Abstract
Objectives
To retrospectively evaluate diagnostic accuracy and complications of magnetic resonance imaging (MRI)-guided biopsy of radiologically indeterminate solid renal masses (RM).
Methods
Electronic records of all consecutive patients undergoing MRI-guided biopsy of solid RM (using free-breathing T2-BLADE and BEAT-IRTTT sequences) between April 2014 and October 2018 were reviewed; 101 patients (69 men, 32 women; median age 68 years; range 32–76) were included. Patient and RM characteristics, procedural details/complications, pathologic diagnosis, and clinical management were recorded. Diagnostic accuracy was calculated on an intention-to-diagnose basis. Diagnostic yield was also evaluated. Multi-variable analysis was performed for variables with p < .20, including patient age/sex; RM size/location/contact with vascular pedicle, RENAL score, number and total length of biopsy samples, and biopsy tract embolization, to determine factors associated with diagnostic samples, diagnostic accuracy, and complications.
Results
Median RM size was 2.4 cm (range 1–8.4 cm). There were 86 (85%; 95%CI 77–91%) diagnostic and 15 (15%; 95%CI 9–23%) non-diagnostic samples; 6/15 (40%) non-diagnostic biopsies were repeated with 50% malignancy rate. Sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy were 96% (95%CI 89–99%), 100% (95%CI 77–100%), 100% (95%CI 95–100%), 82% (95%CI 57–96%), and 97% (95%CI 90–99%), respectively. Primary and secondary diagnostic yields were 85% (95%CI 77–91%) and 91% (95%CI 84–96%), respectively. Seven (7%; 95%CI 1–10%) complications were observed. No tested variables were associated with diagnostic samples, diagnostic accuracy, or complications.
Conclusions
MRI-guided biopsy of solid RM is associated with high diagnostic accuracy and low complication rate. The technique might be helpful for inaccessible tumors.
Key Points
• MRI-guided biopsy of radiologically indeterminate solid renal masses (RM) appears safe, with a low rate of minor self-limiting hemorrhagic complications.
• Diagnostic accuracy and primary/secondary diagnostic yield are high and appear similar to reported estimates for US- and CT-guided RM biopsy.
• MRI guidance may be particularly useful for RM with poor conspicuity on US and CT, for relatively inaccessible tumors (e.g., tumors requiring double-oblique steep-angled approaches), and for young patients or those with renal failure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The incidence of solid renal masses (RM) has increased in the last decades [1, 2]. The majority of the increase comprises small (< 4 cm) localized RM, a minority of which are benign or of low malignant potential [3, 4]. In these cases, imaging has limited diagnostic accuracy [5], and biopsy is used to characterize radiologically indeterminate RM to avoid overtreatment [4, 6].
Ultrasound (US) and computed tomography (CT) are the most common imaging modalities used to guide percutaneous RM biopsy [7,8,9,10,11,12,13,14,15,16,17,18,19], given their easy availability on a large scale, real-time imaging and no radiation exposure (US), and large field of view (CT).
Recently, magnetic resonance imaging (MRI) has emerged as an alternative imaging guidance modality offering superior contrast resolution, large field of view, and real-time multi-planar 3D imaging capabilities, without radiation exposure or need for contrast agent administration to identify the target [20, 21]. In particular, MRI guidance has been sporadically applied to percutaneous renal tumor ablation [21], and for a range of abdominal biopsies [20, 22, 23] including RM in a small population [24]. However, no large data reporting on the diagnostic performance and safety of MRI-guided RM biopsy are currently available.
In our institution, we have the opportunity to perform a rather large number of MR-guided biopsies due to local preferences and machine availability, which allows diagnostic performance and safety analyses of MRI-guided RM biopsy in a relatively large population. Accordingly, the aim of our retrospective non-comparative study was to evaluate the diagnostic accuracy and safety of percutaneous MRI-guided biopsy in patients with radiologically indeterminate RM referred to our interventional radiology department over a 4.5-year period. Although our study did not aim at advising for a systematic adoption of MR guidance instead of more established modalities (i.e., US/CT), its results may contribute to clarify whether: (a) MRI guidance may be considered a valid alternative to US/CT for RM biopsy and (b) there are some specific clinical scenarios that may particularly benefit from this relatively unusual modality of guidance.
Materials and methods
This retrospective study was approved by the institutional review board with permission to perform chart review and a waiver of written informed consent.
Patient selection
All consecutive patients referred to our Department of Interventional Radiology for percutaneous MRI-guided biopsy of radiologically indeterminate solid RM between April 2014 and October 2018 were identified by searching our radiology information system (Xplore; EDL). Three keywords were used (“MRI,” “biopsy,” and “kidney”) and 104 consecutive patients were identified. Three patients were excluded: one undergoing peri-renal pseudo-mass biopsy (final diagnosis: Erdheim-Chester disease), and two without visible RM on pre-procedural planning MRI sequences (one small suspected renal metastasis in a cancer patient, which was not identified on planning MRI probably due to lesion regression under chemotherapy; and one obese (hindering US) patient with renal insufficiency, who received MRI-guided renal parenchymal biopsy, and was erroneously selected by the radiology information system search according to the three entered keywords). Therefore, the final study population comprised 101 patients (69 (68%) men; 32 (32%) women; median age 68 years (range 32–76, inter-quartile ranges [IQR] 60–76 years); 69 years for men (IQR 61–77 years) and 64 years for women (IQR 56–72 years); p = .04)) (Fig. 1).
All study participants were referred for biopsy by a multi-disciplinary tumor board involving oncologists, urologists, and (diagnostic and interventional) radiologists on the basis of their clinical history and previous cross-sectional imaging (i.e., contrast-enhanced CT or MRI showing RM with predominantly solid [> 50%] appearances and absence of internal macroscopic fat to suggest benignity, thus being considered indeterminate and referred for biopsy). The interventional radiologist taking part in the tumor board decided the best suited imaging modality to guide the biopsy. At our institution, MR-guided biopsy has become the first-line procedure for tissue sampling of RM in non-urgent cases since April 2014, due to the easy access we have to an interventional MRI suite with a 1.5-T large-bore unit (Magnetom Aera; Siemens; bore diameter 70 cm, length 140 cm) where one-third of the scanner time is dedicated for interventions. Therefore, we reserve US and CT guidance for patients with contra-indications to MRI (e.g., severe obesity [body mass index ≥ 35], known claustrophobia, non-MRI compatible indwelling devices), or for urgent biopsies where the interventional MRI suite is unavailable at short notice (fully booked for diagnostic/research scanning during two-thirds of the scanner time). Accordingly, a majority of multidisciplinary tumor board referrals (predominantly non-urgent cases) were selected to undergo MR-guided biopsy; and a minority of patients presenting acutely and requiring urgent biopsy, or with contra-indications to MRI, underwent US-/CT-guided biopsy, excluding them from the study population.
Percutaneous MRI-guided renal mass biopsy
All procedures were performed by five interventional radiologists with ≥ 5 years’ experience in percutaneous MRI-guided procedures (R.L.C., 5 years; J.C. and G.K., 6 years; and J.G. and A.G., 11 years) on an inpatient basis under local anesthesia. The integrated body coil was used without intravenous contrast medium (Fig. 2). Free-breathing T2 BLADE sequences (TE/TR 178/3420 ms; flip angle 150°; 30 slices; field-of-view 400 mm × 400 mm; reconstructed in-plane resolution 2 mm × 2 mm; slice thickness 4 mm; total acquisition time 96 s) were used to localize RM (i.e., no diagnostic MRI was performed on the day of the procedure) and to rule out complications at the end of the procedure. Continuous MR-fluoroscopic guidance, facilitated using a multiplanar real-time free-breathing sequence (Video) (BEAT-IRTTT, Siemens; TE/ TR 2.2/5.35 ms, flip angle 50°, field-of-view 400 mm × 400 mm, reconstructed in-plane resolution 1.8 mm × 1.8mm, slice thickness 4 mm, acquisition time per slice 815 ms) and with continuous manual acquisition-plane adjustment to simultaneously demonstrate the needle long-axis and target lesion (Fig. 3), was used to administer local anesthesia using a 22-G spinal needle (KIM-22; ITP); and thereafter to advance a 16-G co-axial cannula (KIM-16; ITP) inside the target RM. A semi-automatic 18-G needle (BIM-18; ITP) was co-axially introduced to obtain one or more needle-core samples depending on subjective operator evaluation of sample adequacy in the absence of an on-site pathologist. Samples were sent for pathological analysis performed by a specialist genito-urinary pathologist (V.L., 15 years’ experience). Embolization of the biopsy tract was performed by injection of a hemostatic matrix (Curaspon®; Curamedical) through the co-axial cannula when bleeding was noted through it [17]. Patients were admitted overnight to the urology department and discharged the following day if well.
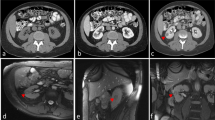
A 65-year-old female presenting with a 13-mm, radiologically indeterminate, endophytic left renal mass. a Axial unenhanced CT image demonstrates poor lesion conspicuity. Following contrast administration, the lesion was transiently visible on (b, arrow) arterial and (c, arrow) portal venous phase axial CT images. d B-Mode US image demonstrates multiple hyperechoic pseudo-nodules (arrows); the lesion seen on CT was not definitively identified. e Axial prone unenhanced T2-weighted MRI and (f) real-time MR fluoroscopy images clearly delineate the target-lesion (with overlaid planned needle trajectory in e). g–i Placement of biopsy system in the renal mass (arrows) under multi-planar MR-fluoroscopy guidance ((g) axial, (h) sagittal , and (i) coronal planes). The histopathological examination of the biopsy revealed a clear cell papillary renal cell carcinoma (ISUP grade 2) in hematoxylin and eosin (j), with characteristic immunohistochemical profile (negativity for α-methylacyl-CoA racemase/AMACR (k); diffuse reactivity for cytokeratin 7 (l); and membranous cup-like staining by carbonic anhydrase IX (m))
A 69-year-old female with a 35-mm radiologically indeterminate left upper pole RM, seen on coronal oblique MR fluoroscopy image (a). b, c Static images from axial oblique and coronal oblique real-time MR fluoroscopy sequences simultaneously demonstrate the needle long-axis and target lesion. A double-oblique steep-angle approach was used to avoid the costo-diaphragmatic recess and pleural margin (dotted line in c). Biopsy was performed accurately and safely, with pathologic examination revealing a clear cell carcinoma
Data collection
Chart review was performed in consensus by two interventional radiologists (L.L., 3 years’ experience; R.L.C., 5 years’ experience) blinded to imaging and procedural information at the time of clinical/pathological data collection. Inter- and intra-observer variabilities were not specifically assessed.
The following data were collected: patient characteristics (age, sex, suspected extra-renal malignancy); RM characteristics (size, location, contact with the main vascular pedicle, calculated RENAL score); procedural details (procedure time, number of biopsy samples, total length of biopsy sample, biopsy tract embolization, complications); pathologic diagnosis (histopathology and surgical pathology if available); and subsequent clinical management.
RM size was measured as the largest diameter on axial T2 BLADE planning sequences. RM location and RENAL score were assessed according to Kutikov and Uzzo [25], and included evaluation of side of renal involvement (left/right), location relative to pyelic axis (anterior/posterior) and renal polar lines (above, below, or in-between), and whether lesions were exophytic (> 50% volume projecting outside cortex), endophytic (< 50% volume projecting outside cortex), or intra-parenchymal (wholly within the kidney). Contact with the main vascular pedicle was defined as absence of the fat plane between the RM and main branch renal vessels. Procedural time was calculated as the interval between planning and post-procedural MRI sequences. Complications were classified according to Clavien et al [26].
Biopsy samples were classified as non-diagnostic on the basis of insufficient material for analysis, non-renal tissue, or normal renal parenchyma [16]. Non-diagnostic samples were considered negative for malignancy and included in diagnostic accuracy calculations where appropriate. Diagnostic accuracy was determined using the following definitions: (a) true positive (TP): malignant histopathology; (b) false positive (FP): defined as zero based on TP definition and supported by minimal FP rates described in prior studies [13, 18]; (c) true negative (TN): benign/non-diagnostic histopathology and RM stability/regression on minimum 12-month MRI follow-up; (d) false negative (FN): benign histopathology and RM progression (e.g., RM increasing in size, metastatic spread) on minimum 12-month MRI follow-up, or non-diagnostic sample with malignant histology on repeat biopsy.
Statistical analysis
Descriptive statistics were used to summarize patient and RM characteristics, procedural details, pathologic results, and clinical management. Categorical variables were expressed as numbers and percentages. Continuous variables were expressed as medians with interquartile ranges (IQR), and analyzed using Wilcoxon’s test. Complications, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated using an intention-to-diagnose analysis. Exact (Clopper-Pearson) 95% confidence intervals (CI) were obtained. Primary and secondary diagnostic yields were also assessed.
The following data were analyzed to identify factors associated with complications, diagnostic samples, and diagnostic accuracy: patient age/sex; RM size; RM location (relative to kidney side; renal parenchyma; pyelic axis; polar lines; and contact with the main vascular pedicle); RENAL score; number and total length of biopsy samples; and biopsy tract embolization.
Optimal cutoff values for RM size and total length of biopsy sample were determined using the Youden method, based on ROC curves outlining the highest combination of sensitivity and specificity for obtaining diagnostic samples (Supplementary Fig. 1).
Odds ratios and p values were calculated using Fisher’s exact test or a generalized linear model, as appropriate. Variables with p value < .20 were tested in a multivariable logistic regression model. P values < .05 were considered statistically significant. Statistical analysis was performed using R v3.5.3 (R Foundation for Statistical Computing).
Results
Baseline patient characteristics
Patient and tumor characteristics are summarized in Table 1. Indications for biopsy were differentiation between primary and secondary tumors in 3 patients with known extra-renal malignancy (one melanoma, one lymphoma, and one neuroendocrine tumor), and characterization of symptomatic or incidentally detected solid RM (in the remainder). Eleven (11/101; 11%) patients had suspected metastatic disease. No patients had undergone prior surgery or ablation.
Median RM size was 2.4 cm (range 1–8.4 cm; IQR 1.9–3.0 cm); 7% (7/101) RM in non-surgical patients measured > 4 cm. Optimal size cutoff for obtaining diagnostic samples was 2.3 cm, with 39% (39/101) RM measuring < 2.3 cm, and 61% (62/101) ≥ 2.3 cm. Thirty-six percent (36/101) RM were above the upper renal line, 50% (51/101) anterior to the pyelic axis, 55% (56/101) intra-parenchymal or endophytic, and 18% (18/101) contacted the main vascular pedicle. Median RENAL score was 6 (range 4–12; IQR 4–7), with 37% (37/101) RM scoring 7–12 (moderate/high complexity).
Median procedure time was 41 min (range 20–70; IQR 35–50). A median of 3 samples was obtained per procedure (range 1–6; IQR 2–3; < 3 samples in 34% [34/101] RM; ≥ 3 samples in 66% [67/101] RM). Median total length of biopsy sample was 2.4 cm (range 0.3–7.2 cm; IQR 13–38). Optimal cutoff for obtaining diagnostic samples was 1.2 cm, with 16% (16/101) samples measuring < 1.2 cm, and 84% (85/101) ≥ 1.2 cm. Biopsy tract embolization was performed in 53% (54/101) RM.
Pathologic results and clinical management
Histopathological results and clinical management are summarized in Table 2. There were 86/101 (85%; 95%CI 77–91%) diagnostic and 15/101 (15%; 95%CI 9–23%) non-diagnostic biopsies. After applying the uni- (Supplementary Table 1) and multi-variable models, only female sex was associated with non-obtaining a diagnostic sample (OR, 0.2 [95%CI 0.1–0.8]; p = .02).
Among 86/101 diagnostic biopsies, 71/101 (70%; 95%CI 60–79%) were malignant and 15/101 (15%; 95%CI 9–23%) benign. The most common malignancy was renal cell carcinoma (RCC; n = 68). RCC subtyping was always possible, and the International Society of Urologic Pathologists (ISUP) grade [27] was available for 57/68 (84%) samples (mean ISUP 2; median 2; range 1–4; IQR 2–2). Non-renal tumors included two metastases and one lymphoma. The most common benign tumor was oncocytoma (n = 11).
Of 71 patients with malignant biopsies, 21 (30%; all RCC; ISUP grade available in 16/21 specimens; mean ISUP grade 2; median 2; range 1–4; IQR 2–3) underwent surgical resection. Histological concordance between biopsy and surgery was 100% for malignancy and RCC subtype. Among the 12 (of 21) operated RM with available ISUP grade both on biopsy and surgery, concordance was 33% (4/12), with 67% (8/12) biopsies reporting ISUP grades one point lower than surgery. A further 45 patients (63%) were treated with percutaneous cryoablation, and 3 (4%) with chemotherapy. Only 2 patients (3%) with melanoma and lymphoma, respectively, underwent active surveillance (6–12 monthly MRI follow-up).
Among 15 patients with benign biopsies, 6 (40%) demonstrated RM stability at 12-month MRI follow-up, and 9 did not complete the minimum 12-month follow-up. Of the 15 patients with non-diagnostic biopsies, 6 (40%) underwent repeat MRI-guided biopsy demonstrating 3 clear cell RCC (50% malignancy rate; all treated with cryoablation), 1 oncocytoma, and 2 samples of normal renal parenchyma (identical to initial biopsy; considered TN based on stability at 12-month MRI follow-up). A further 5 patients demonstrated RM stability at 12-month MRI follow-up, and 4 were lost to follow-up.
Among 27 patients with benign or non-diagnostic biopsies scheduled for 6–12 monthly MRI surveillance, 14 (52%) completed ≥ 12-month follow-up (median 14 months; range 12–42; IQR 12–33), with all RM remaining stable.
Diagnostic accuracy
After excluding patients with diagnostic benign (n = 9) or non-diagnostic (n = 4) findings on the first biopsy who had insufficient follow-up (Fig. 1), there were 71 TP (81%; 95%CI 71–88%), 14 TN (16%; 95%CI 9–25%), 3 FN (3%; 95%CI 1–10%), and no FP results (Table 2). Sensitivity, specificity, PPV, NPV, and diagnostic accuracy were calculated using an intention-to-diagnose analysis (Table 3). None of the tested variables was associated with increased diagnostic accuracy (Supplementary Table 2).
Primary and secondary (accounting for repeat biopsies) diagnostic yields were 85% (86/101; 95%CI 77–91%) and 91% (92/101; 95%CI 84–96%), respectively.
Complications
Seven (7%; 95%CI 1–10%) grade 1 (i.e., any deviation from normal postoperative course without need for pharmacological, surgical, endoscopic, or radiological interventions in accordance with Clavien et al [26]) complications were observed, all being small self-limiting peri-renal hematomas on post-procedural MRI sequences. These were managed conservatively with routine overnight observation and intravenous fluid administration; no additional hospitalization, transfusion, or procedures were required. None of the tested variables was associated with occurrence of complications (Supplementary Table 3).
Discussion
MRI-guided RM biopsy demonstrated diagnostic sensitivity, NPV, overall accuracy, and primary and secondary diagnostic yields of 96%, 82%, 97%, 85%, and 91%, respectively. There were 7% minor complications. None of the tested variables was associated with diagnostic accuracy or complications, probably due to low numbers of FN/non-diagnostic biopsies and adverse events. Although female sex was statistically associated with obtaining diagnostic samples, this relationship is of limited significance due to the resulting OR.
Our results are similar to previous estimates of sensitivity (91–99.1%), NPV (50–100%), diagnostic accuracy (85.5–97.1%), primary/secondary diagnostic yield (78–92.8%/86.1–94%), and major/minor complications (0–0.9%/1.8–20.3%) from series and meta-analyses of US-/CT-guided RM biopsies [4, 11,12,13,14,15,16,17, 28,28,29,30,31,32,33,35].
We had anticipated that superior RM conspicuity and real-time multi-planar imaging guidance granted by MRI guidance would result in fewer non-diagnostic biopsies. However, our non-diagnostic rate (15%) was similar to that of US/CT guidance (7.2–22%) [4, 12,13,14,15,16,17, 29,29,30,31,32,34]. Prior studies have reported associations between non-diagnostic biopsies and small RM (< 2 cm), upper, anterior, and endophytic locations, longer skin lesion distances, and cystic/non-enhancing tumors [4, 12, 13, 17, 31, 32]. Since in our study, 39% RM were small (< 2.3 cm), 36% and 50% superiorly and anteriorly located, respectively, and 55% endophytic, it is possible that these factors may have compromised our diagnostic yield. However, similar non-diagnostic rates have been widely reported across literature [4, 12,13,14, 16, 17, 29,29,30,31,32,34], and it is possible that these results occur secondary to tumor heterogeneity/fragmentation and difficulties in histopathological analysis, rather than technical factors [32].
In terms of diagnostic accuracy, comparison between our study and prior reports should be undertaken with caution due to multiple confounding factors including heterogeneous study definitions and analysis of non-diagnostic results [6, 14]. In fact, we treated non-diagnostic biopsies as negative for malignancy and included them in diagnostic accuracy calculations, since (a) it was unclear whether non-diagnostic biopsies were related to the presence of underlying tumor, and (b) clinical management resembles that of benign biopsies undergoing imaging follow-up and/or repeat biopsy [29, 36]. Although this approach differs from many studies excluding non-diagnostic biopsies from analysis [14], it was considered appropriate for our series due to otherwise suboptimal sensitivity for FN detection in the absence of surgical correlation for benign biopsies, and given the limited ability of 12-month MRI surveillance to detect significant interval growth of biopsy-benign indolent RM. Excluding non-diagnostic results with repeat malignancy-positive biopsies would reduce our FN to zero, and fail to communicate the clinically suboptimal NPV of RM biopsy observed herein and throughout literature [14, 28, 30, 33, 34]. Further potential sources of study heterogeneity include our relatively low referral and selection bias since a vast majority of patients with RM undergo MRI-guided biopsy at our institution; and relatively high differential verification bias since, due to our increasing surveillance and percutaneous cryoablation of RM, only 30% malignant vs. 0% benign biopsies underwent surgery. Overall, given the potentially significant heterogeneity between studies, further direct comparative investigations are required to confirm equivalent diagnostic accuracy between MRI- and CT-/US-guided RM biopsy.
The most frequently reported complications of CT-/US-guided RM biopsy are peri-renal hematomas (1.8–15%), pain (1.2–5%), hematuria (~ 1%), hemorrhage (~ 0.4%), and pneumothorax (~ 1%) (12, 14–17, 33). Our study’s minor peri-renal hematoma rate (7%) is similar to that reported above, and likely reflects greater dependence of hemorrhagic complications (and pain) on physical tumor sampling and bleeding risk, rather than on imaging guidance modality. In contradistinction, pneumothorax more convincingly may depend on operator visualization of the pleura, and may potentially correlate with imaging guidance modality. The risk is greatest for anterior/superior RM, often requiring double-oblique steep-angle approaches with needle trajectories adjacent to the pleural margin. Despite a significant proportion of unfavorably located RM (36% above the upper renal line), we observed no pneumothoraces in our study, in accordance with prior similar experiences reporting very steep needle angulations [23, 24]. However, due to the low number of reported events in literature, comparison with US/CT studies is limited, and it remains unclear whether MRI guidance is associated with lower pneumothorax rates than US/CT guidance.
Given the apparently similar diagnostic performance and complication rate among MRI and US/CT guidance for RM biopsy, and considering the principal disadvantages (i.e., long procedure times; co-axial needle-related artifacts on MR fluoroscopy obscuring small RM; relatively high cost; limited availability; need for specialized radiographers) and technical advantages (i.e., high contrast resolution; no need for contrast medium; no radiation exposure; MR fluoroscopy) of MRI guidance, one may figure out some specific clinical scenarios which may particularly benefit from this modality including:
-
(a)
RM with poor US/CT conspicuity (e.g., small, iso-attenuating, endophytic tumors; obese patients).
-
(b)
RM in young patients or those with renal failure, to avoid radiation and contrast exposure.
-
(c)
RM in challenging locations (e.g., adjacent to non-target organs, requiring double-oblique steep-angle approaches, located in the upper renal pole or highly mobile due to respiratory motion); these may benefit from the large MRI field of view and MR fluoroscopy allowing multi-planar real-time needle navigation with compensation for respiratory motion. Although US, CT (with 30° gantry tilt), and cone beam CT have been reported for these indications, these techniques offer relatively limited needle-tip identification and/or real-time needle guidance [9].
Our study has several limitations. Firstly, the retrospective protocol precluded an US-/CT-guided control group, and the low number of observed complications and non-diagnostic samples limits statistical power to demonstrate associations between variables. We also did not evaluate the effect of operator experience on results, and inter-/intra-observer variability was not assessed. Secondly, our study is subject to referral and selection bias, since acutely presenting patients were effectively excluded due to scanner unavailability; however, these represent a small minority of referrals only. Moreover, there is differential verification bias because different reference tests were used for positive, negative, and non-diagnostic biopsy results (surgery, 12-month imaging follow-up, and repeat biopsy/12-month imaging follow-up, respectively). This is unavoidable and commonly observed throughout the literature [4, 16, 17, 29, 30], and reflects current urologic practice in which fewer RM undergo surgery (21% herein; 20–33% reported elsewhere [4, 12, 16, 17]), and a majority are managed with ablation or imaging follow-up, thereby obviating use of a single surgical reference standard. Thirdly, our analysis of non-diagnostic biopsies, and our TN and FN definitions, are open to interpretation. As described, we included non-diagnostic results in our diagnostic accuracy calculations to more accurately reflect our clinical practice. We considered non-diagnostic biopsies with 12-month MRI stability as TN; however, these are technically indeterminate, even when repeated and showing the same result [4, 12, 13, 16, 31, 32]. In practice, our FN results depended entirely on repeat biopsies of non-diagnostic cases because no patients with benign/non-diagnostic biopsies received surgical confirmation, and 12-month MR follow-up did not detect any cases of tumor progression. Our FN rate is therefore likely underestimated because only 40% of non-diagnostic cases underwent repeat biopsy; surgical correlation was absent; 12-month imaging follow-up is insensitive for tumor progression in slow-growing, rarely metastasizing RM < 4 cm (93% of our cases) [2, 10, 18]; and a relatively high proportion of patients did not complete 12-month imaging follow-up (12.8% overall; 43.3% with benign or non-diagnostic biopsies). Despite the above, our results are comparable with literature data [4, 12,13,14, 16, 17, 29,29,30,31,32,34], thus suggesting that many of these limitations are intrinsic to this type of study. Even accounting for our high attrition rate by assuming that 9/13 (70%) incompletely followed up cases were FN (higher than expected according to literature [14, 18, 28]), sensitivity, specificity, and accuracy would still be 85.6%, 100%, and 88.1% respectively, which remains broadly similar to other published studies [14, 28].
In conclusion, MRI-guided biopsy of solid RM is safe with high diagnostic accuracy and yield; it is an alternative to more established modalities of imaging guidance for RM biopsy, and may be particularly useful for inconspicuous and inaccessible lesions. Nevertheless, prospective comparative studies are required to evaluate its potential role in the rapidly evolving management of this common clinical scenario.
Abbreviations
- FN:
-
False Negative
- FP:
-
False Positive
- ISUP:
-
International Society of Urologic Pathologists
- NPV :
-
Negative Predictive Value
- PPV:
-
Positive Predictive Value
- RCC:
-
Renal Cell Carcinoma
- RM:
-
Renal Masses
- TN:
-
True Negative
- TP:
-
True Positive
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Sahni VA, Silverman SG (2014) Imaging management of incidentally detected small renal masses. Semin Intervent Radiol 31(1):9–19
Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H (2003) Solid renal tumors: an analysis of pathological features related to tumor size. J Urol 170(6 Pt 1):2217–2220
Richard PO, Jewett MAS, Bhatt JR et al (2015) Renal tumor biopsy for small renal masses: a single-center 13-year experience. Eur Urol 68(6):1007–1013
Vogel C, Ziegelmüller B, Ljungberg B et al (2019) Imaging in suspected renal-cell carcinoma: systematic review. Clin Genitourin Cancer 17(2):e345–e355
Perera M, Papa N, Ischia J, Christidis D, Bolton D, Lawrentschuk N (2018) Trends in percutaneous renal biopsy: the evolving diagnostic pathway for the small renal mass. Urol Ann 10(3):237–239
Caoili EM, Bude RO, Higgins EJ, Hoff DL, Nghiem HV (2002) Evaluation of sonographically guided percutaneous core biopsy of renal masses. AJR Am J Roentgenol 179(2):373–378
Eshed I, Elias S, Sidi AA (2004) Diagnostic value of CT-guided biopsy of indeterminate renal masses. Clin Radiol 59(3):262–267
Braak SJ, van Melick HHE, Onaca MG, van Heesewijk JPM, van Strijen MJL (2012) 3D cone-beam CT guidance, a novel technique in renal biopsy--results in 41 patients with suspected renal masses. Eur Radiol 22(11):2547–2552
Sanchez A, Feldman AS, Hakimi AA (2018) Current management of small renal masses, including patient selection, renal tumor biopsy, active surveillance, and thermal ablation. J Clin Oncol 36(36):3591–3600
Marconi L, Dabestani S, Lam TB et al (2016) Systematic review and meta-analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Urol 69(4):660–673
Park SY, Park BK, Kim CK, Kwon GY (2013) Ultrasound-guided core biopsy of small renal masses: diagnostic rate and limitations. J Vasc Interv Radiol 24(1):90–96
Prince J, Bultman E, Hinshaw L et al (2015) Patient and tumor characteristics can predict nondiagnostic renal mass biopsy findings. J Urol 193(6):1899–1904
Patel HD, Johnson MH, Pierorazio PM et al (2016) Diagnostic accuracy and risks of biopsy in the diagnosis of a renal mass suspicious for localized renal cell carcinoma: systematic review of the literature. J Urol 195(5):1340–1347
Schmidbauer J, Remzi M, Memarsadeghi M et al (2008) Diagnostic accuracy of computed tomography-guided percutaneous biopsy of renal masses. Eur Urol 53(5):1003–1011
Leveridge MJ, Finelli A, Kachura JR et al (2011) Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur Urol 60(3):578–584
Volpe A, Mattar K, Finelli A et al (2008) Contemporary results of percutaneous biopsy of 100 small renal masses: a single center experience. J Urol 180(6):2333–2337
Rybicki FJ, Shu KM, Cibas ES, Fielding JR, vanSonnenberg E, Silverman SG (2003) Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol 180(5):1281–1287
Kim MH (2017) CT-guided biopsy of entirely endophytic small renal masses: diagnostic rates and complications using standard-dose and reduced-dose CT protocols. AJR Am J Roentgenol 208(5):1030–1036
Kühn JP, Langner S, Hegenscheid K et al (2010) Magnetic resonance-guided upper abdominal biopsies in a high-field wide-bore 3-T MRI system: feasibility, handling, and needle artefacts. Eur Radiol 20(10):2414–2421
Cazzato RL, Garnon J, Shaygi B et al (2018) How to perform a routine cryoablation under MRI guidance. Top Magn Reson Imaging 27(1):33–38
Moche M, Heinig S, Garnov N et al (2016) Navigated MRI-guided liver biopsies in a closed-bore scanner: experience in 52 patients. Eur Radiol 26(8):2462–2470
Koch G, Garnon J, Tsoumakidou G et al (2018) Adrenal biopsy under wide-bore MR imaging guidance. J Vasc Interv Radiol 29(2):285–290
Garnon J, Schlier A, Buy X et al (2015) Evaluation of percutaneous biopsies of renal masses under MRI-guidance: a retrospective study about 26 cases. Eur Radiol 25(3):617–623
Kutikov A, Uzzo RG (2009) The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 182(3):844–53
Clavien PA, Barkun J, de Oliveira ML et al (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250(2):187–196
Delahunt B, Cheville JC, Martignoni G et al (2013) The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol 37(10):1490–1504
Paterson C, Ghaemi J, Alashkham A et al (2018) Diagnostic accuracy of image-guided biopsies in small (<4 cm) renal masses with implications for active surveillance: a systematic review of the evidence. Br J Radiol 91(1090):20170761
Salem S, Ponsky LE, Abouassaly R et al (2013) Image-guided biopsy of small renal masses in the era of ablative therapies. Int J Urol 20(6):580–584
Shannon BA, Cohen RJ, de Bruto H, Davies RJ (2008) The value of preoperative needle core biopsy for diagnosing benign lesions among small, incidentally detected renal masses. J Urol 180(4):1257–1261
Posielski NM, Bui A, Wells SA et al (2019) Risk factors for complications and nondiagnostic results following 1,155 consecutive percutaneous core renal mass biopsies. J Urol 201(6):1080–1087
Seager MJ, Patel U, Anderson CJ, Gonsalves M (2018) Image-guided biopsy of small (≤4 cm) renal masses: the effect of size and anatomical location on biopsy success rate and complications. Br J Radiol 91(1085):20170666
Sofikerim M, Tatlisen A, Canoz O, Tokat F, Demirtas A, Mavili E (2010) What is the role of percutaneous needle core biopsy in diagnosis of renal masses? Urology 76(3):614–618
Wang R, Wolf JS, Wood DP, Higgins EJ, Hafez KS (2009) Accuracy of percutaneous core biopsy in management of small renal masses. Urology 73(3):586–590
Halverson SJ, Kunju LP, Bhalla R et al (2013) Accuracy of determining small renal mass management with risk stratified biopsies: confirmation by final pathology. J Urol 189(2):441–446
Shinkins B, Thompson M, Mallett S, Perera R (2013) Diagnostic accuracy studies: how to report and analyse inconclusive test results. BMJ 346:f2778
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Afshin Gangi.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Supplementary Figure. Receiver Operating Characteristic analysis for Renal Mass Size and Total Length of Biopsy Sample demonstrating assessment of optimal cut-off values for obtaining diagnostic samples. (DOCX 229 kb)
ESM 2
Video. Multiplanar real-time free-breathing MR-fluoroscopy sequence obtained during MRI-guided biopsy of a 15 mm endophytic right renal nodule in a 73-year-old man. The biopsy sample revealed a clear cell renal cell carcinoma. (MP4 14,642 kb)
Rights and permissions
About this article
Cite this article
Cazzato, R.L., De Marini, P., Auloge, P. et al. Diagnostic accuracy and safety of percutaneous MRI-guided biopsy of solid renal masses: single-center results after 4.5 years. Eur Radiol 31, 580–590 (2021). https://doi.org/10.1007/s00330-020-07160-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07160-6