was of 22%, which is slightly higher than the 1–19% reported in the literature [13]. As a tertiary center specialized in ophthalmological diseases, we tend to recruit more patients with ophthalmological complications of GCA, such as diplopia, which might explain this discrepancy. Our patients presented mostly with 3rd and 6th cranial nerve impairment, which is in line with previous studies’ showing that 4th nerve palsy was rarer among patients with GCA [15].
We showed that HR MRI could detect signal abnormalities of the 3rd cranial nerve in all but one patient presenting with 3rd cranial nerve impairment. These signal abnormalities were visible in the orbit and the superior orbital fissure for all patients. A typical imaging pattern, which we referred to as the “check mark sign,” was visible at the orbital apex for all of them. The “check mark sign” corresponds to the division of the 3rd cranial nerve in two branches when arriving in the superior orbital fissure. The superior branch, which appears short and lateral in the axial plane on HR MRI, provides motor innervation to the superior rectus and levator palpebrae superioris, whereas the inferior branch, which appears long and medial in the axial plane, provides motor innervation to the inferior rectus, medial rectus, and inferior oblique. We used black-blood fat-suppressed 3D HR MRI with an isotropic voxel of 0.55 mm, which clearly distinguishes cranial nerves from vessels by nulling vessel lumen [21,22,23]. This technique highlights signal abnormalities of cranial nerves. However, identifying cranial nerves in the superior orbital fissure and in the orbit remains challenging due to the many various small structures crossing and entering the orbit at this location. Properly identifying the “check mark sign” might help clinicians distinguish the 3rd cranial nerve signal abnormalities from other structures.
Interestingly, signal abnormalities of the 3rd cranial nerve were never seen in its cisternal portion. Because HR MRI allows excellent visualization of the cisternal portion of the 3rd cranial nerve, this finding is probably not due to a technical limitation. This could be explained by the specific vascularization of the 3rd cranial nerve [24]. Indeed, its initial cisternal portion is supplied by branches arising from the posterior cerebral artery, whereas its distal portions including the cavernous sinus, the superior orbital fissure, and the orbit itself are vascularized by branches arising not only from the ophthalmic artery but also from the internal carotid artery [24]. We showed that a vast majority of GCA patients presenting with diplopia included in our study had inflammatory changes of the ophthalmic artery visible on HR MRI. This suggests that diplopia in GCA patients might be due to microvascular ischemia secondary to inflammatory changes of the ophthalmic artery, leading to demyelination and neurogenic impairment, as hypothesized by clinical studies [25].
Only one patient presenting with diplopia secondary to 3rd cranial nerve impairment had no signal abnormalities on HR MRI. This patient presented with transient diplopia. This suggests that HR MRI might have prognostic value by detecting only severe cranial nerve impairments leading to fixed diplopia. However, one should remain cautious given that this observation was made in only one patient.
Our study detected no signal abnormalities of the 4th and 6th cranial nerves. This might be due to spatial resolution remaining too low despite HR MRI. Indeed, the size of the 6th and 4th cranial nerves is substantially smaller than that of the 3rd cranial nerve, especially in their distal portion in the superior orbital fissure and the orbit [26, 27]. This might also be due to a different pathophysiological process as compared to the 3rd cranial nerve, leading to undetectable changes on HR MRI.
Our study has limitations: firstly, the overall number of patients remains small in a single center. However, GCA is a relatively rare disease, and diplopia in GCA patients is even rarer. While HR MRI seems to present encouraging performance, our results should be taken with caution and require further and larger studies to be confirmed. Secondly, this study has been conducted in a tertiary referral center specialized in ophthalmological diseases, which might have led to a selection bias, by recruiting more GCA patients presenting with ophthalmological complications. Thirdly, we used 3D HR MRI with an isotropic voxel of 0.55 mm at 3 T, which might not be practical across all medical centers worldwide and limits its generalization. Fourthly, we included patients with GCA only; thus, we could not specify whether 3rd cranial nerve signal abnormalities are specific of GCA-related diplopia. It would be valuable to determine whether the presence of an enhancement of the 3rd cranial nerve as well as its particular localization might help to distinguish GCA from other causes of 3rd cranial nerve impairment, such as non-arteritic microvascular cranial nerve palsy. Properly and quickly identifying the cause of a 3rd cranial nerve palsy using HR MRI would be valuable in clinical practice to adapt management and start prompt, urgent treatment of corticosteroids for patients with GCA-related diplopia. We could not evaluate the specificity of the “check mark sign,” which we described in this study. This striking pattern was described by all readers. It appeared to be useful to localize 3rd cranial nerve signal abnormalities. It might be a valuable sign to diagnose GCA as well. However, our study remains only exploratory and does not provide enough data to answer this question. Further prospective studies enrolling patients presenting with 3rd cranial nerve palsy from various causes are needed. Meanwhile, we recommend searching for GCA when signal abnormalities of the 3rd cranial nerve are visible on MRI. Fourthly, inter- and intrareader agreement was good for assessing enhancement of the 3rd cranial nerve alone but only moderate for assessing enhancement of any of the 3rd, 4th, and/or 6th cranial nerves. This discrepancy between readers highlights the need for specific learning and training to increase reading performance. Finally, we found that a majority of GCA patients with or without diplopia presented with inflammatory changes of the ophthalmic artery or of the posterior ciliary arteries. Inflammatory changes of orbital arteries have already been reported as very specific to GCA patients presenting with AION [16, 17]. Our results suggest that these inflammatory changes might be relevant diagnostic findings for GCA in all patients with a suspected diagnosis of GCA, regardless the presence of AION. However, one should remain cautious given the lack of a control group consisting of non-GCA patients. Further studies might determine whether HR MRI could be useful to confirm cranial nerve signal abnormalities and diagnosing GCA at the same time.
Conclusion
Our study showed that high-resolution MRI had excellent sensitivity and specificity when detecting signal abnormalities of the 3rd cranial nerve in GCA patients presenting with 3rd cranial nerve impairment.
Abbreviations
- AION:
-
Anterior ischemic optic neuropathy
- CN:
-
Cranial nerve
- GCA:
-
Giant cell arteritis
- HR MRI:
-
High-resolution magnetic resonance imaging
- MRI:
-
Magnetic resonance imaging
- WI:
-
Weighted imaging
References
Lie JT (2010) Illustrated histopathologic classification criteria for selected vasculitis syndromes. Arthritis Rheum 33:1074–1087. https://doi.org/10.1002/art.1780330804
Salvarani C, Cantini F, Boiardi L, Hunder GG (2002) Polymyalgia rheumatica and giant-cell arteritis. N Engl J Med 347:261–271. https://doi.org/10.1056/NEJMra011913
Klink T, Geiger J, Both M et al (2014) Giant cell arteritis: diagnostic accuracy of MR imaging of superficial cranial arteries in initial diagnosis—results from a multicenter trial. Radiology 273:844–852. https://doi.org/10.1148/radiol.14140056
Rhéaume M, Rebello R, Pagnoux C et al (2017) High-resolution magnetic resonance imaging of scalp arteries for the diagnosis of giant cell arteritis: results of a prospective cohort study. Arthritis Rheum 69:161–168. https://doi.org/10.1002/art.39824
Bley TA, Wieben O, Vaith P et al (2004) Magnetic resonance imaging depicts mural inflammation of the temporal artery in giant cell arteritis. Arthritis Care Res 51:1062–1063. https://doi.org/10.1002/art.20840
Poillon G, Collin A, Benhamou Y et al (2019) Increased diagnostic accuracy of giant cell arteritis using three-dimensional fat-saturated contrast-enhanced vessel-wall magnetic resonance imaging at 3 T. Eur Radiol. https://doi.org/10.1007/s00330-019-06536-7
Siemonsen S, Brekenfeld C, Holst B et al (2015) 3T MRI reveals extra- and intracranial involvement in giant cell arteritis. AJNR Am J Neuroradiol 36:91–97. https://doi.org/10.3174/ajnr.A4086
Remond P, Attyé A, Lecler A et al (2017) The central bright spot sign: a potential new MR imaging sign for the early diagnosis of anterior ischemic optic neuropathy due to giant cell arteritis. AJNR Am J Neuroradiol 38:1411–1415. https://doi.org/10.3174/ajnr.A5205
Bley TA, Markl M, Schelp M et al (2008) Mural inflammatory hyperenhancement in MRI of giant cell (temporal) arteritis resolves under corticosteroid treatment. Rheumatology (Oxford) 47:65–67. https://doi.org/10.1093/rheumatology/kem283
Bley TA, Uhl M, Carew J et al (2007) Diagnostic value of high-resolution MR imaging in giant cell arteritis. ANJR Am J Neuroradiol 28:1722–1727. https://doi.org/10.3174/ajnr.A0638
Dejaco C, Ramiro S, Duftner C et al (2018) EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 77:636–643. https://doi.org/10.1136/annrheumdis-2017-212649
Hellmich B, Agueda A, Monti S, et al (2019) 2018 update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 79(1):19–30. https://doi.org/10.1136/annrheumdis-2019-215672
Vodopivec I, Rizzo JF (2018) Ophthalmic manifestations of giant cell arteritis. Rheumatology (Oxford) 57:ii63–ii72. https://doi.org/10.1093/rheumatology/kex428
Hayreh SS, Podhajsky PA, Zimmerman B (1998) Ocular manifestations of giant cell arteritis. Am J Ophthalmol 125:509–520. https://doi.org/10.1016/s0002-9394(99)80192-5
Ross A, Jivraj I, Rodriguez G et al (2019) Retrospective, multicenter comparison of the clinical presentation of patients presenting with diplopia from giant cell arteritis vs other causes. J Neuroophthalmol 39:8–13. https://doi.org/10.1097/WNO.0000000000000656
Mohammed-Brahim N, Clavel G, Charbonneau F et al (2019) Three Tesla 3D high-resolution vessel wall MRI of the orbit may differentiate arteritic from nonarteritic anterior ischemic optic neuropathy. Invest Radiol 54:712. https://doi.org/10.1097/RLI.0000000000000595
Sommer NN, Treitl KM, Coppenrath E et al (2018) Three-dimensional high-resolution black-blood magnetic resonance imaging for detection of arteritic anterior ischemic optic neuropathy in patients with giant cell arteritis. Invest Radiol 53:698–704. https://doi.org/10.1097/RLI.0000000000000500
Weisenburger-Lile D, Obadia M, Cahuzac A, Lecler A (2018) Ophthalmic artery MRI in an arteritis-related central retinal artery occlusion. Neurology 90:188–189. https://doi.org/10.1212/WNL.0000000000004864
Bossuyt PM, Reitsma JB, Bruns DE et al (2015) STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Radiology 277:826–832. https://doi.org/10.1148/radiol.2015151516
Hunder GG, Bloch DA, Michel BA et al (1990) The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 33:1122–1128
Mandell DM, Mossa-Basha M, Qiao Y et al (2017) Intracranial vessel wall MRI: principles and expert consensus recommendations of the American Society of Neuroradiology. ANJR Am J Neuroradiol 38:218–229. https://doi.org/10.3174/ajnr.A4893
Tan HW, Chen X, Maingard J et al (2018) Intracranial vessel wall imaging with magnetic resonance imaging: current techniques and applications. World Neurosurg 112:186–198. https://doi.org/10.1016/j.wneu.2018.01.083
Lindenholz A, van der Kolk AG, Zwanenburg JJM, Hendrikse J (2018) The use and pitfalls of intracranial vessel wall imaging: how we do it. Radiology 286:12–28. https://doi.org/10.1148/radiol.2017162096
Ozanne A, Pereira V, Krings T et al (2008) Arterial vascularization of the cranial nerves. Neuroimaging Clin 18:431–439. https://doi.org/10.1016/j.nic.2007.12.010
Thurtell MJ, Longmuir RA (2014) Third nerve palsy as the initial manifestation of giant cell arteritis. J Neuroophthalmol 34:243–245. https://doi.org/10.1097/WNO.0000000000000116
Kim JH, Hwang J-M (2017) Imaging of cranial nerves III, IV, VI in congenital cranial dysinnervation disorders. Korean J Ophthalmol 31:183–193. https://doi.org/10.3341/kjo.2017.0024
Ramkumar M, Sharma S, Jacob TG, Bhardwaj DN, Nag TC, Roy TS (2014) The human trochlear and abducens nerves at different ages - a morphometric study. Aging Dis 6:6–16. https://doi.org/10.14336/AD.2014.0310
Acknowledgements
Laura McMaster provided professional English-language medical editing of this article.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Augustin Lecler.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise. No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
•retrospective
•case-control study; diagnostic study/observational
•performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 2821 kb)
Rights and permissions
About this article
Cite this article
Mournet, S., Sené, T., Charbonneau, F. et al. High-resolution MRI demonstrates signal abnormalities of the 3rd cranial nerve in giant cell arteritis patients with 3rd cranial nerve impairment. Eur Radiol 31, 4472–4480 (2021). https://doi.org/10.1007/s00330-020-07595-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07595-x